GC-MS Phytochemical Profiling, Pharmacological Properties, and In Silico Studies of Chukrasia velutina Leaves: A Novel Source for Bioactive Agents
Abstract
1. Introduction
2. Results
2.1. Qualitative Phytochemical Screening
2.2. Gas Chromatography-Mass Spectroscopy (GC-MS) Analysis
2.3. Acute Toxicity Test
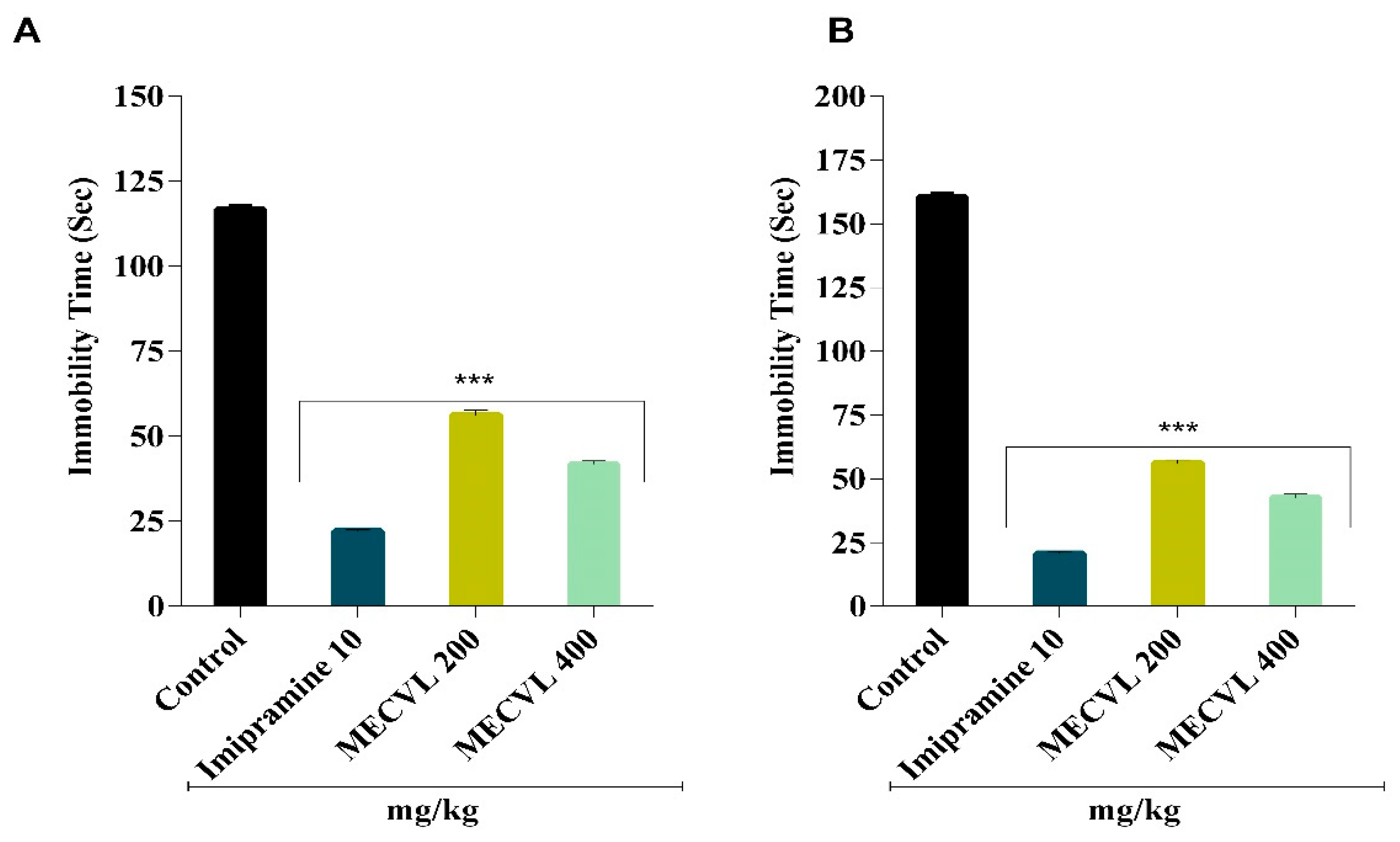
2.4. Antidepressant Activity
2.4.1. Effect of MECVL on Force Swimming Test
2.4.2. Effect of MECVL on Tail Suspension Test
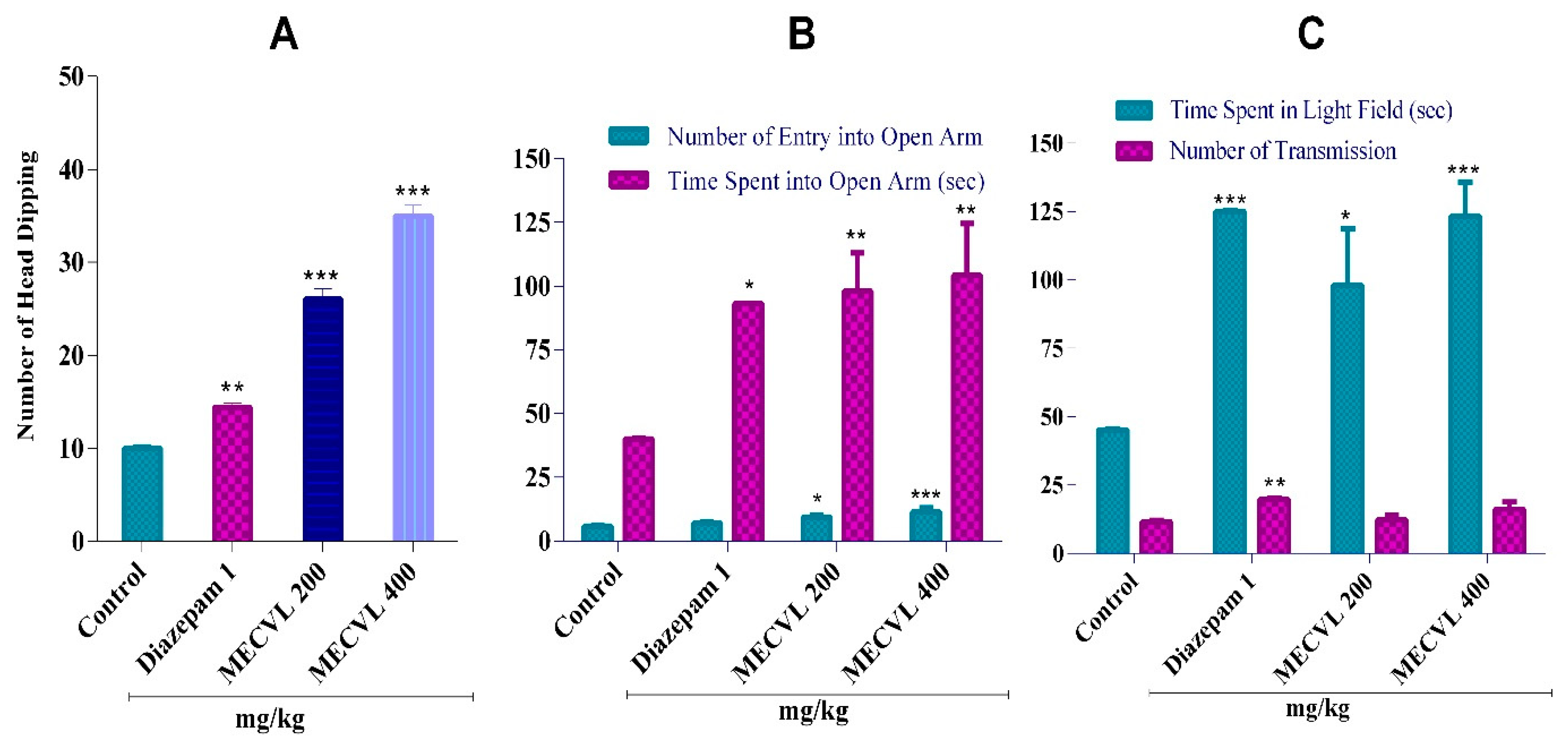
2.5. Anxiolytic Activity
2.5.1. Effect of MECVL on Hole Board Test
2.5.2. Effect of MECVL on Elevated Plus Maze Test
2.5.3. Effect of MECVL on Light/Dark Box Test
2.6. Sedative Activity
2.6.1. Effect of MECVL on Hole Cross Test
2.6.2. Effect of MECVL on Open Field Test
2.7. In Silico Studies: Pharmacokinetic Parameter Analysis by SwissADME
2.8. Molecular Docking Study for Antidepressant Activity
2.9. Molecular Docking Study for Anxiolytic Activity
2.10. Molecular Docking Study for Sedative Activity
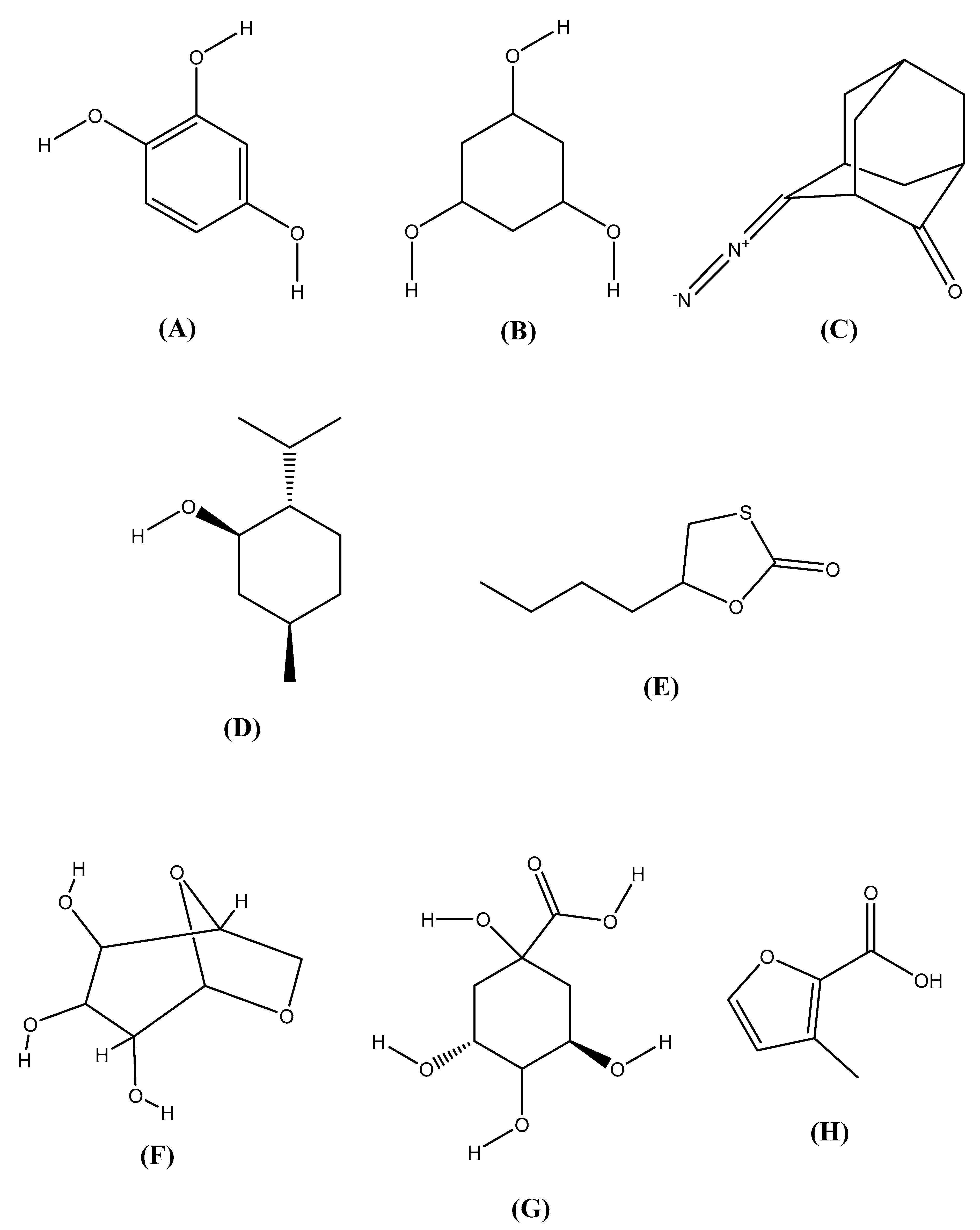
2.11. Chemical Structures
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Plant Collection, Identification, and Extract Preparation
4.3. Qualitative Phytochemical Screening of MECVL
4.4. GC-MS Analysis of MECVL
4.5. Animals and Ethical Statements
4.6. Acute Toxicity Testing of MECVL
4.7. Dosing Groups
4.8. Antidepressant Activity of MECVL
4.8.1. Force Swimming Test
4.8.2. Tail Suspension Test
4.9. Anxiolytic Activity Analysis of MECVL
4.9.1. Hole Board Test
4.9.2. Elevated Plus Maze Test
4.9.3. Light/Dark Box Test
4.10. Sedative Effect of MECVL
4.10.1. Hole Cross Test
4.10.2. Open Field Test
4.11. In Silico Molecular Docking
4.11.1. Determination of Pharmacokinetic Parameters by SwissADME
4.11.2. Molecular Docking
Ligand Preparation
Receptor/Enzyme Preparation
Glide Standard Precision Docking
5. Statistical Analysis
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Taylor, D.J.; Lichstein, K.L.; Durrence, H.H.; Reidel, B.W.; Bush, A.J. Epidemiology of insomnia, depression, and anxiety. Sleep 2005, 28, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Chy, M.N.U.; Kamal, A.T.M.M.; Chowdhury, K.A.A.; Rahman, M.A.; Reza, A.S.M.A.; Moniruzzaman, M.; Rony, S.R.; Nasrin, M.S.; Azad, M.O.K.; et al. Intervention in Neuropsychiatric Disorders by Suppressing Inflammatory and Oxidative Stress Signal and Exploration of in Silico Studies for Potential Lead Compounds from Holigarna caustica (Dennst.) Oken leaves. Biomolecules 2020, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Al Mahmud, Z.; Bachar, S.C.; Hasan, C.M.; Emran, T.B.; Qais, N.; Uddin, M.M.N. Phytochemical investigations and antioxidant potential of roots of Leea macrophylla (Roxb.). BMC Res. Notes 2017, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska, E.; Dudka, J.; Poleszak, E.; Kotlińska, J.H. Antidepressant and anxiolytic-like activity of sodium selenite after acute treatment in mice. Pharmacol. Rep. 2017, 69, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Dziubina, A.; Szmyd, K.; Zygmunt, M.; Sapa, J.; Dudek, M.; Filipek, B.; Drabczyńska, A.; Załuski, M.; Pytka, K.; Kieć-Kononowicz, K. Evaluation of antidepressant-like and anxiolytic-like activity of purinedione-derivatives with affinity for adenosine A2A receptors in mice. Pharmacol. Rep. 2016, 68, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Barua, N.; Aziz, M.A.I.; Tareq, A.M.; Sayeed, M.A.; Alam, N.; ul Alam, N.; Uddin, M.A.; Lyzu, C.; Emran, T. Bin in vivo and in vitro evaluation of pharmacological activities of Adenia trilobata (Roxb.). Biochem. Biophys. Rep. 2020, 23, 100772. [Google Scholar] [PubMed]
- Adnan, M.; Chy, M.; Uddin, N.; Kama, A.T.M.; Azad, M.; Kalam, O.; Chowdhury, K.A.A.; Kabir, M.S.H.; Gupta, S.D.; Chowdhury, M. Comparative Study of Piper sylvaticum Roxb. Leaves and Stems for Anxiolytic and Antioxidant Properties through in vivo, in vitro, and in silico Approaches. Biomedicines 2020, 8, 68. [Google Scholar] [CrossRef]
- Da Silva Oliveira, G.Z.; Cavalcanti, I.M.F.; Santos-Magalhães, N.S.; Rolim, H.M.L.; de Freitas, R.M. Development and evaluation of liposomal formulation containing nimodipine on anxiolytic activity in mice. Pharmacol. Biochem. Behav. 2014, 116, 64–68. [Google Scholar]
- Clinton, C.D.; Islam, M.; Talukder, M.B. In vivo sedative and hypnotic activities of methanol extract from the leaves of Jacquemontia paniculata (Burm.f.) Hallier f. in Swiss Albino mice. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 115–121. [Google Scholar]
- Moniruzzaman, M.; Mannan, M.A.; Khan, M.F.H.; Abir, A.B.; Afroze, M. The leaves of Crataeva nurvala Buch-Ham. modulate locomotor and anxiety behaviors possibly through GABAergic system. BMC Complement. Altern. Med. 2018, 18, 283. [Google Scholar] [CrossRef]
- Islam, A.; Hussain, M.S.; Sen, N.; Abedin, F.; Millat, M.S.; Islam, M.S.; Das, A.; Kar, A.; Hossain, M.M. Investigation of in vitro thrombolytic and anti-helminthic activity and in vivo anxiolytic and antidepressant potentiality with phytochemical nature of methanolic extract of Leucas lavandulifolia. Sustain. Chem. Pharm. 2017, 6, 61–66. [Google Scholar] [CrossRef]
- Momin, M.A.M.; Bellah, S.F.; Rahman, S.M.R.; Rahman, A.A.; Murshid, G.M.M.; Emran, T. Bin Phytopharmacological evaluation of ethanol extract of Sida cordifolia L. roots. Asian Pac. J. Trop. Biomed. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- Uddin, J.; Reza, A.S.M.A.; Kabir, M.S.H. Antinociceptive and Anxiolytic and Sedative Effects of Methanol Extract of Anisomeles indica: An Experimental Assessment in Mice and Computer Aided Models. Front. Pharmacol. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Thind, T.S.; Singh, B.; Arora, S. Inhibition of lipid peroxidation by extracts/subfractions of Chickrassy (Chukrasia tabularis A. Juss.). Naturwissenschaften 2009, 96, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Kalinganire, A.; Pinyopusarerk, K. Chukrasia: Biology, Cultivation and Utilisation; Australian Centre for International Agricultural Research: Canberra, Australia, 2000.
- Nagalakshmi, M.A.H.; Thangadurai, D.; Pullaiah, T. In vitro antimicrobial efficacy of leaf essential oils of Chukrasia tabularis Adr. Juss. and Melia dubia Cav.(Meliaceae). Phyther. Res. An Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Kong, F.-D.; Wang, H.; Mei, W.-L.; Liu, S.-B.; Zhao, Y.-X.; Dai, H.-F. Six New Phragmalin Limonoids from the Stems of Chukrasia tabularis A. Juss. Molecules 2018, 23, 3024. [Google Scholar] [CrossRef]
- Al Mahmud, Z.; Emran, T.B.; Qais, N.; Bachar, S.C.; Sarker, M.; Uddin, M.M.N. Evaluation of analgesic, anti-inflammatory, thrombolytic and hepatoprotective activities of roots of Premna esculenta (Roxb). J. Basic Clin. Physiol. Pharmacol. 2016, 27, 63–70. [Google Scholar] [CrossRef]
- Rahman, M.S.; Sultan, R.A.; Emran, T. Bin Evaluation of the anti-diarrheal activity of methanol extract and its fractions of Urena sinuata L.(Borss) leaves. J. Appl. Pharm. Sci. 2016, 6, 56–60. [Google Scholar]
- Emran, T.B.; Rahman, M.A.; Uddin, M.M.N.; Rahman, M.M.; Uddin, M.Z.; Dash, R.; Layzu, C. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro thrombolysis. BMC Complement. Altern. Med. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Adnan, M.; Uddin Chy, M.N.; Kamal, A.T.M.M.; Barlow, J.W.; Faruque, M.O.; Yang, X.; Uddin, S.B. Evaluation of anti-nociceptive and anti-inflammatory activities of the methanol extract of Holigarna caustica (Dennst.) Oken leaves. J. Ethnopharmacol. 2019, 236, 401–411. [Google Scholar] [CrossRef]
- Kabir, M.S.H.; Hossain, M.M.; Kabir, M.I.; Rahman, M.M.; Hasanat, A.; Emran, T.B.; Rahman, M.A. Phytochemical screening, Antioxidant, Thrombolytic, alpha-amylase inhibition and cytotoxic activities of ethanol extract of Steudnera colocasiifolia K. Koch leaves. J. Young Pharm. 2016, 8, 391. [Google Scholar] [CrossRef]
- Wanda, G.J.M.K.; Djiogue, S.; Gamo, F.Z.; Ngitedem, S.G.; Njamen, D. Anxiolytic and sedative activities of aqueous leaf extract of Dichrocephala integrifolia (Asteraceae) in mice. J. Ethnopharmacol. 2015, 176, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Uddin Mazumdar, M.M.; Islam, M.A.; Hosen, M.T.; Alam, M.S.; Alam, M.N.; Faruk, M.; Rahman, M.M.; Sayeed, M.A.; Rahman, M.M.; Uddin, S.B. Estimation of in vivo neuropharmacological and in vitro antioxidant effects of Tetracera sarmentosa. Cogent Biol. 2017, 3, 1300990. [Google Scholar] [CrossRef]
- Uddin, M.M.N.; Ahmed, S.; Kabir, M.S.H.; Rahman, M.S.; Sultan, R.A.; Emran, T. Bin In vivo analgesic, anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic fruits extract from Daemonorops robusta Warb. J. Appl. Pharm. Sci. 2017, 7, 104–113. [Google Scholar] [CrossRef]
- Shajib, M.; Akter, S.; Ahmed, T.; Imam, M.Z. Antinociceptive and neuropharmacological activities of methanol extract of Phoenix sylvestris fruit pulp. Front. Pharmacol. 2015, 6, 212. [Google Scholar] [CrossRef]
- Begum, A.; Hossen, A.; Moly, A.A.; Bhuiyan, M.M.R.; Shahed-Al, M.S.-A.-M. In Vivo Sedative and Anxiolytic Activities of Thunbergia erecta (Acanthaceae) Leaves Activate Gamma-Aminobutyric Acid (GABA) Mediated Hyperpolarization in Swiss Albino Mice. Pharmacol. Pharm. 2019, 10, 177–193. [Google Scholar]
- Goyal, M.; Ghosh, M.; Nagori, B.P.; Sasmal, D. Analgesic and anti-inflammatory studies of cyclopeptide alkaloid fraction of leaves of Ziziyphus nummularia. Saudi J. Biol. Sci. 2013, 20, 365–371. [Google Scholar] [CrossRef]
- Adnan, M.; Chy, N.U.; Mostafa Kamal, A.T.M.; Azad, M.O.K.; Paul, A.; Uddin, S.B.; Barlow, J.W.; Faruque, M.O.; Park, C.H.; Cho, D.H. Investigation of the Biological Activities and Characterization of Bioactive Constituents of Ophiorrhiza rugosa var. prostrata (D. Don) & Mondal Leaves through in Vivo, in Vitro, and in Silico Approaches. Molecules 2019, 24, 1367. [Google Scholar]
- Adnan, M.; Chy, M.N.U.; Kamal, A.T.M.M.; Chowdhury, M.R.; Islam, M.S.; Hossain, M.A.; Tareq, A.M.; Bhuiyan, M.I.H.; Uddin, M.N.; Tahamina, A.; et al. Unveiling Pharmacological Responses and Potential Targets Insights of Identified Bioactive Constituents of Cuscuta reflexa Roxb. Leaves through in Vivo and in Silico Approaches. Pharmaceuticals 2020, 13, 50. [Google Scholar] [CrossRef]
- Emran, T.B.; Ahmed, S.; Zahan, S.; Rakib, A.; Hasan, M.S.; Amin, M.N.; Mow, T.R.; Uddin, M.M.N. Sedative, Anxiolytic, Antinociceptive, Anti-inflammatory and Antipyretic Effects of a Chloroform Extract from the Leaves of Urena sinuata in Rodents. J. Appl. Life Sci. Int. 2018, 1–19. [Google Scholar] [CrossRef]
- Marques, T.H.C.; de Melo, C.H.S.; de Freitas, R.M. In vitro evaluation of antioxidant, anxiolytic and antidepressant-like effects of the Bellis perennis extract. Rev. Bras. Farmacogn. 2012, 22, 1044–1052. [Google Scholar] [CrossRef]
- Mahendran, G.; Vijayan, R. Neuropharmacological and molecular docking studies of xanthones from Swertia corymbosa. J. Recept. Signal Transduct. 2018, 38, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.E.; Islam, A.M.T.; Chowdhury, M.A.U.; Rahman, M.K.; Islam, M.S.; Islam, M.R. Sedative and analgesic activities of Ludwigia repens. Phytopharmacology 2012, 2, 202–211. [Google Scholar]
- Tona, M.R.; Tareq, A.M.; Sayeed, M.A.; Mahmud, M.H.; Jahan, I.; Sakib, S.A.; Shima, M.; Emran, T. Bin Phytochemical screening and in vitro pharmacological activities of methanolic leaves extract of Caryota mitis. J. Adv. Biotechnol. Exp. Ther. 2020, 3, 109–115. [Google Scholar] [CrossRef]
- Shajib, M.S.; Rashid, R.B.; Ming, L.C.; Islam, S.; Sarker, M.M.R.; Nahar, L.; Sarker, S.D.; Datta, B.K.; Rashid, M.A. Polymethoxyflavones from Nicotiana plumbaginifolia (Solanaceae) exert antinociceptive and neuropharmacological effects in mice. Front. Pharmacol. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Rahman, A.; Emran, T. Bin Sedative, anxiolytic and analgesic effects of Urena sinuata L. leaf extract in animal models. Int. Food Res. J. 2014, 21, 2069–2075. [Google Scholar]
- Tareq, A.M.; Farhad, S.; Uddin, A.B.M.N.; Hoque, M.; Nasrin, M.S.; Uddin, M.M.R.; Hasan, M.; Sultana, A.; Munira, M.S.; Lyzu, C. Chemical profiles, pharmacological properties, and in silico studies provide new insights on Cycas pectinata. Heliyon 2020, 6, e04061. [Google Scholar] [CrossRef]
- Rashid, M.M.U.; Kabir, H.; Sayeed, M.A.; Alam, R.; Kabir, M.F. Sedative and Cytotoxic Properties of the Leaf Extract of Desmodium paniculatum. J. Pharmacogn. Phytochem. 2013, 2, 63–67. [Google Scholar]
- Rakib, A.; Ahmed, S.; Islam, M.A.; Uddin, M.M.N.; Paul, A.; Chy, M.N.U.; Emran, T.B.; Seidel, V. Pharmacological studies on the antinociceptive, anxiolytic and antidepressant activity of Tinospora crispa. Phyther. Res. 2020. [Google Scholar] [CrossRef]
- Bulbul, M.; Rakibul, H.; Rahman, M.; Emran, T.B.; Afroze, M.; Khan, M.; Chowdhury, M.A.H.; Ibrahim, M.A.; Chowdhury, M.S. Leea macrophylla (Roxb.) root extract reverses CCl4 induced liver injury through upregulation of antioxidative gene expression: A molecular interaction for therapeutic inception. Orient. Pharm. Exp. Med. 2020, 20, 35–52. [Google Scholar] [CrossRef]
- Uddin, M.Z.; Rana, M.S.; Hossain, S.; Ferdous, S.; Dutta, E.; Dutta, M.; Emran, T. Bin In vivo neuroprotective, antinociceptive, anti-inflammatory potential in Swiss albino mice and in vitro antioxidant and clot lysis activities of fractionated Holigarna longifolia Roxb. bark extract. J. Complement. Integr. Med. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Nasir Uddin, M.M.; Rahman, A.; Uddin, Z.; Islam, M. Phytochemical, antimicrobial, cytotoxic, analgesic and anti-inflammatory properties of Azadirachta indica: A therapeutic study. J. Bioanal. Biomed. S 2015, 12, 2. [Google Scholar] [CrossRef]
- Rahman, M.A.; bin Imran, T.; Islam, S. Antioxidative, antimicrobial and cytotoxic effects of the phenolics of Leea indica leaf extract. Saudi J. Biol. Sci. 2013, 20, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Patel, T.R.; Whalen, M. Detoxification mechanisms for 1,2,4-benzenetriol employed by a Rhodococcus sp. BPG-8. Arch. Microbiol. 1993, 159, 136–140. [Google Scholar] [CrossRef]
- Guo, L.; Wu, J.; Han, T.; Cao, T.; Rahman, K.; Qin, L. Chemical composition, antifungal and antitumor properties of ether extracts of Scapania verrucosa Heeg. and its endophytic fungus Chaetomium fusiforme. Molecules 2008, 13, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, G.; Murugan, M.; Mohan, V.R. GC-MS analysis of bioactive components of Hugonia mystax L.(Linaceae). Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 301–308. [Google Scholar]
- Mohammed, G.J.; Omran, A.M.; Hussein, H.M. Antibacterial and phytochemical analysis of Piper nigrum using gas chromatography-mass Spectrum and Fourier-transform infrared spectroscopy. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 977–996. [Google Scholar]
- Altaee, N.; Fahdil, A.; Yousif, E.; Sudesh, K. Recovery and subsequent characterization of polyhydroxybutyrate from Rhodococcus equi cells grown on crude palm kernel oil. J. Taibah Univ. Sci. 2016, 10, 543–550. [Google Scholar] [CrossRef]
- Lantzouraki, D.Z.; Sinanoglou, V.J.; Tsiaka, T.; Proestos, C.; Zoumpoulakis, P. Total phenolic content, antioxidant capacity and phytochemical profiling of grape and pomegranate wines. RSC Adv. 2015, 5, 101683–101692. [Google Scholar] [CrossRef]
- Kurashov, E.A.; Fedorova, E.V.; Krylova, J.V.; Mitrukova, G.G. Assessment of the potential biological activity of low molecular weight metabolites of freshwater macrophytes with QSAR. Scientifica 2016, 2016. [Google Scholar] [CrossRef]
- Pivazyan, V.A.; Ghazaryan, E.A.; Shainova, R.S.; Tamazyan, R.A.; Ayvazyan, A.G.; Yengoyan, A.P. Synthesis and Growth Stimulant Properties of 2-Acetyl-3, 7-dimethyl-5H-thiazolo [3, 2-a] pyrimidin-5-one Derivatives. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Aly, A.A.; Brown, A.B.; Bedair, T.M.I.; Ishak, E.A. Dithiocarbamate salts: Biological activity, preparation, and utility in organic synthesis. J. Sulfur Chem. 2012, 33, 605–617. [Google Scholar] [CrossRef]
- Igwe, O.U.; Okwu, D.E. GC-MS evaluation of bioactive compounds and antibacterial activity of the oil fraction from the stem bark of Brachystegia eurycoma Harms. Int. J. Chem. Sci. 2013, 11, 357–371. [Google Scholar]
- Mohan, S.C.; Anand, T. In vitro Antioxidant Activity of Leaf and Bark Extracts of Barringtonia acutangula Linn. Int. Res. J. Biol. Sci. 2019, 1, 37–40. [Google Scholar]
- Sun, Y.; Hayakawa, S.; Puangmanee, S.; Izumori, K. Chemical properties and antioxidative activity of glycated α-lactalbumin with a rare sugar, D-allose, by Maillard reaction. Food Chem. 2006, 95, 509–517. [Google Scholar] [CrossRef]
- Rjabova, J.; Rjabovs, V.; Vargas, A.J.M.; Clavijo, E.M.; Turks, M. Synthesis of novel 3-deoxy-3-C-triazolylmethyl-allose derivatives and evaluation of their biological activity. Cent. Eur. J. Chem. 2012, 10, 386–394. [Google Scholar] [CrossRef]
- Stranden, M.; Liblikas, I.; König, W.A.; Almaas, T.J.; Borg-Karlson, A.-K.; Mustaparta, H. (–)-Germacrene D receptor neurones in three species of heliothine moths: Structure-activity relationships. J. Comp. Physiol. A 2003, 189, 563–577. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Pani, F.; Porcedda, S.; Ballero, M. Extraction, separation and isolation of essential oils from natural matrices by supercritical CO2. Flavour Fragr. J. 2003, 18, 505–509. [Google Scholar] [CrossRef]
- Arjouni, M.Y.; Bahri, F.; Romane, A.; Fels, M.A.E.A. El Chemical composition and antimicrobial activity of essential oil of Cupressus atlantica. Nat. Prod. Commun. 2011, 6, 1934578X1100601028. [Google Scholar]
- Mimaki, Y.; Yokosuka, A.; Kuroda, M.; SASHIDA, Y. Cytotoxic activities and structure-cytotoxic relationships of steroidal saponins. Biol. Pharm. Bull. 2001, 24, 1286–1289. [Google Scholar] [CrossRef]
- Carev, I.; Maravic, A.; Bektasevic, M.; Ruscic, M.; Siljak-Yakovlev, S.; Politeo, O. Centaurea rupestris L.: Cytogenetics, essential oil chemistry and biological activity. Croat. Chem. Acta 2018, 91, 11–19. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and biological activities of decanal, linalool, valencene, and octanal from sweet orange oil. J. Food Sci. 2012, 77, C1156–C1161. [Google Scholar] [CrossRef] [PubMed]
- Gideon, V.A. GC-MS analysis of phytochemical components of Pseudoglochidion anamalayanum Gamble: An endangered medicinal tree. Asian J. Plant Sci. Res. 2015, 5, 36–41. [Google Scholar]
- Bertelli, A.; Donati, L.; Lami, V.; Primo, G.; Rossano, M.A. Gamma-amino acid esters and the central nervous system. Int. J. Neuropharmacol. 1968, 7, 149–154. [Google Scholar] [CrossRef]
- Kontogiorgis, C.A.; Hadjipavlou-Litina, D.J. Synthesis and biological evaluation of novel coumarin derivatives with a 7-azomethine linkage. Bioorg. Med. Chem. Lett. 2004, 14, 611–614. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Gao, H. A dual antibacterial mechanism involved in membrane disruption and DNA binding of 2R, 3R-dihydromyricetin from pine needles of Cedrus deodara against Staphylococcus aureus. Food Chem. 2017, 218, 463–470. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.; Thuong, P.T.; Su, N.D.; Sok, D.; Song, K.S.; Seong, Y.H.; Bae, K. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef]
- Kwon, H.C.; Jung, C.M.; Shin, C.G.; Lee, J.K.; Choi, S.U.; Kim, S.Y.; Lee, K.R. A new caffeoly quinic acid from aster scaber and its inhibitory activity against human immunodeficiency virus-1 (HIV-1) integrase. Chem. Pharm. Bull. 2000, 48, 1796–1798. [Google Scholar] [CrossRef]
- Mannaa, M.; Kim, K.D. Effect of temperature and relative humidity on growth of Aspergillus and Penicillium spp. and biocontrol activity of Pseudomonas protegens AS15 against aflatoxigenic Aspergillus flavus in stored rice grains. Mycobiology 2018, 46, 287–295. [Google Scholar] [CrossRef]
- Ezekiel, A.; Abiodun, O.; Justina, T.; Funmilayo, A.; Omolara, P. Evaluation of Ascorbic Acid Contents in Selected Fruits using Iodometric method and UV Spectrophotometer. Adv. Nat. Appl. Sci. 2018, 12, 21–24. [Google Scholar]
- Bharathy, V.; Sumathy, B.M.; Uthayakumari, F. Determination of phytocomponents by GC-MS in leaves of Jatropha gossypifolia L. Sci. Res. Rep. 2012, 2, 286–290. [Google Scholar]
- Nice, K.J. Antimicrobial screening of secondary metabolites from Solanaceae. Ph.D. Thesis, Royal Holloway University of London, Egham, UK, September 2012. [Google Scholar]
- Thebti, A.; Sanhoury, M.A.K.; Ouzari, H.I.; Barhoumi-Slimi, T. Synthesis and Evaluation of Biological Activity of New Arylphosphoramidates. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Bhat, K.I.; Sufeera, K.; Kumar, P.C.S. Synthesis, characterization and biological activity studies of 1, 3, 4-Oxadiazole analogs. J. Young Pharm. 2011, 3, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.S.; Kumar, P.; Kumar, R.M. Hemodynamic changes in preterm neonates with septic shock: A prospective observational study. Pediatr. Crit. Care Med. 2014, 15, 443–450. [Google Scholar] [CrossRef]
- Tatipamula, V.B.; Kolli, M.K.; Lagu, S.B.; Paidi, K.R.; Reddy, R.; Yejella, R.P. Novel indolizine derivatives lowers blood glucose levels in Streptozotocin-induced diabetic rats: A histopathological approach. Pharmacol. Rep. 2019, 71, 233–242. [Google Scholar] [CrossRef]
- Schein, P.S.; Anderson, T.; McMenamin, M.G.; Bull, J. Streoptozotocin, Chlorozotocin and Related Nitrosourea Antitumour Agents. In Chemotherapy; Plenum Press: New York, NY, USA, 1976; pp. 159–166. [Google Scholar]
- Panasci, L.C.; Green, D.; Schein, P.S. Chlorozotocin: Mechanism of reduced bone marrow toxicity in mice. J. Clin. Investig. 1979, 64, 1103–1111. [Google Scholar] [CrossRef]
- Zylicz, Z.; Wagener, D.J.T.; van Rennes, H.; van der Kleijn, E.; Lelieveld, P.; van den Broek, L.A.G.M.; Ottenheijm, H.C.J. In vivo antitumor activity of sparsomycin and its analogues in eight murine tumor models. Investig. New Drugs 1988, 6, 285–292. [Google Scholar] [CrossRef]
- Balamurugan, J.; Li, C.; Thanh, T.D.; Park, O.-K.; Kim, N.H.; Lee, J.H. Hierarchical design of Cu 1− x Ni x S nanosheets for high-performance asymmetric solid-state supercapacitors. J. Mater. Chem. A 2017, 5, 19760–19772. [Google Scholar] [CrossRef]
- Abdelmageed, A.H.A.; Faridah, Q.Z.; Norhana, F.M.A.; Julia, A.A.; Kadir, M.A. Micropropagation of Etlingera elatior (Zingiberaceae) by using axillary bud explants. J. Med. Plants Res. 2011, 5, 4465–4469. [Google Scholar]
- Mohammed, H.A.; Abuobeida, I.A.M.A.; Vuthaluru, H.B.; Liu, S. Two-phase forced convection of nanofluids flow in circular tubes using convergent and divergent conical rings inserts. Int. Commun. Heat Mass Transf. 2019, 101, 10–20. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Xie, Y.; Feng, X.-L.; Huang, L.-F. Study of the volatile constituents in radix flemingiae macrophyllae and a substitute by gas chromatography-mass spectrometry and chemometric methods. Molecules 2012, 17, 14111–14125. [Google Scholar] [CrossRef] [PubMed]
- Erwin, E.; Soemardi, T.P.; Surjosatyo, A.; Nugroho, Y.S.; Nugraha, K.; Andayani, R.D. Analysis of near wake recovery scale model vawt hybrid wind turbin in wind tunnel. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Jakarta, Indonesia, 22–23 November 2018; IOP Publishing: Bristol, UK, 2019; Volume 508, p. 12068. [Google Scholar]
- Ubaid, J.M.; Kadhim, M.J.; Hameed, I.H. Study of bioactive methanolic extract of Camponotus fellah using Gas chromatography–mass spectrum. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 434–439. [Google Scholar]
- Tian, W.; Zhou, H.; Li, L. Hybrid organic–inorganic perovskite photodetectors. Small 2017, 13, 1702107. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Guerrero, M.P.; Santos-Sánchez, N.F.; Salas-Coronado, R.; Valadez-Blanco, R.; Hernández-Carlos, B.; Guadarrama-Mendoza, P.C. Productivity and Antioxidant Activity of Wild, Reconstituted, and Hybrid Strains of the Pink Oyster Mushroom, Pleurotus djamor (Agaricomycetes), from Mexico. Int. J. Med. Mushrooms 2018, 20, 607–621. [Google Scholar] [CrossRef]
- Fleury, L.M.; Kosal, A.D.; Masters, J.T.; Ashfeld, B.L. Cooperative Titanocene and Phosphine Catalysis: Accelerated C–X Activation for the Generation of Reactive Organometallics. J. Org. Chem. 2013, 78, 253–269. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; Mahmoud, A.Z.; Farshori, N.N.; Alfaraj, R.; Al-sheddi, E.S.; Alsarra, I.A. Chemical composition and antimicrobial, antioxidant, and anti-inflammatory activities of Lepidium sativum seed oil. Saudi J. Biol. Sci. 2019, 26, 1089–1092. [Google Scholar] [CrossRef]
- Hassan, W.; Noreen, H.; Castro-Gomes, V.; Mohammadzai, I.; Batista Teixeira da Rocha, J.; Landeira-Fernandez, J. Association of oxidative stress with psychiatric disorders. Curr. Pharm. Des. 2016, 22, 2960–2974. [Google Scholar] [CrossRef]
- Sitarek, P.; Rijo, P.; Garcia, C.; Skała, E.; Kalemba, D.; Białas, A.J.; Szemraj, J.; Pytel, D.; Toma, M.; Wysokińska, H. Antibacterial, anti-inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxid. Med. Cell. Longev. 2017, 2017, 7384061. [Google Scholar] [CrossRef]
- Popaj, K.; Hesse, M. Syntheses of the Macrocyclic Spermine Alkaloids (±)-Budmunchiamine A–C. Helv. Chim. Acta 2001, 84, 180–186. [Google Scholar] [CrossRef]
- Bae, J.-Y.; Ali, Z.; Wang, Y.-H.; Chittiboyina, A.G.; Zaki, A.A.; Viljoen, A.M.; Khan, I.A. Anthraquinone-based specialized metabolites from rhizomes of Bulbine natalensis. J. Nat. Prod. 2019, 82, 1893–1901. [Google Scholar] [CrossRef]
- Huang, R.-J.; Zhang, Y.; Bozzetti, C.; Ho, K.-F.; Cao, J.-J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef]
- Namgaladze, D.; Lips, S.; Leiker, T.J.; Murphy, R.C.; Ekroos, K.; Ferreiros, N.; Geisslinger, G.; Brüne, B. Inhibition of macrophage fatty acid β-oxidation exacerbates palmitate-induced inflammatory and endoplasmic reticulum stress responses. Diabetologia 2014, 57, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Akhter, S.; Rahman, M.D.; Aklima, J.; Akhter, S.; Merry, S.R.; Jubair, S.M.; Dash, R.; Emran, T. Bin Antithrombotic effects of five organic extracts of Bangladeshi plants in vitro and mechanisms in in silico models. Evid.-Based Complement. Altern. Med. 2015, 2015, 782742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Emran, T.B.; Rahman, M.A.; Uddin, M.M.N.; Dash, R.; Hossen, M.F.; Mohiuddin, M.; Alam, M.R. Molecular docking and inhibition studies on the interactions of Bacopa monnieri’s potent phytochemicals against pathogenic Staphylococcus aureus. DARU J. Pharm. Sci. 2015, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Rakib, A.; Ahmed, S.; Islam, M.A.; Haye, A.; Uddin, S.M.N.; Uddin, M.M.N.; Hossain, M.K.; Paul, A.; Emran, T. Bin Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci. Nutr. 2020, 8, 547–556. [Google Scholar] [CrossRef]
- Ahmed, S.; Rakib, A.; Islam, M.A.; Khanam, B.H.; Faiz, F.B.; Paul, A.; Chy, M.N.U.; Bhuiya, N.M.M.A.; Uddin, M.M.N.; Ullah, S.M.A.; et al. In vivo and in vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico approaches. Clin. Phytoscience 2019, 5, 36. [Google Scholar] [CrossRef]
- Dash, R.; Ahsan, T.; Hosen, S.Z.; Rahman, M.G.; Emran, T.B.; Muhammad, M. Evolution of selective COX-2 inhibitor from Alangium salvifolium: An in silico approach. J. Appl. Pharm. Sci. 2015, 2015, 5. [Google Scholar] [CrossRef]
- Dutta, T.; Paul, A.; Majumder, M.; Sultan, R.A.; Emran, T. Bin Pharmacological evidence for the use of Cissus assamica as a medicinal plant in the management of pain and pyrexia. Biochem. Biophys. Rep. 2020, 21, 100715. [Google Scholar]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and extraction: A review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S. The protein data bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Lenaeus, M.J.; Burdette, D.; Wagner, T.; Focia, P.J.; Gross, A. Structures of KcsA in complex with symmetrical quaternary ammonium compounds reveal a hydrophobic binding site. Biochemistry 2014, 53, 5365–5373. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABA A receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
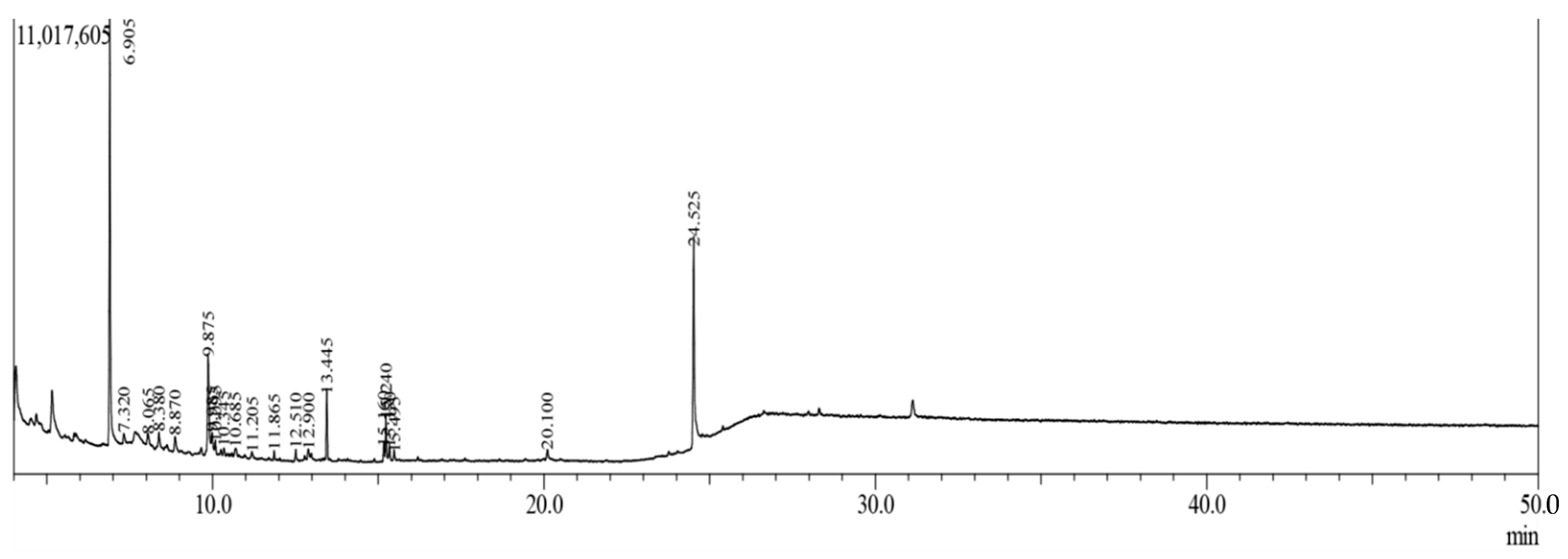
| S.N. | R.T. (min) | Compound Name | m/z | Area | Molecular Formula | Nature of Molecules |
|---|---|---|---|---|---|---|
| 1 | 6.902 | 1,2,4 Benzenetriol | 126.00 | 6106391 | C6H6O3 | Phenol |
| 2 | 6.902 | 3-Methyl-2-furoic acid | 126.00 | 6106391 | C6H6O3 | Carboxylic acid |
| 3 | 6.903 | 2,4-Octadienoic acid, 7-hydroxy-6-methyl | 63.00 | 341570 | C9H14O3 | Unsaturated carboxylic acid |
| 4 | 6.903 | Diethyl mercaptal of d-mannose | 63.00 | 341570 | C12H26O4S3 | Carbohydrate |
| 5 | 8.469 | 1,2,4-Cyclopentanetriol | 63.00 | 35297 | C5H10O3 | Cycloalkane |
| 6 | 8.469 | dl-Allo-cystathionine | 63.00 | 35297 | C7H14N2O4S | Amino acid |
| 7 | 8.469 | Phloroglucinol | 63.00 | 35297 | C6H12O3 | Phenol |
| 8 | 8.469 | Acetoacetic acid, 1,3-dithio-, S-ethyl ester | 63.00 | 35297 | C6H10OS2 | Ethyl ester of acetoacetic acid |
| 9 | 8.469 | 1-Deoxy-d-arabitol | 63.00 | 35297 | C5H12O4 | Sugar alcohol |
| 10 | 8.383 | β-d-Glucopyranose, 1,6-anhydro- | 60.00 | 256706 | C6H10O5 | Carbohydrate |
| 11 | 8.383 | d-Mannoheptulose | 60.00 | 256706 | C19H26O13 | Monosaccharide |
| 12 | 8.383 | D-Allose | 60.00 | 256706 | C6H12O6 | Aldohexose sugar |
| 13 | 8.383 | d-erythro-Pentose, 2-deoxy- | 60.00 | 256706 | C5H10O4 | Monosaccharide |
| 14 | 8.628 | Germacrene D | 63.00 | 22058 | C15H24 | Sesquiterpene |
| 15 | 8.628 | Cis-muurola-3,5-diene | 63.00 | 22058 | C15H24 | Isopropyl or carbocyclic compound |
| 16 | 8.628 | β-copaene | 63.00 | 22058 | C15H24 | Sesquiterpene |
| 17 | 9.872 | Decanal | 60.00 | 769010 | C10H20O | Saturated fatty adehyde |
| 18 | 9.872 | Dodecanoic acid, 3-hydroxy- | 60.00 | 769010 | C12H24O3 | Fatty acid |
| 19 | 9.872 | Butanoic acid, octyl ester | 60.00 | 769010 | C12H24O2 | Carboxylic ester |
| 20 | 9.872 | Decanoic acid, 2-ethylhexyl ester | 60.00 | 769010 | C18H36O2 | Carboxylic acid |
| 21 | 9.872 | Quinic acid | 60.00 | 769010 | C7H12O6 | Carboxylic acid |
| 22 | 9.872 | 1-Heptanol, 2,4-dimethyl-, (R,R)-(+)- | 60.00 | 769010 | C9H20O | Alcohol |
| 23 | 9.872 | d-Mannitol, 1-decylsulfonyl- | 43.00 | 700880 | C16H34O7S | Pentose alcohol |
| 24 | 9.872 | d-Mannitol, 1-thiohexyl-1-deoxy- | 43.00 | 700880 | C12H26O5S | Pentose alcohol |
| 25 | 9.873 | Sorbitol | 73.00 | 166357 | C6H14O6 | Sugar alcohol |
| 26 | 9.873 | d-glycero-d-manno-Heptitol | 73.00 | 166357 | C7H14O7 | Mannoheptulose |
| 27 | 9.875 | 4-Diazodamantanone | 44.00 | 117524 | C10H12N2O | Ester |
| 28 | 9.875 | 5alpha-Androstan-12-one, cyclic ethylene mercaptole | 44.00 | 117524 | C21H34S2 | Terpenoid |
| 29 | 11.013 | 3-Nonyn-2-ol | 44.00 | 15411 | C9H16O | Secondary alcohol |
| 30 | 11.013 | Pseduosarsasapogenin-5,20-dien | 44.00 | 15411 | C27H42O3 | Sapogenins |
| 31 | 11.013 | Chlorozotocin | 44.00 | 15411 | C9H16ClN3O7 | Amino sugar |
| 32 | 11.013 | Sparsomycin | 44.00 | 15411 | C13H19N3O5S2 | Amino acid |
| 33 | 11.860 | l-Gala-l-ido-octose | 73.00 | 77890 | C8H16O8 | Carbohydrate |
| 34 | 12.284 | 9-Dodecen-1-ol, acetate, (Z)- | 44.00 | 13463 | C14H26O2 | Diterpene |
| 35 | 12.284 | Cis-7-Tetradecen-1-ol | 44.00 | 13463 | C14H28O | Secondary alcohol |
| 36 | 12.284 | 3-Chloropropionic acid, 10-undecenyl ester | 44.00 | 13463 | C14H25ClO2 | Ester |
| 37 | 12.284 | Levomenthol | 44.00 | 13463 | C10H20O | Phenol |
| 38 | 12.890 | Dimethylmuconic acid | 153.00 | 200436 | C8H10O4 | Ethyl ester |
| 39 | 12.890 | 1,5-Hexadien-3-ol, trifluoroacetate | 153.00 | 200436 | C8H9F3O2 | Ester |
| 40 | 13.452 | Hexadecanoic acid, methyl ester | 74.00 | 620535 | C17H34O2 | Terpenoid |
| 41 | 13.452 | Tridecanoic acid, 12-methyl-, methyl ester | 74.00 | 620535 | C17H34O2 | Terpenoid |
| 42 | 13.452 | Pentadecanoic acid, 14-methyl-, methyl ester | 74.00 | 620535 | C17H34O2 | Terpenoid |
| 43 | 13.452 | Octadecanoic acid, 17-methyl-, methyl ester | 74.00 | 620535 | C20H40O2 | Terpenoid |
| 44 | 14.960 | 9,12-Octadecadienoic acid, methyl ester, (E,E) | 44.00 | 41709 | C19H34O2 | Terpenoid |
| 45 | 14.960 | 13-Tetradece-11-yn-1-ol | 44.00 | 41709 | C14H24O | Alcohol |
| 46 | 14.960 | 11,14-Eicosadienoic acid, methyl ester | 44.00 | 41709 | C21H38O2 | Terpenoid |
| 47 | 14.960 | 9,12-Octadecadien-1-ol, (Z,Z)- | 44.00 | 41709 | C18H34O | Fatty alcohol |
| 48 | 14.960 | Linoelaidic acid | 44.00 | 41709 | C18H32O2 | Fatty acid |
| 49 | 14.960 | 9,12-Octadecadienoic acid (Z,Z)- | 44.00 | 41709 | C18H32O2 | Fatty acid |
| 50 | 15.233 | 8,11,14-Eicosatrienoic acid, (Z,Z,Z)- | 55.00 | 118092 | C20H34O2 | Organic compound |
| 51 | 15.336 | Undecanal | 71.00 | 97968 | C11H22O | Organic compound |
| 52 | 15.336 | Dodecanal | 71.00 | 97968 | C12H24O | Aldehyde |
| 53 | 15.156 | 1-Deoxy-d-arabitol | 44.00 | 35550 | C5H12O4 | Secondary alcohol |
| 54 | 20.205 | Glycerol 1-palmitate | 44.00 | 27326 | C19H38O4 | Saturated fatty acid |
| 55 | 20.205 | Octadecanoic acid, 2,3-dihydroxypropyl ester | 44.00 | 27326 | C21H42O4 | Ester |
| 56 | 24.520 | 13-Docosenamide, (Z)- | 59.00 | 1596681 | C22H43NO | Amines |
| 57 | 24.520 | Nonadecanamide | 59.00 | 1596681 | C19H39NO | Amines |
| Treatment | Dose | Number of Hole Crossed | ||||
|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | ||
| Control | 10 mL/kg | 16.00 ± 0.32 | 12.40 ± 0.24 | 8.60 ± 0.24 | 6.20 ± 0.37 | 4.00 ± 0.32 |
| Diazepam | 1 mg/kg | 13.40 ± 0.51 *** | 5.60 ± 0.51 *** | 4.00 ± 0.32 *** | 2.00 ± 0.32 *** | 0.78 ± 0.89 *** |
| MECVL | 200 | 15.60 ± 0.51 | 11.40 ± 0.51 | 8.00 ± 0.32 | 4.60 ± 0.24 ** | 3.20 ± 0.37 |
| MECVL | 400 | 14.40 ± 0.24 * | 7.40 ± 0.24 *** | 6.20 ± 0.37 *** | 3.80 ± 0.37 *** | 2.40 ± 0.24 *** |
| Treatment | Dose | Number of Squares Crossed | ||||
|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | ||
| Control | 10 mL/kg | 75.60 ± 0.98 | 55.00 ± 0.71 | 53.00 ± 0.71 | 47.00 ± 0.71 | 37.00 ± 0.71 |
| Diazepam | 1 mg/kg | 72.60 ± 0.93 | 51.00 ± 0.71 *** | 27.00 ± 0.71 *** | 17.00 ± 0.71 *** | 13.00 ± 0.71 *** |
| MECVL | 200 | 74.00 ± 0.71 | 54.00 ± 0.71 | 51.00 ± 1.58 | 42.00 ± 1.42 | 32.00 ± 0.95 *** |
| MECVL | 400 | 73.00 ± 0.49 | 52.00 ± 0.86 | 49.00 ± 2.53 | 38.00 ± 2.53 *** | 25.00 ± 0.86 *** |
| Compound Name | MW 1 | HBA 2 | HBD 3 | LogP 4 | MR 5 | ROF 6 |
|---|---|---|---|---|---|---|
| 1,2,4 Benzenetriol (PubChem CID: 10787) | 126.11 | 3 | 3 | 0.70 | 32.51 | 0 |
| 3-Methyl-2-furoic acid (PubChem CID: 78127) | 126.11 | 3 | 1 | 0.97 | 30.63 | 0 |
| 2,4-Octadienoic acid, 7-hydroxy-6-methyl (PubChem CID: 5364229) | 170.21 | 3 | 2 | 1.28 | 47.36 | 0 |
| Diethyl mercaptal of d-mannose (PubChem CID: 124044) | 330.53 | 4 | 4 | 1.44 | 87.22 | 0 |
| dl-Allo-cystathionine (PubChem CID: 834) | 222.26 | 6 | 4 | −2.58 | 52.31 | 0 |
| Phloroglucinol (PubChem CID: 230351) | 132.16 | 3 | 3 | −0.30 | 32.33 | 0 |
| Acetoacetic acid, 1,3-dithio-, S-ethyl ester (PubChem CID: 547875) | 162.27 | 1 | 0 | 1.92 | 46.54 | 0 |
| β-d-Glucopyranose, 1,6-anhydro- (PubChem CID: 79029) | 162.14 | 5 | 3 | −1.26 | 32.38 | 0 |
| d-Allose (PubChem CID: 439507) | 180.16 | 6 | 5 | −2.26 | 35.75 | 0 |
| Germacrene D (PubChem CID: 5317570) | 204.35 | 0 | 0 | 4.30 | 70.68 | 0 |
| cis-muurola-3,5-diene (PubChem CID: 51351708) | 204.35 | 0 | 0 | 4.14 | 69.04 | 0 |
| β-copaene (PubChem CID: 87529) | 204.35 | 0 | 0 | 4.40 | 67.14 | 0 |
| Decanal (PubChem CID: 8175) | 156.27 | 1 | 0 | 3.17 | 50.38 | 0 |
| Dodecanoic acid, 3-hydroxy- (PubChem CID: 94216) | 216.32 | 3 | 2 | 2.86 | 62.73 | 0 |
| Butanoic acid, octyl ester (PubChem CID: 61030) | 200.32 | 2 | 0 | 3.68 | 61.08 | 0 |
| Quinic acid (PubChem CID: 6508) | 192.17 | 6 | 5 | −1.75 | 40.11 | 0 |
| 1-Heptanol, 2,4-dimethyl-, (R,R)-(+)- (PubChem CID: 87650) | 144.25 | 1 | 1 | 2.62 | 46.54 | 0 |
| d-Mannitol, 1-decylsulfonyl- (PubChem CID: 568528) | 370.50 | 7 | 5 | 1.28 | 93.80 | 0 |
| d-Mannitol, 1-thiohexyl-1-deoxy- (PubChem CID: 537501) | 282.40 | 5 | 5 | 0.63 | 73.20 | 0 |
| 4-Diazodamantanone (PubChem CID: 561686) | 176.22 | 3 | 0 | 1.28 | 47.69 | 0 |
| 3-Nonyn-2-ol (PubChem CID: 536232) | 140.22 | 1 | 1 | 2.34 | 44.70 | 0 |
| Chlorozotocin (PubChem CID: 451706) | 313.69 | 8 | 5 | −1.36 | 66.04 | 0 |
| Sparsomycin (PubChem CID: 9543443) | 361.44 | 5 | 4 | 0.01 | 91.61 | 0 |
| 9-Dodecen-1-ol, acetate, (Z)- (PubChem CID: 5363405) | 226.36 | 2 | 0 | 4.11 | 70.22 | 0 |
| Cis-7-Tetradecen-1-ol (PubChem ID: 5362795) | 212.37 | 1 | 1 | 4.38 | 70.10 | 0 |
| 3-Chloropropionic acid, 10-undecenyl ester (PubChem ID: 543975) | 260.80 | 2 | 0 | 4.58 | 75.02 | 0 |
| Levomenthol (PubChem CID: 16666) | 156.27 | 1 | 1 | 2.58 | 49.23 | 0 |
| Dimethylmuconic acid (PubChem CID: 5369045) | 170.16 | 4 | 2 | 0.83 | 43.17 | 0 |
| 1,5-Hexadien-3-ol, trifluoroacetate (PubChem CID: 238297) | 194.15 | 5 | 0 | 2.21 | 40.69 | 0 |
| Tridecanoic acid, 12-methyl-, methyl ester (PubChem CID: 21204) | 242.40 | 2 | 0 | 4.75 | 75.50 | 0 |
| 13-Tetradece-11-yn-1-ol (PubChem CID: 543337) | 208.34 | 1 | 1 | 4.12 | 68.26 | 0 |
| Undecanal (PubChem CID: 8186) | 170.29 | 1 | 0 | 3.55 | 55.19 | 0 |
| Dodecanal (PubChem CID: 8194) | 184.32 | 1 | 0 | 3.94 | 60.00 | 0 |
| 5-Butyl-1,3-oxathiolan-2-one (PubChem CID: 535042) | 160.23 | 2 | 0 | 2.14 | 42.91 | 0 |
| Glycerol 1-palmitate (PubChem CID: 14900) | 330.50 | 4 | 2 | 4.64 | 97.06 | 0 |
| Compounds Name | Docking Score (kcal/mol) | ||
|---|---|---|---|
| 5I6X | 4UUJ | 4COF | |
| 1,2,4 Benzenetriol | −5.18 | −5.771 | −5.447 |
| 3-Methyl-2-furoic acid | −3.593 | −4.085 | −5.608 |
| 2,4-Octadienoic acid, 7-hydroxy-6-methyl | −2.416 | −1.871 | −3.355 |
| Diethyl mercaptal of d-mannose | - | - | - |
| dl-Allo-cystathionine | - | - | - |
| Phloroglucinol | −4.741 | −5.955 | −6.151 |
| Acetoacetic acid, 1,3-dithio-, S-ethyl ester | −3.145 | −1.829 | −4.042 |
| β-d-Glucopyranose, 1,6-anhydro- | - | −4.675 | −5.895 |
| d-Allose | −3.192 | −2.932 | −5.357 |
| Germacrene D | - | - | - |
| Cis-muurola-3,5-diene | - | - | - |
| β-copaene | - | - | - |
| Decanal | 2.673 | 2.998 | 2.594 |
| Dodecanoic acid, 3-hydroxy- | 2.717 | 2.952 | 1.136 |
| Butanoic acid, octyl ester | 2.634 | 2.715 | 2.271 |
| Quinic acid | - | −4.42 | −6.942 |
| 1-Heptanol, 2,4-dimethyl-, (R,R)-(+)- | - | - | - |
| d-Mannitol, 1-decylsulfonyl- | - | - | - |
| d-Mannitol, 1-thiohexyl-1-deoxy- | - | - | 0.428 |
| 4-Diazodamantanone | −4.171 | −3.539 | −3.734 |
| 3-Nonyn-2-ol | −1.407 | −0.79 | −0.909 |
| Chlorozotocin | - | - | - |
| Sparsomycin | - | - | - |
| 9-Dodecen-1-ol, acetate, (Z)- | - | - | - |
| Cis-7-Tetradecen-1-ol | - | - | - |
| 3-Chloropropionic acid, 10-undecenyl ester | 1.46 | 2.832 | 1.339 |
| Levomenthol | −3.911 | −4.647 | −4.417 |
| Dimethylmuconic acid | −2.71 | −2.726 | −4.814 |
| 1,5-Hexadien-3-ol, trifluoroacetate | - | - | - |
| Tridecanoic acid, 12-methyl-, methyl ester | 1.42 | 2.602 | 1.292 |
| 13-Tetradece-11-yn-1-ol | 4.354 | 4.228 | 4.67 |
| Undecanal | 2.431 | 3.537 | 2.216 |
| Dodecanal | 3.097 | 2.879 | 2.894 |
| 5-Butyl-1,3-oxathiolan-2-one | −3.831 | −3.895 | −4.03 |
| Glycerol 1-palmitate | −0.992 | 0.902 | −1.954 |
| Reference drug (Imipramine/Diazepam) | −5.35 | −4.035 | −5.961 |
| Compound Name | Biological Activity | References |
|---|---|---|
| 1,2,4 Benzenetriol | Anti-microbial activity | [45] |
| 3-Methyl-2-furoic acid | Anti-fungal and anti-tumor activity | [46] |
| 2,4-Octadienoic acid, 7-hydroxy-6-methyl | Analgesic and anti-inflammatory activity | [47] |
| Diethyl mercaptal of d-mannose | Antibacterial and anti-fungal activity | [48] |
| dl-Allo-cystathionine | Analgesic, anti-inflammatory, antifungal, antibacterial activity | [49] |
| Phloroglucinol | Antioxidant, antineoplastic, anti-inflammatory, antimicrobial, antifungal activity | [50,51] |
| Acetoacetic acid, 1,3-dithio-, S-ethyl ester | Analgesic, antipyretic, antimalarial and antibiotic effect | [52,53] |
| β-D-Glucopyranose, 1,6-anhydro- | Anti-bacterial and antioxidant effect | [54,55] |
| D-Allose | Antioxidant, antibacterial and antiviral activity | [56,57] |
| Germacrene D | Neurological activity, cytotoxicity and antimicrobial activity | [58,59] |
| Cis-muurola-3,5-diene | Antimicrobial activity | [60] |
| β-copaene | Cytotoxic and antioxidant effect | [61,62] |
| Decanal | Antioxidant | [63] |
| Dodecanoic acid, 3-hydroxy- | Antibacterial, antitumor and antioxidant effect | [64] |
| Butanoic acid, octyl ester | Effect on CNS, antioxidant, analgesic and anti-inflammatory activity | [65,66] |
| Quinic acid | Antibacterial, antiviaral, antioxidant and hepatoprotective activity | [67,68,69] |
| 1-Heptanol, 2,4-dimethyl-, (R,R)-(+)- | Antifungal activity | [70] |
| d-Mannitol, 1-decylsulfonyl- | Anti-diabetic, anti-microbial activity | [71,72] |
| d-Mannitol, 1-thiohexyl-1-deoxy- | Anti-microbial activity | [73] |
| 4-Diazodamantanone | Antimicrobial, anti-inflammatory and antiacetylcholinesterase activities | [74,75] |
| 3-Nonyn-2-ol | Anti-inflammatory, anti-septic, anti-tumor activity | [76,77] |
| Chlorozotocin | Anti-tumor, reduction in bone marrow toxicity | [78,79] |
| Sparsomycin | Anti-tumor antibiotic | [80] |
| 9-Dodecen-1-ol, acetate, (Z)- | - | - |
| Cis-7-Tetradecen-1-ol | Anti-fungal, anti-bacterial, antioxidant, cytotoxic, anti-inflammatory, antinociceptive and hepatoprotective activty | [81,82,83,84] |
| 3-Chloropropionic acid, 10-undecenyl ester | Antioxidant, anti-inflammatory, antitherogenic, hypocholesteromia activities, antibacterial activity | [85,86] |
| Levomenthol | Phytotoxic, antimicrobial, nematocidal, cytotoxic, anti-influenza and inflammation-promoting activities, antioxidant effect | [87,88] |
| Dimethylmuconic acid | - | - |
| 1,5-Hexadien-3-ol, trifluoroacetate | Antioxidant Activity | [89] |
| Tridecanoic acid, 12-methyl-, methyl ester | Antimicrobial, antioxidant, and anti-inflammatory activities | [90] |
| 13-Tetradece-11-yn-1-ol | Antibacterial, antifungal and antioxidant effect | [91] |
| Undecanal | Cytotoxic effect, antimicrobial, anti-inflammatory, and antioxidant activities | [92] |
| Dodecanal | Analgesic effect, antimicrobial, anti-inflammatory and cytotoxic activities | [93,94] |
| 5-Butyl-1,3-oxathiolan-2-one | Anti-viral activity | [95] |
| Glycerol 1-palmitate | Palmitate-induced inflammatory effect on microphage | [96] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, I.; Tona, M.R.; Sharmin, S.; Sayeed, M.A.; Tania, F.Z.; Paul, A.; Chy, M.N.U.; Rakib, A.; Emran, T.B.; Simal-Gandara, J. GC-MS Phytochemical Profiling, Pharmacological Properties, and In Silico Studies of Chukrasia velutina Leaves: A Novel Source for Bioactive Agents. Molecules 2020, 25, 3536. https://doi.org/10.3390/molecules25153536
Jahan I, Tona MR, Sharmin S, Sayeed MA, Tania FZ, Paul A, Chy MNU, Rakib A, Emran TB, Simal-Gandara J. GC-MS Phytochemical Profiling, Pharmacological Properties, and In Silico Studies of Chukrasia velutina Leaves: A Novel Source for Bioactive Agents. Molecules. 2020; 25(15):3536. https://doi.org/10.3390/molecules25153536
Chicago/Turabian StyleJahan, Israt, Marzia Rahman Tona, Sanjida Sharmin, Mohammed Aktar Sayeed, Fatamatuz Zuhura Tania, Arkajyoti Paul, Md. Nazim Uddin Chy, Ahmed Rakib, Talha Bin Emran, and Jesus Simal-Gandara. 2020. "GC-MS Phytochemical Profiling, Pharmacological Properties, and In Silico Studies of Chukrasia velutina Leaves: A Novel Source for Bioactive Agents" Molecules 25, no. 15: 3536. https://doi.org/10.3390/molecules25153536
APA StyleJahan, I., Tona, M. R., Sharmin, S., Sayeed, M. A., Tania, F. Z., Paul, A., Chy, M. N. U., Rakib, A., Emran, T. B., & Simal-Gandara, J. (2020). GC-MS Phytochemical Profiling, Pharmacological Properties, and In Silico Studies of Chukrasia velutina Leaves: A Novel Source for Bioactive Agents. Molecules, 25(15), 3536. https://doi.org/10.3390/molecules25153536












