i-Propylammonium Lead Chloride Based Perovskite Photocatalysts for Depolymerization of Lignin Under UV Light
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. Powder X-ray Diffraction (PXRD) and Scanning Electron Microscopic (SEM) Analysis
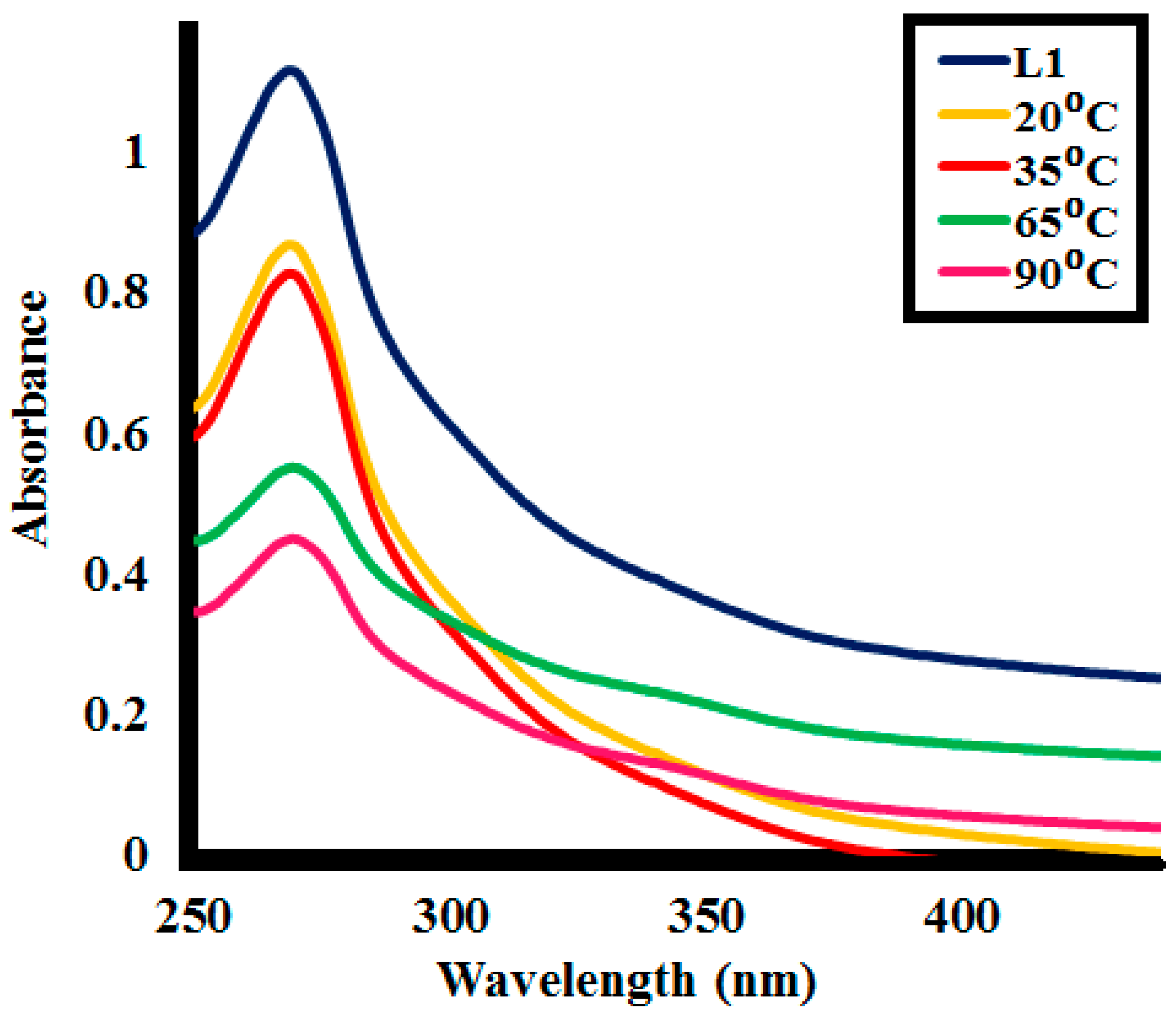
2.1.2. UV and Photoluminescence (PL) Analysis
2.1.3. FTIR Analysis
2.2. Photocatalytic Depolymerization of Lignin Under UV Light
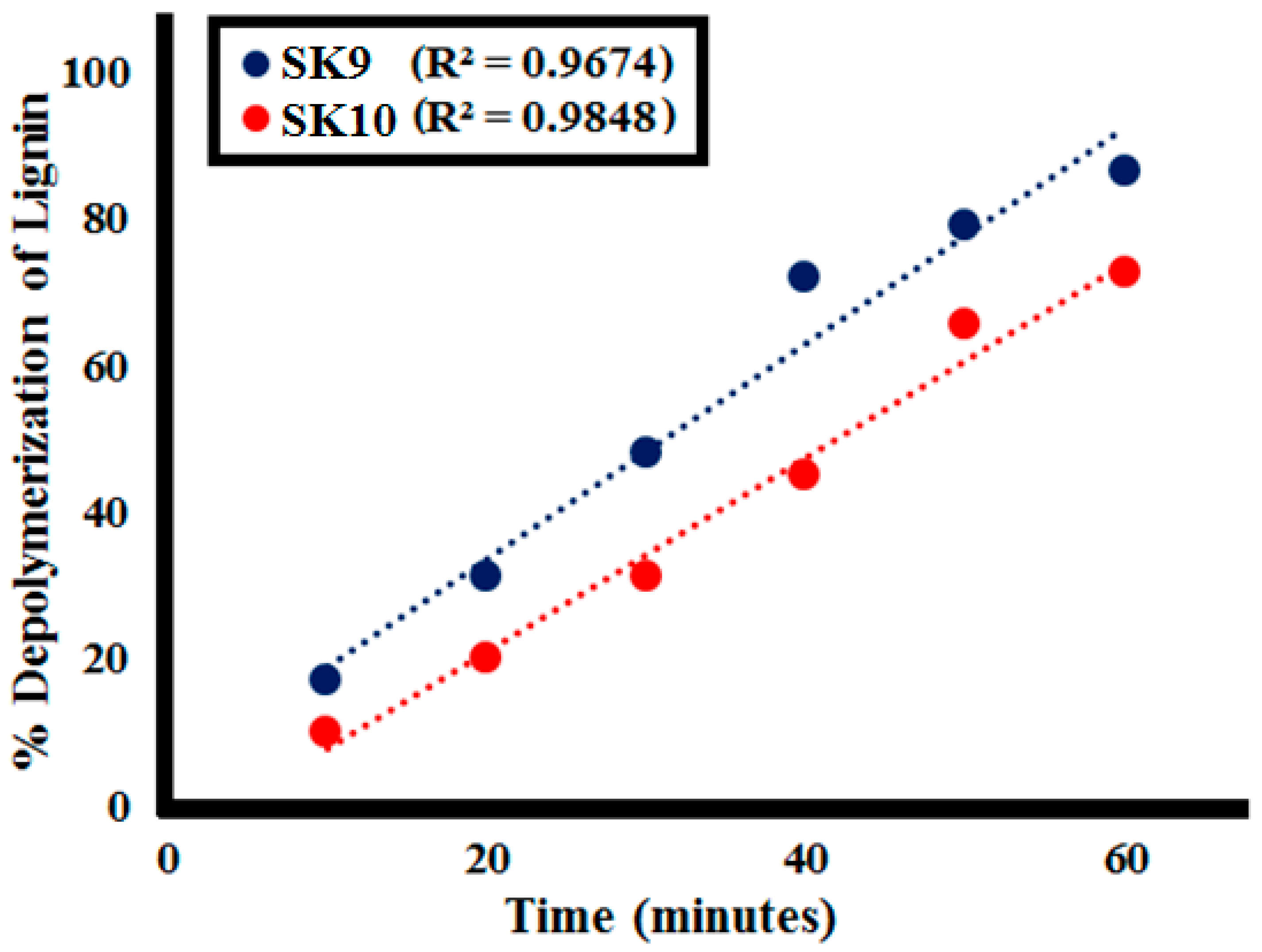
2.2.1. Effect of Time
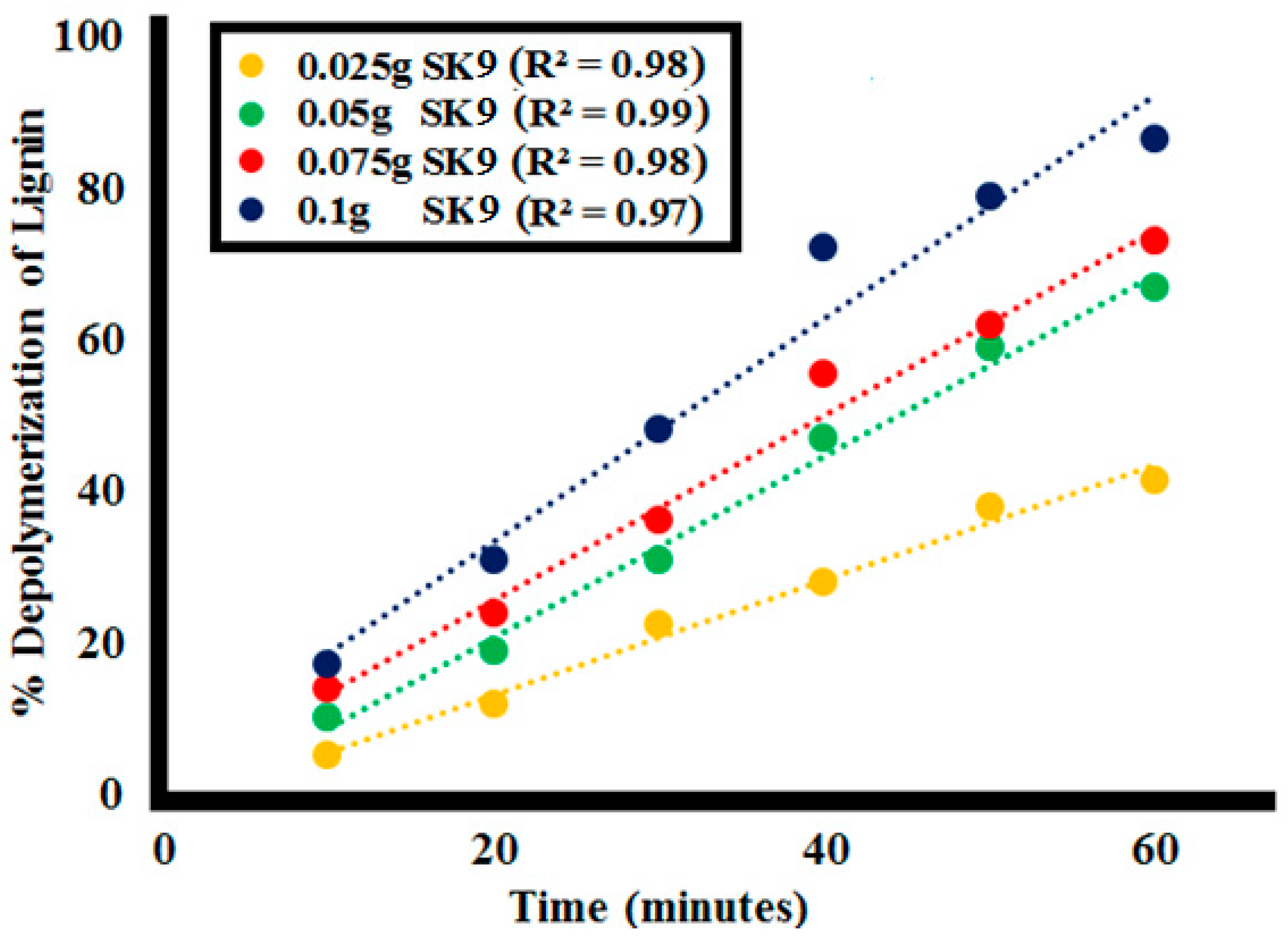
2.2.2. Effect of Catalyst Dosage
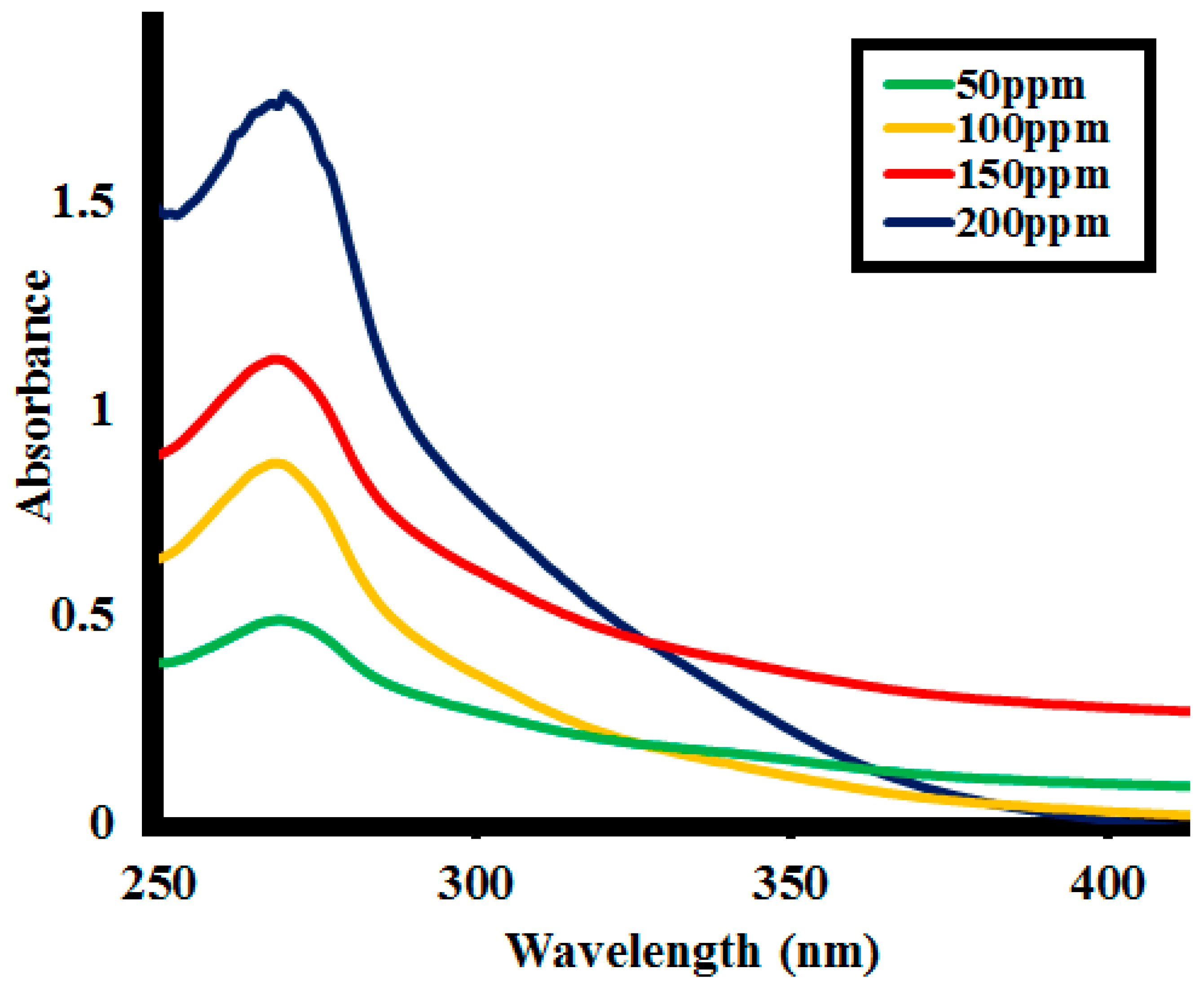
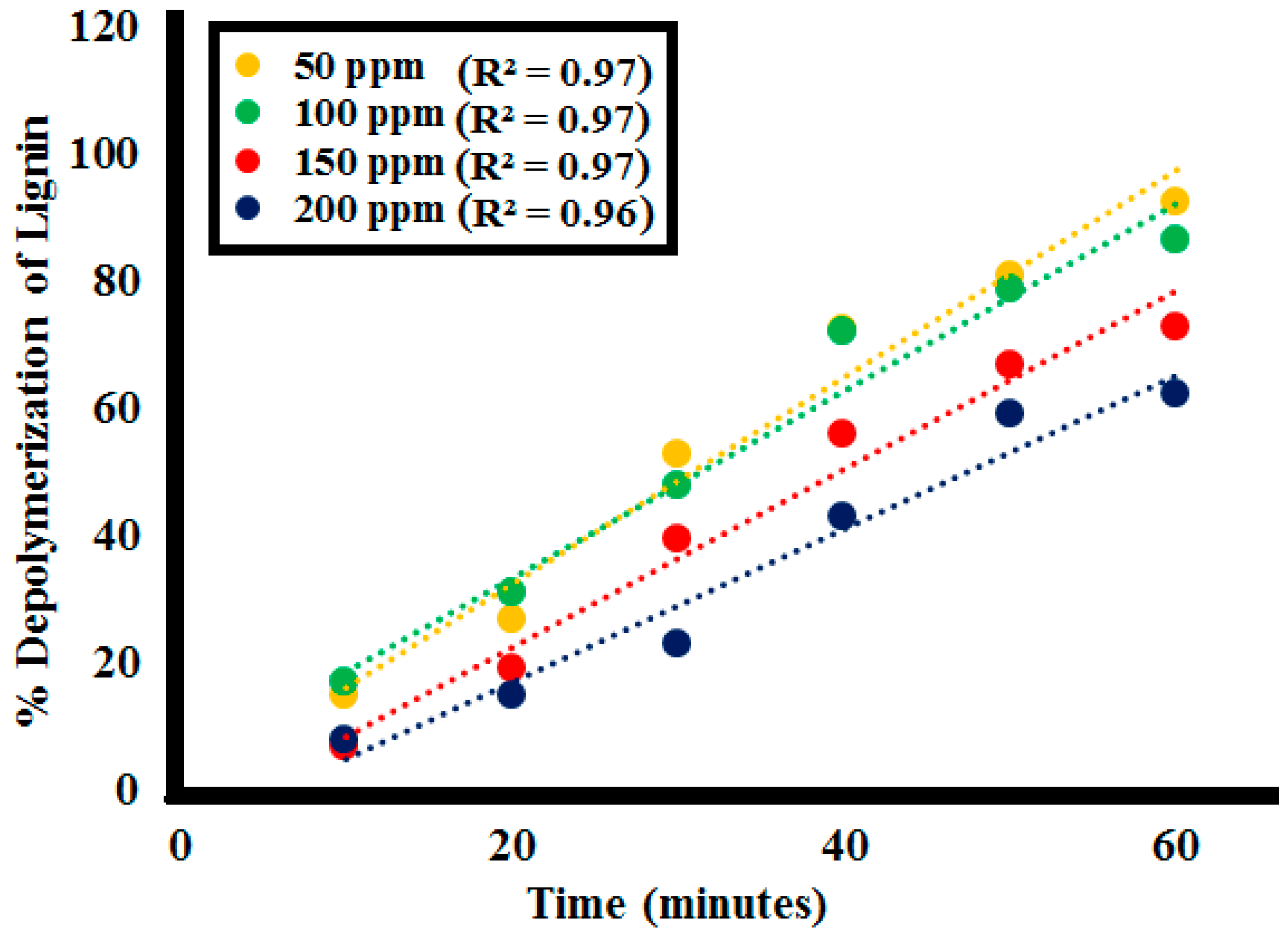
2.2.3. Effect of Initial Lignin Concentration
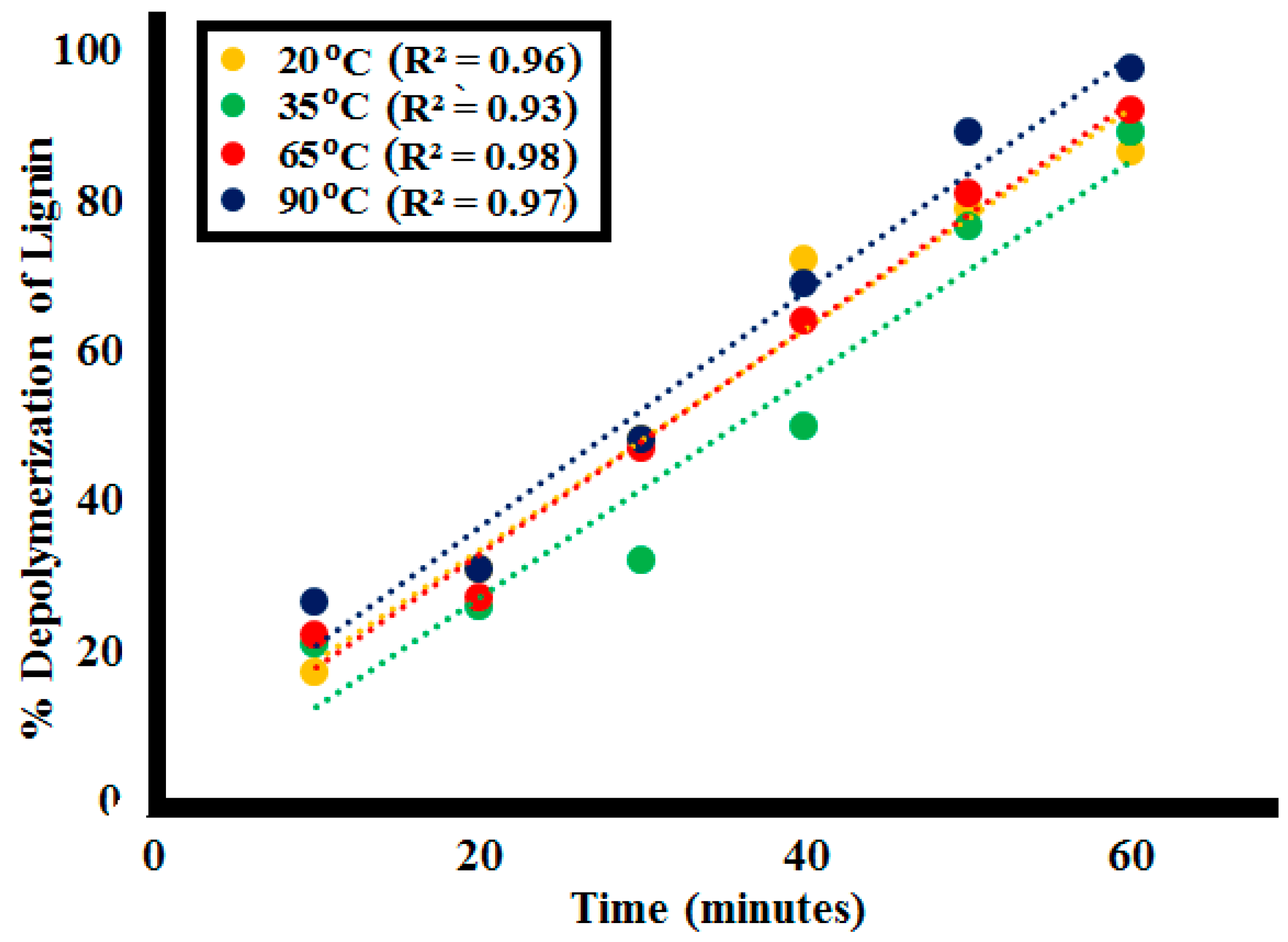
2.2.4. Effect of Variating Temperature
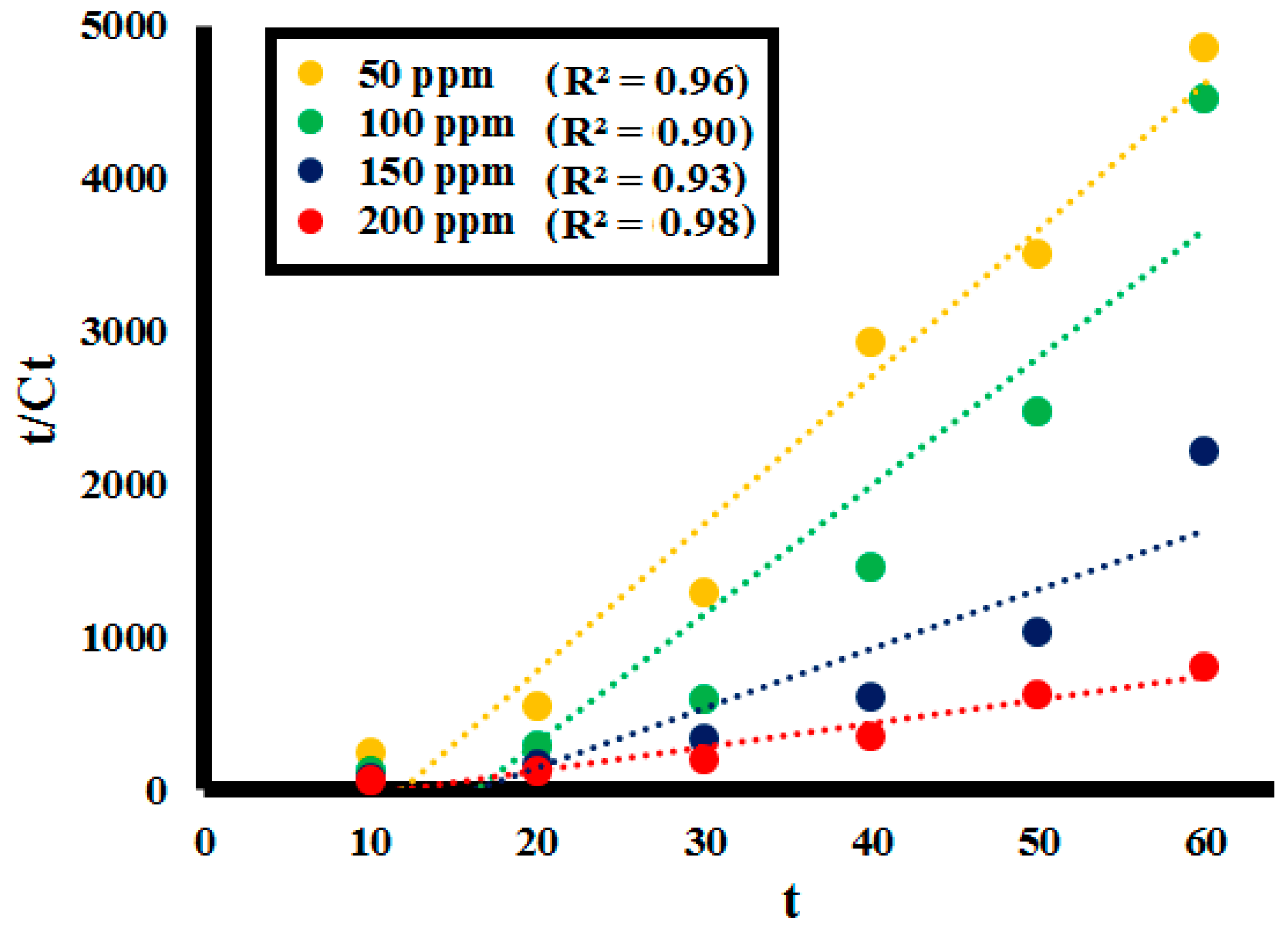
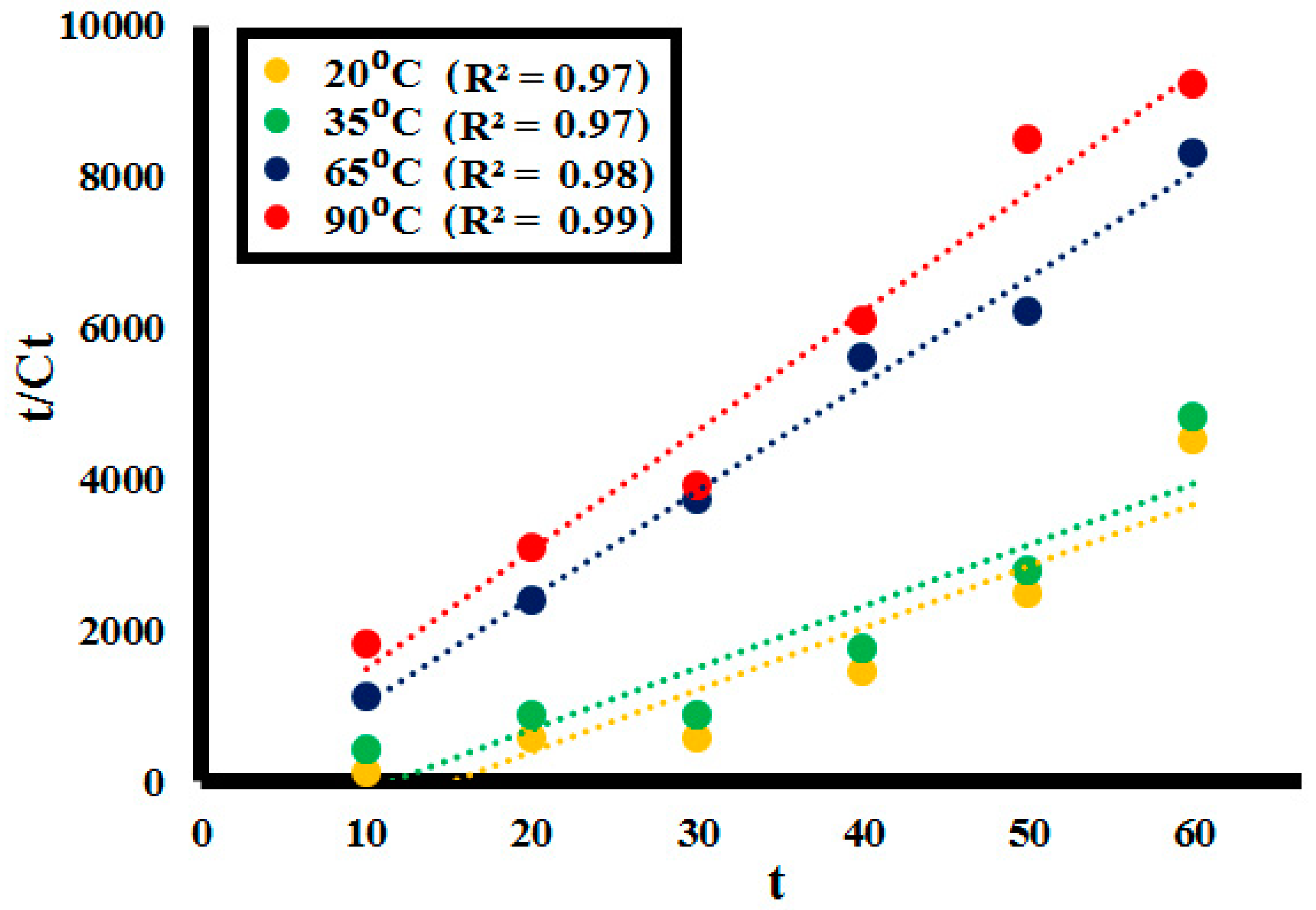
2.3. Kinetic Analysis
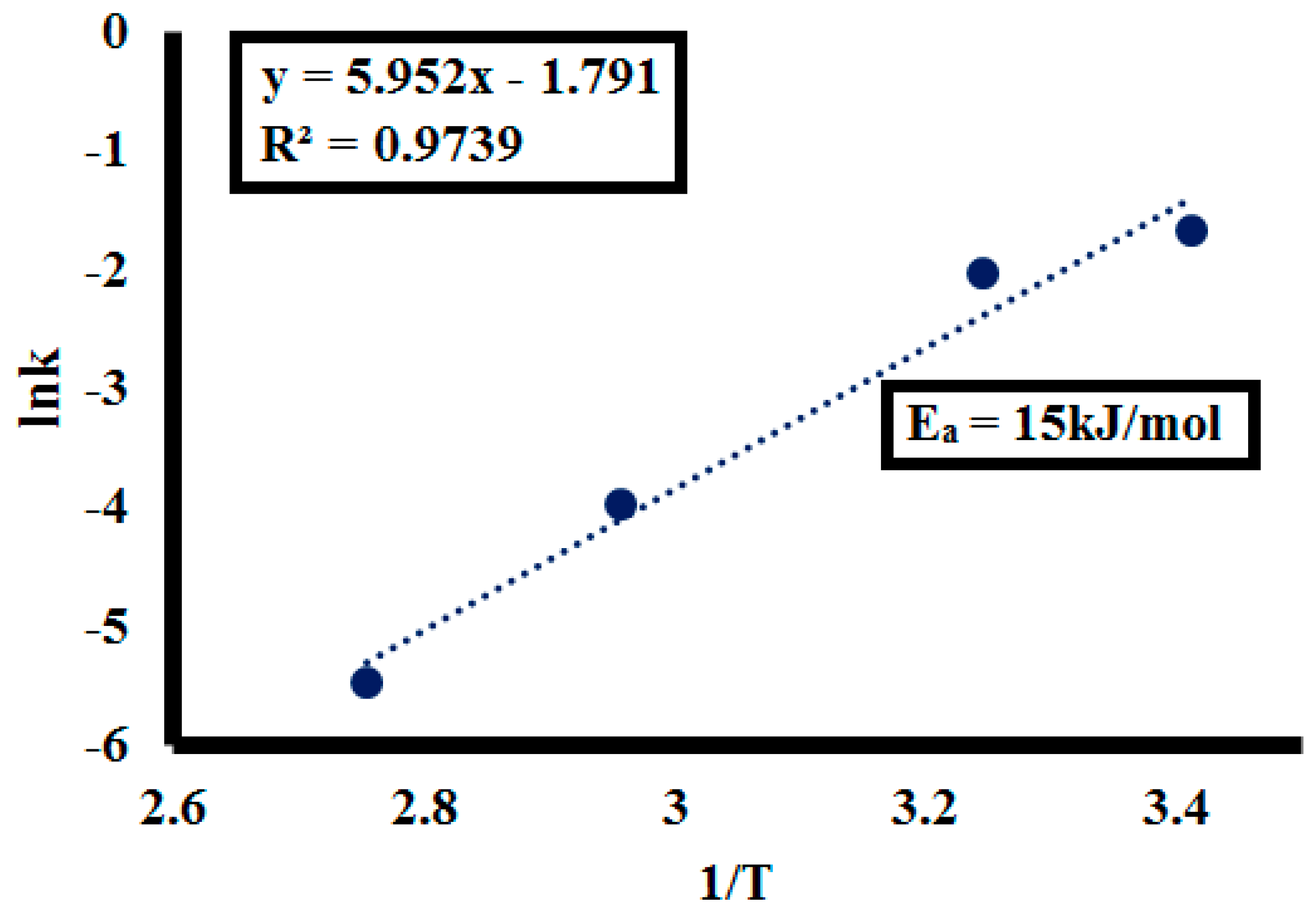
Activation Energy (Ea)
2.4. Characterization of Depolymerization Products
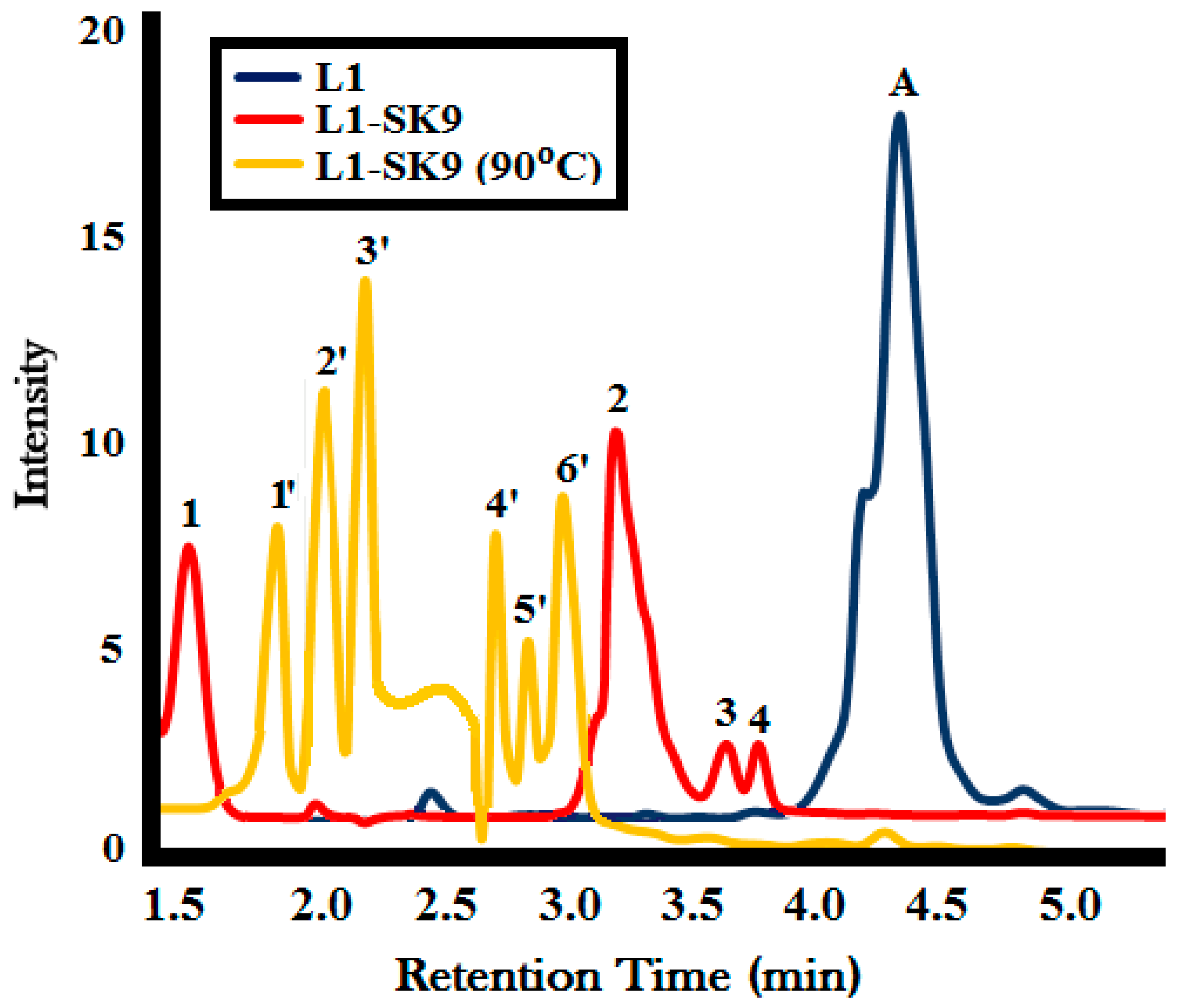
GC-MS Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of iso-Propyl Amine Lead Chloride Perovskites (IAPbCl3) (SK9–SK10)
3.3. Photocatalytic Activity under UV Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dapsens, P.Y.; Mondelli, C.; Pérez-Ramírez, J. Biobased Chemicals from Conception toward Industrial Reality: Lessons Learned and To Be Learned. ACS Catal. 2012, 2, 1487–1499. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.; Herrero-Davila, L.; Campelo, J.M.; Clark, J.H.; Hidalgo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Biofuels: A technological perspective. Energy Environ. Sci. 2008, 1, 542–564. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Pérez, E.; Tuck, C.O.; Poliakoff, M. Valorisation of lignin by depolymerisation and fractionation using supercritical fluids and conventional solvents. J. Supercrit. Fluids 2018, 133, 690–695. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Flores, F.G.; Dobado, J.A. Lignin as Renewable Raw Material. ChemSusChem 2010, 3, 1227–1235. [Google Scholar] [CrossRef]
- Ma, R.; Xu, Y.; Zhang, X. ChemInform Abstract: Catalytic Oxidation of Biorefinery Lignin to Value-Added Chemicals to Support Sustainable Biofuel Production. Cheminform 2015, 46, 24–51. [Google Scholar] [CrossRef]
- Lavoie, J.-M.; Baré, W.; Bilodeau, M. Depolymerization of steam-treated lignin for the production of green chemicals. Bioresour. Technol. 2011, 102, 4917–4920. [Google Scholar] [CrossRef]
- Grabber, J.H. How Do Lignin Composition, Structure, and Cross-Linking Affect Degradability? A Review of Cell Wall Model Studies. Crop. Sci. 2005, 45, 820–831. [Google Scholar] [CrossRef]
- Deuss, P.J.; Barta, K.; De Vries, J.G. Homogeneous catalysis for the conversion of biomass and biomass-derived platform chemicals. Catal. Sci. Technol. 2014, 4, 1174–1196. [Google Scholar] [CrossRef]
- Deuss, P.J.; Barta, K. From models to lignin: Transition metal catalysis for selective bond cleavage reactions. Coord. Chem. Rev. 2016, 306, 510–532. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ohta, H.; Fukuoka, A. Conversion of lignocellulose into renewable chemicals by heterogeneous catalysis. Catal. Sci. Technol. 2012, 2, 869–883. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Thermochemical conversion of lignin to functional materials: A review and future directions. Green Chem. 2015, 17, 4888–4907. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Brink, D.P.; Prothmann, J.; Ravi, K.; Sun, M.; García-Hidalgo, J.; Sandahl, M.; Hulteberg, C.P.; Turner, C.; Lidén, G.; et al. Biological valorization of low molecular weight lignin. Biotechnol. Adv. 2016, 34, 1318–1346. [Google Scholar] [CrossRef]
- Sun, Z.-H.; Fridrich, B.; De Santi, A.; Elangovan, S.; Barta, K. Bright Side of Lignin Depolymerization: Toward New Platform Chemicals. Chem. Rev. 2018, 118, 614–678. [Google Scholar] [CrossRef]
- Strieth-Kalthoff, F.; James, M.; Teders, M.; Pitzer, L.; Glorius, F. Energy transfer catalysis mediated by visible light: Principles, applications, directions. Chem. Soc. Rev. 2018, 47, 7190–7202. [Google Scholar] [CrossRef]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon–Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K. Study of the effect of photo-irradiation on the surface chemistry of wood. Polym. Degrad. Stab. 2005, 90, 9–20. [Google Scholar] [CrossRef]
- Uğurlu, M.; Karaoğlu, M.H. Removal of AOX, total nitrogen and chlorinated lignin from bleached Kraft mill effluents by UV oxidation in the presence of hydrogen peroxide utilizing TiO2 as photocatalyst. Environ. Sci. Pollut. Res. 2008, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Liu, S.; Colmenares, J.C.; Xu, Y.-J. A sustainable approach for lignin valorization by heterogeneous photocatalysis. Green Chem. 2016, 18, 594–607. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Varma, R.S.; Nair, V. Selective photocatalysis of lignin-inspired chemicals by integrating hybrid nanocatalysis in microfluidic reactors. Chem. Soc. Rev. 2017, 46, 6675–6686. [Google Scholar] [CrossRef]
- Sheldon, R.A. Engineering a more sustainable world through catalysis and green chemistry. J. R. Soc. Interface 2016, 13, 20160087. [Google Scholar] [CrossRef]
- Ma, Y.-S.; Chang, C.-N.; Chiang, Y.-P.; Sung, H.-F.; Chao, A.C. Photocatalytic degradation of lignin using Pt/TiO2 as the catalyst. Chemosphere 2008, 71, 998–1004. [Google Scholar] [CrossRef]
- Wu, W.-B.; Wong, Y.-C.; Tan, Z.-K.; Wu, J. Photo-induced thiol coupling and C-H activation using nanocrystalline lead-halide perovskite catalysts. Catal. Sci. Technol. 2018, 8, 4257–4263. [Google Scholar] [CrossRef]
- Snaith, H.J. Present status and future prospects of perovskite photovoltaics. Nat. Mater. 2018, 17, 372–376. [Google Scholar] [CrossRef]
- Xu, J.; Huang, W.; Li, P.; Onken, D.R.; Dun, C.; Guo, Y.; Ucer, K.B.; Lu, C.; Wang, H.; Geyer, S.M.; et al. Imbedded Nanocrystals of CsPbBr3 in Cs4 PbBr6: Kinetics, Enhanced Oscillator Strength, and Application in Light-Emitting Diodes. Adv. Mater. 2017, 29, 1703703. [Google Scholar] [CrossRef]
- Wei, H.; Fang, Y.; Mulligan, P.L.; Chuirazzi, W.; Fang, H.-H.; Wang, C.; Ecker, B.; Gao, Y.; Loi, M.A.; Cao, L.; et al. Sensitive X-ray detectors made of methylammonium lead tribromide perovskite single crystals. Nat. Photon. 2016, 10, 333–339. [Google Scholar] [CrossRef]
- Tiep, N.H.; Ku, Z.; Fan, H.J. Recent Advances in Improving the Stability of Perovskite Solar Cells. Adv. Energy Mater. 2015, 6, 1501420. [Google Scholar] [CrossRef]
- Chen, Q.; De Marco, N.; Song, T.-B.; Chen, C.-C.; Zhao, H.; Hong, Z.; Zhou, H.; Yang, Y. (Michael); Yang, Y. Under the spotlight: The organic-inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.G.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Cui, D.; Ren, X.; Sun, J.; Liu, X.; Zhang, J.; Wei, Q.; Fan, H.; Yucheng, L.; et al. Two-Inch-Sized Perovskite CH3NH3PbX3(X = Cl, Br, I) Crystals: Growth and Characterization. Adv. Mater. 2015, 27, 5176–5183. [Google Scholar] [CrossRef]
- Riente, P.; Noël, T.; Paiva, P.R. Application of metal oxide semiconductors in light-driven organic transformations. Catal. Sci. Technol. 2019, 9, 5186–5232. [Google Scholar] [CrossRef]
- Yahya, N.; Nasir, A.M.; Daub, N.A.; Aziz, F.; Aizat, A.; Jaafar, J.; Lau, W.J.; Yusof, N.; Salleh, W.N.W.; Ismail, A.F.; et al. Visible Light–Driven Perovskite-Based Photocatalyst for Wastewater Treatment; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 265–302. [Google Scholar]
- Luciani, G.; Imparato, C.; Vitiello, G. Photosensitive Hybrid Nanostructured Materials: The Big Challenges for Sunlight Capture. Catalysts 2020, 10, 103. [Google Scholar] [CrossRef]
- Fu, Y.; Meng, F.; Rowley, M.B.; Thompson, B.J.; Shearer, M.J.; Ma, D.; Hamers, R.J.; Wright, J.C.; Jin, S. Solution Growth of Single Crystal Methylammonium Lead Halide Perovskite Nanostructures for Optoelectronic and Photovoltaic Applications. J. Am. Chem. Soc. 2015, 137, 5810–5818. [Google Scholar] [CrossRef]
- Mashiyama, H.; Kawamura, Y.; Magoma, E.; Kubota, Y. Displacive character of the cubic-tetragonal transition in CH3NH3PbX3. J. Korean Phys. Soc. 2003, 42, S1026–S1029. [Google Scholar]
- Edri, E.; Kirmayer, S.; Kulbak, M.; Hodes, G.; Cahen, D. Chloride Inclusion and Hole Transport Material Doping to Improve Methyl Ammonium Lead Bromide Perovskite-Based High Open-Circuit Voltage Solar Cells. J. Phys. Chem. Lett. 2014, 5, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Jagodzinski, H. The Role of Munich in X-Ray Crystallography and the Development of Powder Diffraction. Mater. Sci. Forum 1991, 79, 1–16. [Google Scholar] [CrossRef]
- Cullity, B.; Stock, S. Elements of X-Ray Diffraction, 3rd ed.; Prentice Hall: New York, NY, USA, 2001; pp. 174–177. [Google Scholar]
- Saleem, M. Effect of zinc acetate concentration on the structural and optical properties of ZnO thin films deposited by Sol-Gel method. Int. J. Phys. Sci. 2012, 7, 2971–2979. [Google Scholar] [CrossRef]
- Aé, L.; Kieven, D.; Chen, J.; Klenk, R.; Rissom, T.; Tang, Y.; Lux-Steiner, M.C.; Rissom, T. ZnO nanorod arrays as an antireflective coating for Cu(In,Ga)Se2 thin film solar cells. Prog. Photovolt. Res. Appl. 2010, 18, 209–213. [Google Scholar] [CrossRef]
- Bindu, P.; Thomas, S. Estimation of lattice strain in ZnO nanoparticles: X-ray peak profile analysis. J. Theor. Appl. Phys. 2014, 8, 123–134. [Google Scholar] [CrossRef]
- Kanemitsu, Y. Luminescence spectroscopy of lead-halide perovskites: Materials properties and application as photovoltaic devices. J. Mater. Chem. C 2017, 5, 3427–3437. [Google Scholar] [CrossRef]
- Sarkar, P.; Mazumder, J.; Tripathy, S.K.; Baishnab, K.L.; Palai, G. Structural, optoelectronic, and morphological study of indium-doped methylammonium lead chloride perovskites. Appl. Phys. A 2019, 125, 580. [Google Scholar] [CrossRef]
- De Matteis, F.; Vitale, F.; Privitera, S.; Ciotta, E.; Pizzoferrato, R.; Generosi, A.; Paci, B.; Di Mario, L.; Cresi, J.S.P.; Martelli, F.; et al. Optical Characterization of Cesium Lead Bromide Perovskites. Catalysts 2019, 9, 280. [Google Scholar] [CrossRef]
- Labhsetwar, N.K.; Watanabe, A.; Mitsuhashi, T. New improved syntheses of LaRuO3 perovskites and their applications in environmental catalysis. Appl. Catal. B Environ. 2003, 40, 21–30. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Tombe, S.; Adam, G.; Heilbrunner, H.; Apaydin, D.H.; Ulbricht, C.; Sariciftci, N.S.; Arendse, C.J.; Iwuoha, E.I.; Scharber, M.C. Optical and electronic properties of mixed halide (X = I, Cl, Br) methylammonium lead perovskite solar cells. J. Mater. Chem. C 2017, 5, 1714–1723. [Google Scholar] [CrossRef]
- Park, B.-W.; Philippe, B.; Jain, S.M.; Zhang, X.; Edvinsson, T.; Rensmo, H.; Zietz, B.; Boschloo, G. Chemical engineering of methylammonium lead iodide/bromide perovskites: Tuning of opto-electronic properties and photovoltaic performance. J. Mater. Chem. A 2015, 3, 21760–21771. [Google Scholar] [CrossRef]
- Kostka, P.; Lezal, D.; Poulain, M.; Pedlíková, J.; Novotna, M. Glass Formation in the PbCl2-Sb2O3-TeO2 System. Solid State Phenom. 2003, 235–240. [Google Scholar] [CrossRef]
- Zhao, J.; Xiuwen, W.; Hu, J.; Liu, Q.; Shen, D.; Xiao, R. Thermal degradation of softwood lignin and hardwood lignin by TG-FTIR and Py-GC/MS. Polym. Degrad. Stab. 2014, 108, 133–138. [Google Scholar] [CrossRef]
- Sharma, R.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Chan, W.G.; Hajaligol, M.R. Characterization of chars from pyrolysis of lignin. Fuel 2004, 83, 1469–1482. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Kinetic Models for the Sorption of Dye from Aqueous Solution by Wood. Process. Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S. Removal of lead from water using biochars prepared from hydrothermal liquefaction of biomass. J. Hazard. Mater. 2009, 167, 933–939. [Google Scholar] [CrossRef]
- Várhegyi, G.; Antal, M.J.; Jakab, E.; Szabó, P. Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 1997, 42, 73–87. [Google Scholar] [CrossRef]
- Carrier, M.; Auret, L.; Bridgwater, T.; Knoetze, J.H. Using Apparent Activation Energy as a Reactivity Criterion for Biomass Pyrolysis. Energy Fuels 2016, 30, 7834–7841. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Qian, M.; Yadavalli, G. A thermal behavior and kinetics study of the catalytic pyrolysis of lignin. RSC Adv. 2016, 6, 100700–100707. [Google Scholar] [CrossRef]
- Damayanti; Wu, H.-S. Pyrolysis kinetic of alkaline and dealkaline lignin using catalyst. J. Polym. Res. 2017, 25, 7. [Google Scholar] [CrossRef]
- Saito, K.; Kato, T.; Takamori, H.; Kishimoto, T.; Fukushima, K. A New Analysis of the Depolymerized Fragments of Lignin Polymer Using ToF-SIMS. Biomacromolecules 2005, 6, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Evans, L.; Littlewolf, A.; Trudell, D. Batch microreactor studies of lignin and lignin model compound depolymerization by bases in alcohol solvents. Fuel 1999, 78, 1363–1366. [Google Scholar] [CrossRef]
- Ohra-Aho, T.; Tenkanen, M.; Tamminen, T. Direct analysis of lignin and lignin-like components from softwood kraft pulp by Py-GC/MS techniques. J. Anal. Appl. Pyrolysis 2005, 74, 123–128. [Google Scholar] [CrossRef]
- Pecina, R.; Burtscher, P.; Bonn, G.; Bobleter, O. GC-MS and HPLC analyses of lignin degradation products in biomass hydrolyzates. Anal. Bioanal. Chem. 1986, 325, 461–465. [Google Scholar] [CrossRef]
- Watkins, D.; Nuruddin, M.; Hosur, M.; Tcherbi-Narteh, A.; Jeelani, S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Kausar, S.; Altaf, A.A.; Hamayun, M.; Danish, M.; Zubair, M.; Naz, S.; Muhammad, S.; Zaheer, M.; Ullah, S.; Badshah, A. Soft template-based bismuth doped zinc oxide nanocomposites for photocatalytic depolymerization of lignin. Inorg. Chim. Acta 2020, 502, 119390. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
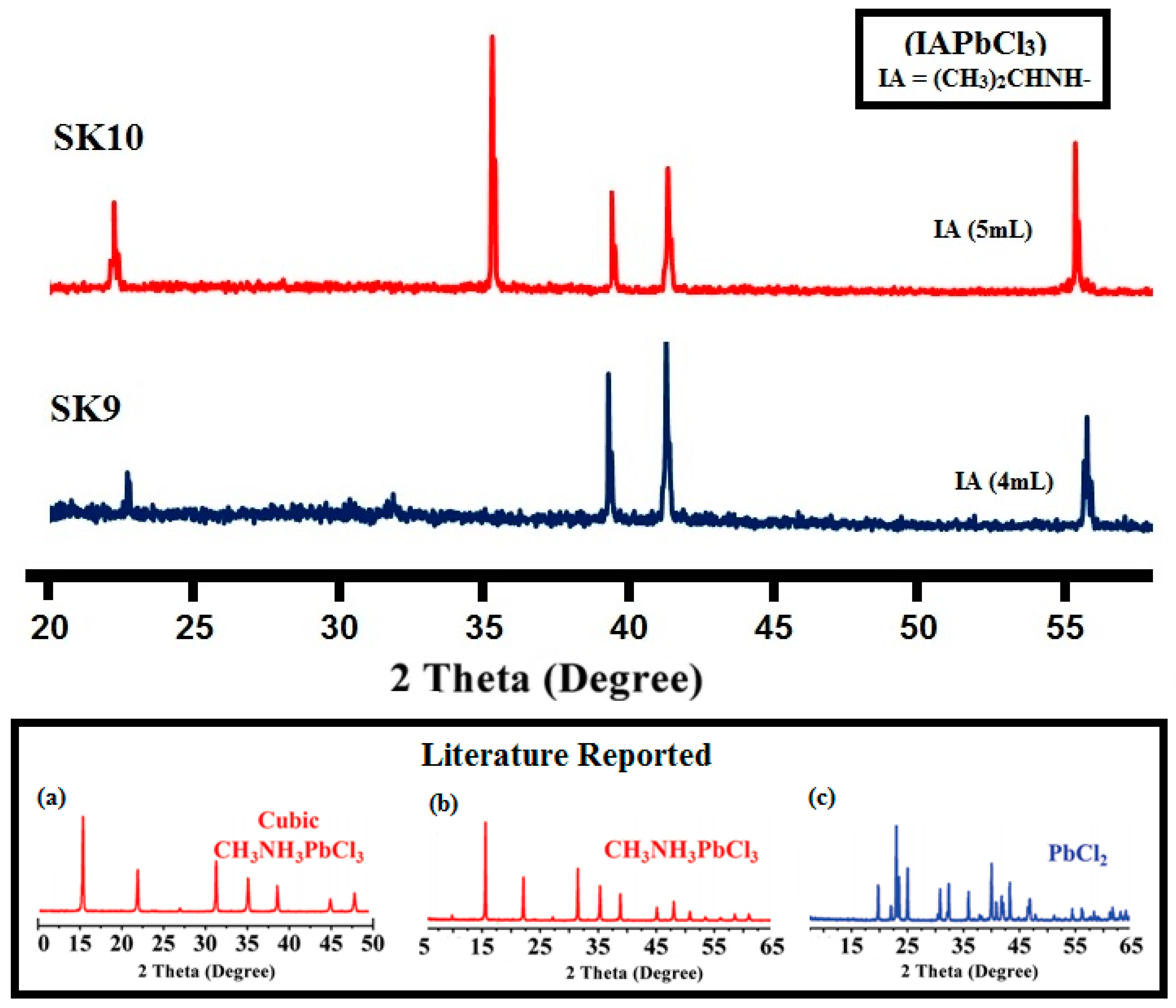
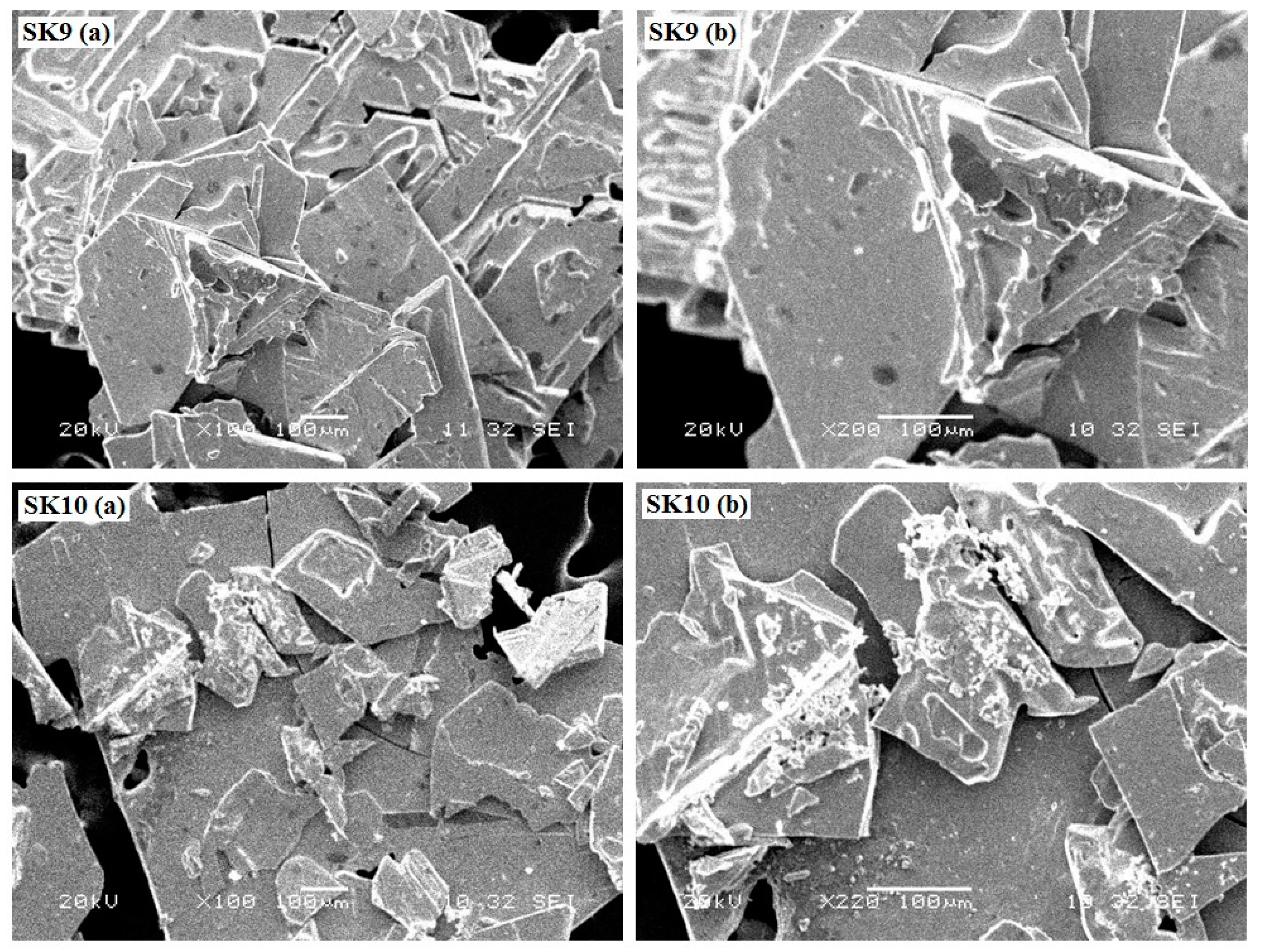


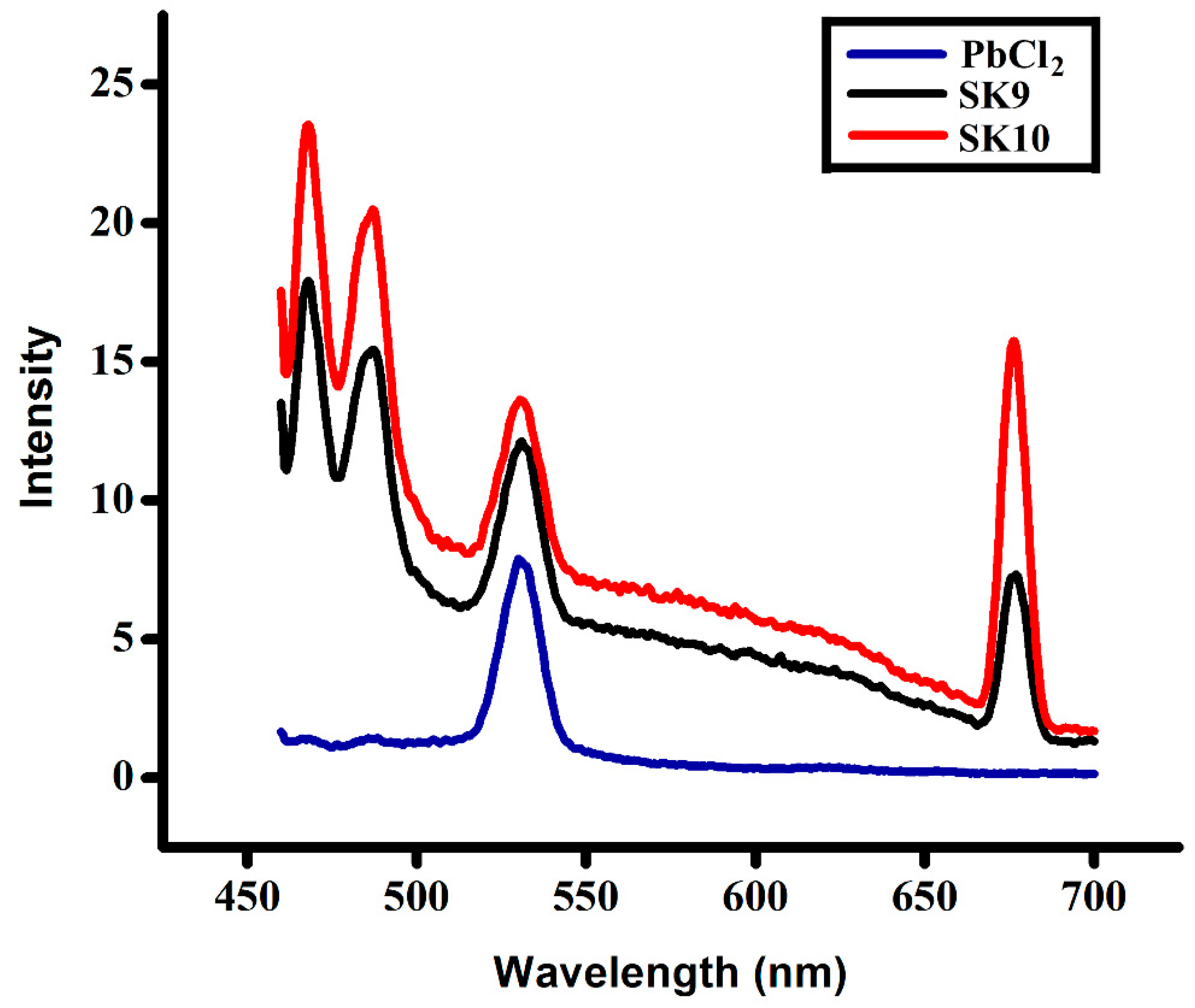
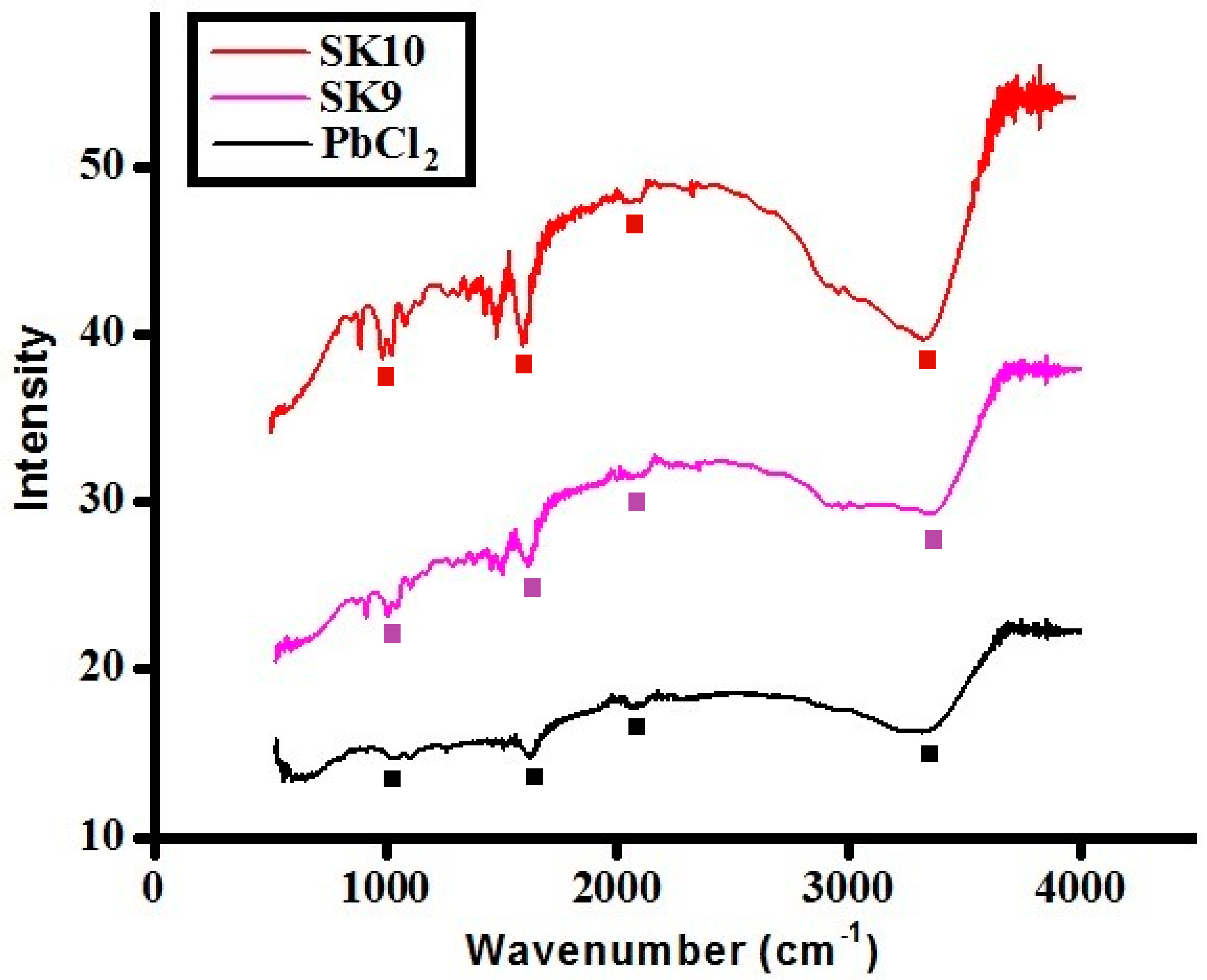
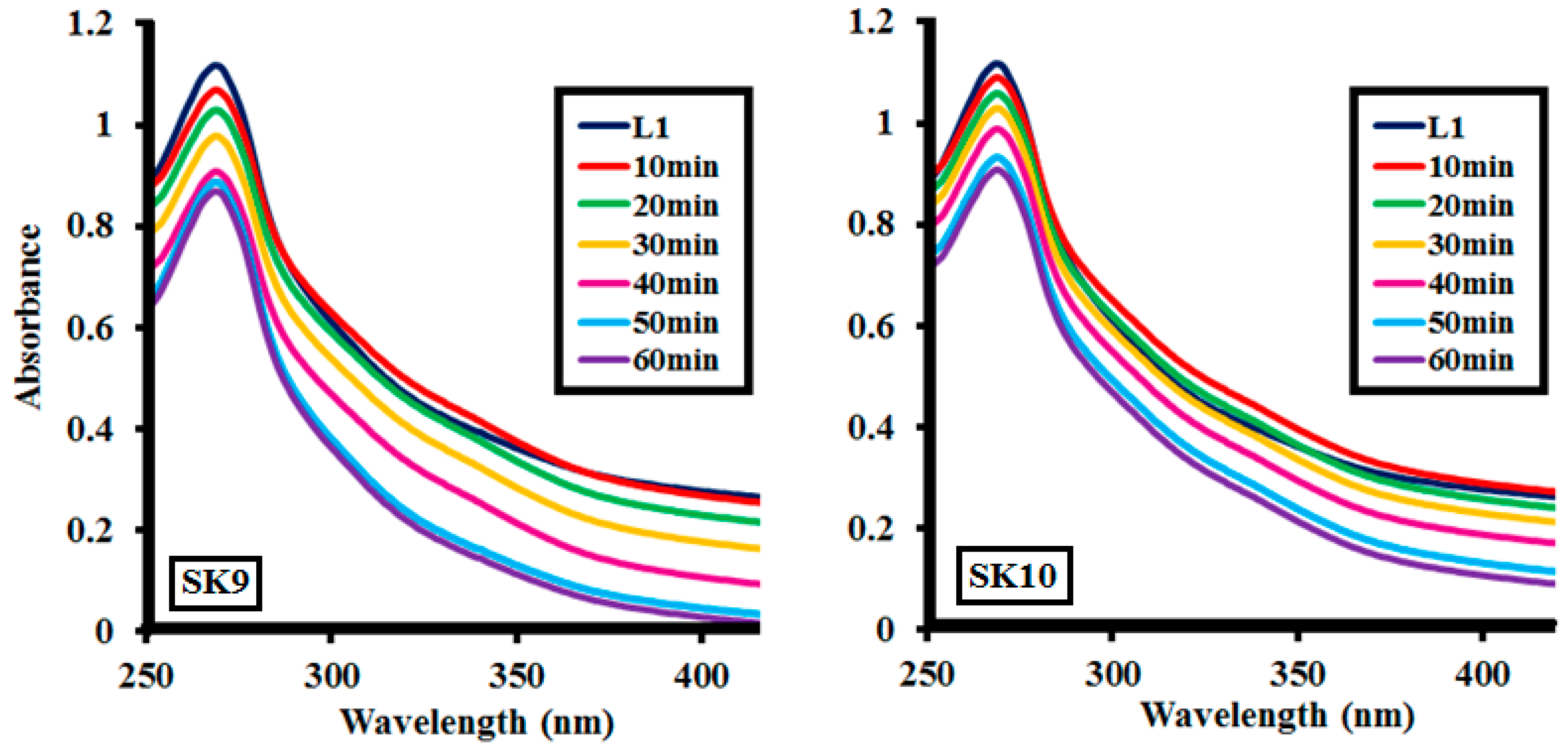


| Samples | SK9 | SK10 |
|---|---|---|
| PXRD Parameters | ||
| Average Crystallite Size D (nm) | 77.10 ± 5 | 75.57 ± 7 |
| Volume V = D3 | 1,162,604 | 1,051,104 |
| Dislocation Density × 10−3 (nm)−2 (δ) | 1.43 × 10−7 | 1.18 × 10−7 |
| Microstrain (ε) | 0.0017 | 0.0016 |
| SEM Parameters | ||
| Material Nature | Shiny Crystalline | Shiny Crystalline |
| Dispersity | Homogenous | Homogenous |
| Structural Appearance | Broad Elliptical Leaflets | Broad Elliptical Leaflets |
| Color | White | White |
| Time | %Lignin (L1100ppm) Depolymerization at Different Lignin Concentrations | |
|---|---|---|
| (min) | SK9 | SK10 |
| 0 | 0 | 0 |
| 10 | 17 | 10 |
| 20 | 31 | 20 |
| 30 | 48 | 31 |
| 40 | 72 | 45 |
| 50 | 79 | 65.5 |
| 60 | 86.5 | 72.5 |
| Time | %Lignin (L1100ppm) Depolymerization at Different Catalyst Doses of SK9 | |||
|---|---|---|---|---|
| (min) | 0.025 g | 0.05 g | 0.075 g | 0.1 g |
| 0 | 0 | 0 | 0 | 0 |
| 10 | 5 | 10 | 14 | 17 |
| 20 | 12 | 19 | 24 | 31 |
| 30 | 22.5 | 31 | 36 | 48 |
| 40 | 28 | 47 | 55.5 | 72 |
| 50 | 38 | 59 | 62 | 79 |
| 60 | 41.5 | 67 | 73 | 86.5 |
| Time | %Lignin (L1) Depolymerization at the Different Lignin Concentrations | |||
|---|---|---|---|---|
| (min) | 50 ppm | 100 ppm | 150 ppm | 200 ppm |
| 0 | 0 | 0 | 0 | 0 |
| 10 | 15 | 17 | 7 | 8 |
| 20 | 27 | 31 | 19 | 15 |
| 30 | 53 | 48 | 39.5 | 23 |
| 40 | 72.5 | 72 | 56 | 43 |
| 50 | 81 | 79 | 67 | 59 |
| 60 | 92.5 | 86.5 | 73 | 62.5 |
| Time | %Lignin (L1100ppm) Depolymerization at Different Temperatures | |||
|---|---|---|---|---|
| (min) | 20 °C | 35 °C | 65 °C | 90 °C |
| 0 | 0 | 0 | 0 | 0 |
| 10 | 17 | 21 | 22 | 26.5 |
| 20 | 31 | 26 | 27 | 31 |
| 30 | 48 | 32 | 47 | 48 |
| 40 | 72 | 50 | 64 | 69 |
| 50 | 79 | 76.5 | 81 | 89 |
| 60 | 86.5 | 89 | 92 | 97.5 |
| Pseudo Second Order Kinetic Parameters at Different Temperatures | ||
|---|---|---|
| Temperature | Rate Constant (k) (g−1min−1) | R2 |
| 20 °C | 0.011 | 0.97 |
| 35 °C | 0.018 | 0.97 |
| 65 °C | 0.132 | 0.98 |
| 90 °C | 0.186 | 0.99 |
| GC Peak No. | Retention Time (RT) | m/z of Molecular Ion Peak | Name | Concentration (%) |
|---|---|---|---|---|
| L1-SK9 | ||||
| 1 | 1.6 | 71 | Butadienol | 18 |
| 2 | 3.1 | 137 | 2-methoxy-4-methylphenol | 39 |
| 3 | 3.6 | 164 | 2-methoxy-5-propenyl phenol | 7 |
| 4 | 3.8 | 244 | 5-[2-(3-hydroxyphenyl)ethyl]-2-methoxyphenol (guaiacyl dimer) | 6 |
| L1-SK9 (90 °C) | ||||
| 1′ | 1.9 | 75 | Methoxypropane | 14 |
| 2′ | 2.1 | 78 | Benzene | 17 |
| 3′ | 2.3 | 81 | Cyclohexene | 19 |
| 4′ | 2.6 | 95 | Phenol | 10 |
| 5′ | 2.8 | 110 | Catechol | 7 |
| 6′ | 3.1 | 137 | 2-methoxy-4-methylphenol | 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, S.; Altaf, A.A.; Hamayun, M.; Rasool, N.; Hadait, M.; Akhtar, A.; Muhammad, S.; Badshah, A.; Shah, S.A.A.; Zakaria, Z.A. i-Propylammonium Lead Chloride Based Perovskite Photocatalysts for Depolymerization of Lignin Under UV Light. Molecules 2020, 25, 3520. https://doi.org/10.3390/molecules25153520
Kausar S, Altaf AA, Hamayun M, Rasool N, Hadait M, Akhtar A, Muhammad S, Badshah A, Shah SAA, Zakaria ZA. i-Propylammonium Lead Chloride Based Perovskite Photocatalysts for Depolymerization of Lignin Under UV Light. Molecules. 2020; 25(15):3520. https://doi.org/10.3390/molecules25153520
Chicago/Turabian StyleKausar, Samia, Ataf Ali Altaf, Muhammad Hamayun, Nasir Rasool, Mahwish Hadait, Arusa Akhtar, Shabbir Muhammad, Amin Badshah, Syed Adnan Ali Shah, and Zainul Amiruddin Zakaria. 2020. "i-Propylammonium Lead Chloride Based Perovskite Photocatalysts for Depolymerization of Lignin Under UV Light" Molecules 25, no. 15: 3520. https://doi.org/10.3390/molecules25153520
APA StyleKausar, S., Altaf, A. A., Hamayun, M., Rasool, N., Hadait, M., Akhtar, A., Muhammad, S., Badshah, A., Shah, S. A. A., & Zakaria, Z. A. (2020). i-Propylammonium Lead Chloride Based Perovskite Photocatalysts for Depolymerization of Lignin Under UV Light. Molecules, 25(15), 3520. https://doi.org/10.3390/molecules25153520






