Horticultural Plant Residues as New Source for Lignocellulose Nanofibers Isolation: Application on the Recycling Paperboard Process
Abstract
1. Introduction
2. Results and Discussion
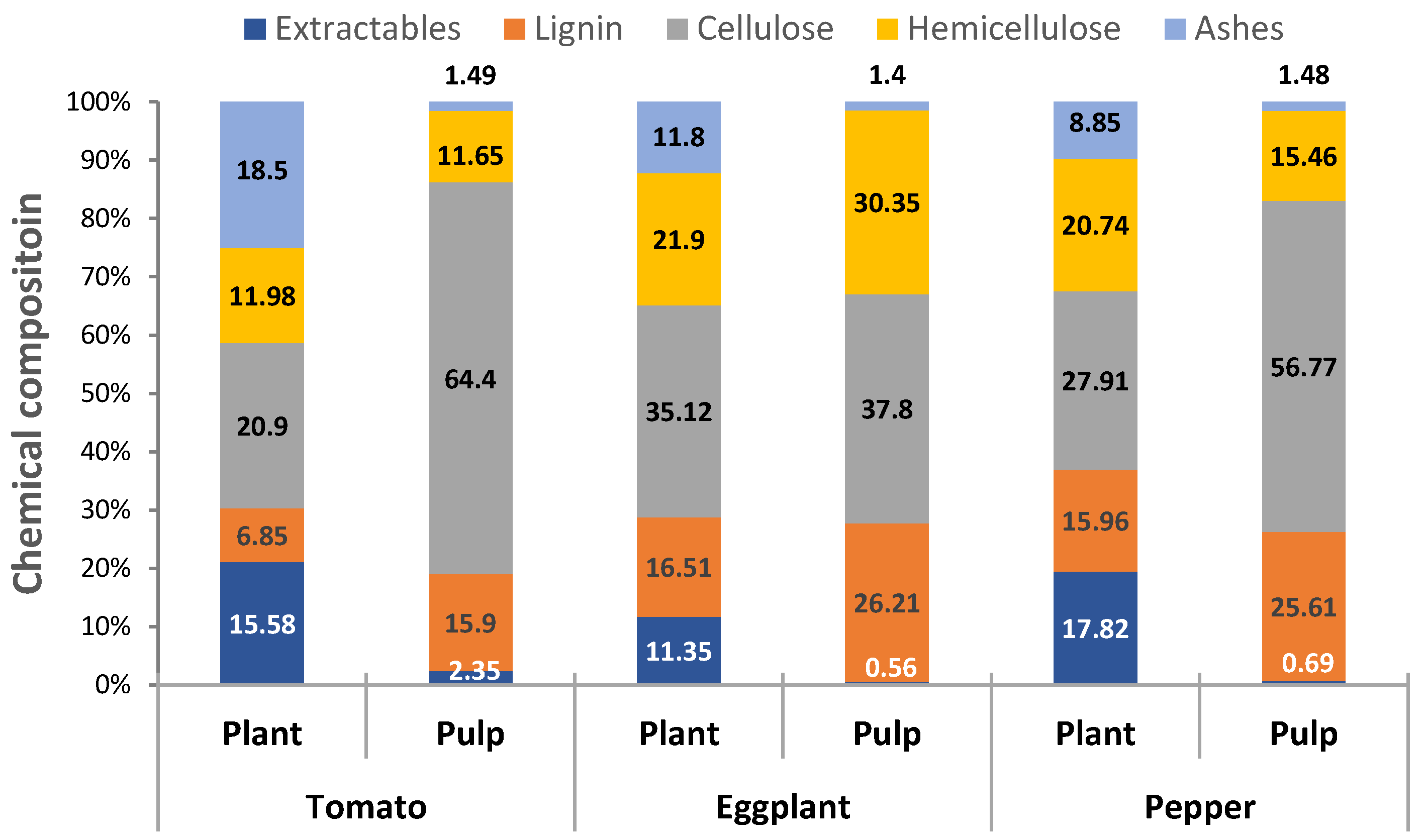
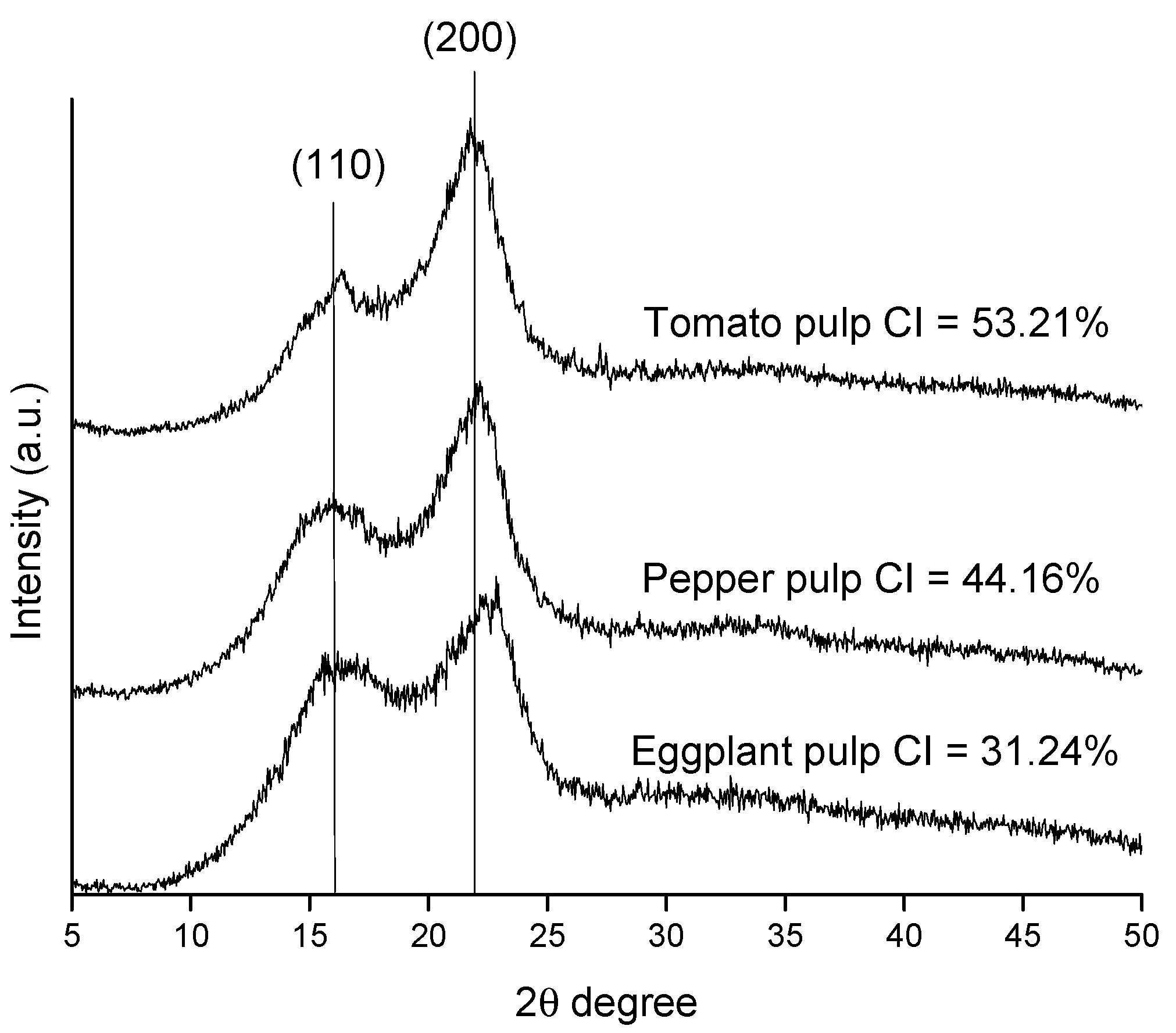
2.1. Chemical Composition of Raw Materials and Obtained Cellulosic Pulps
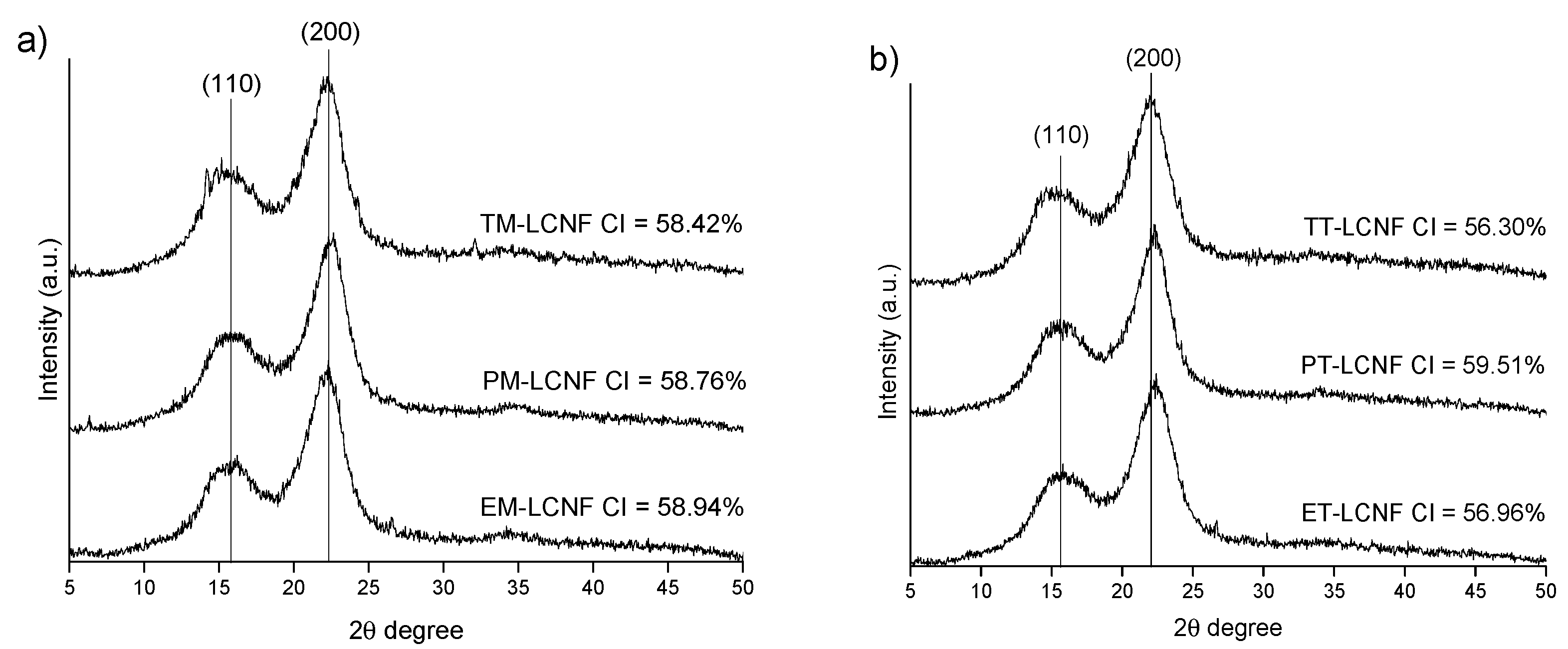
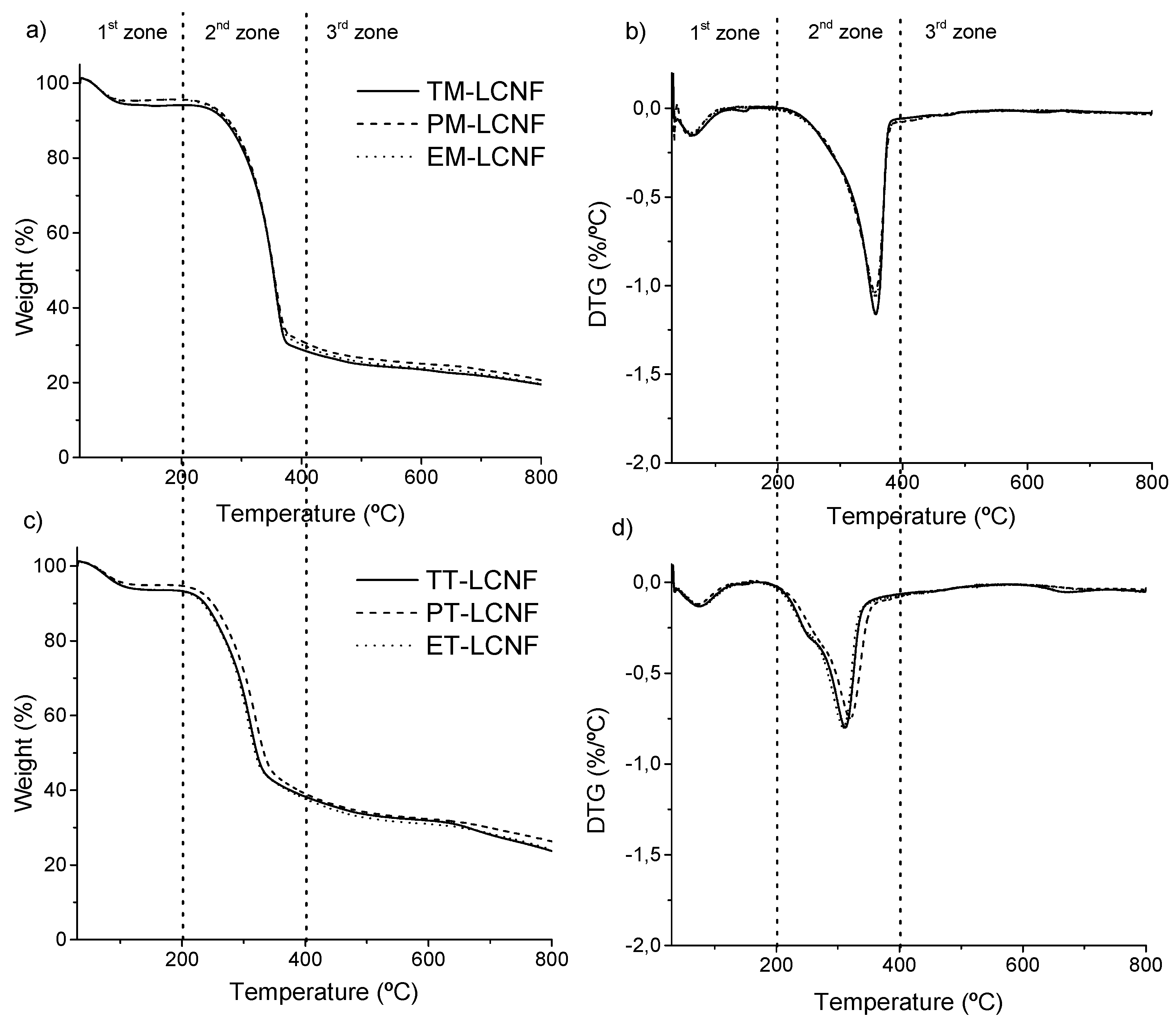
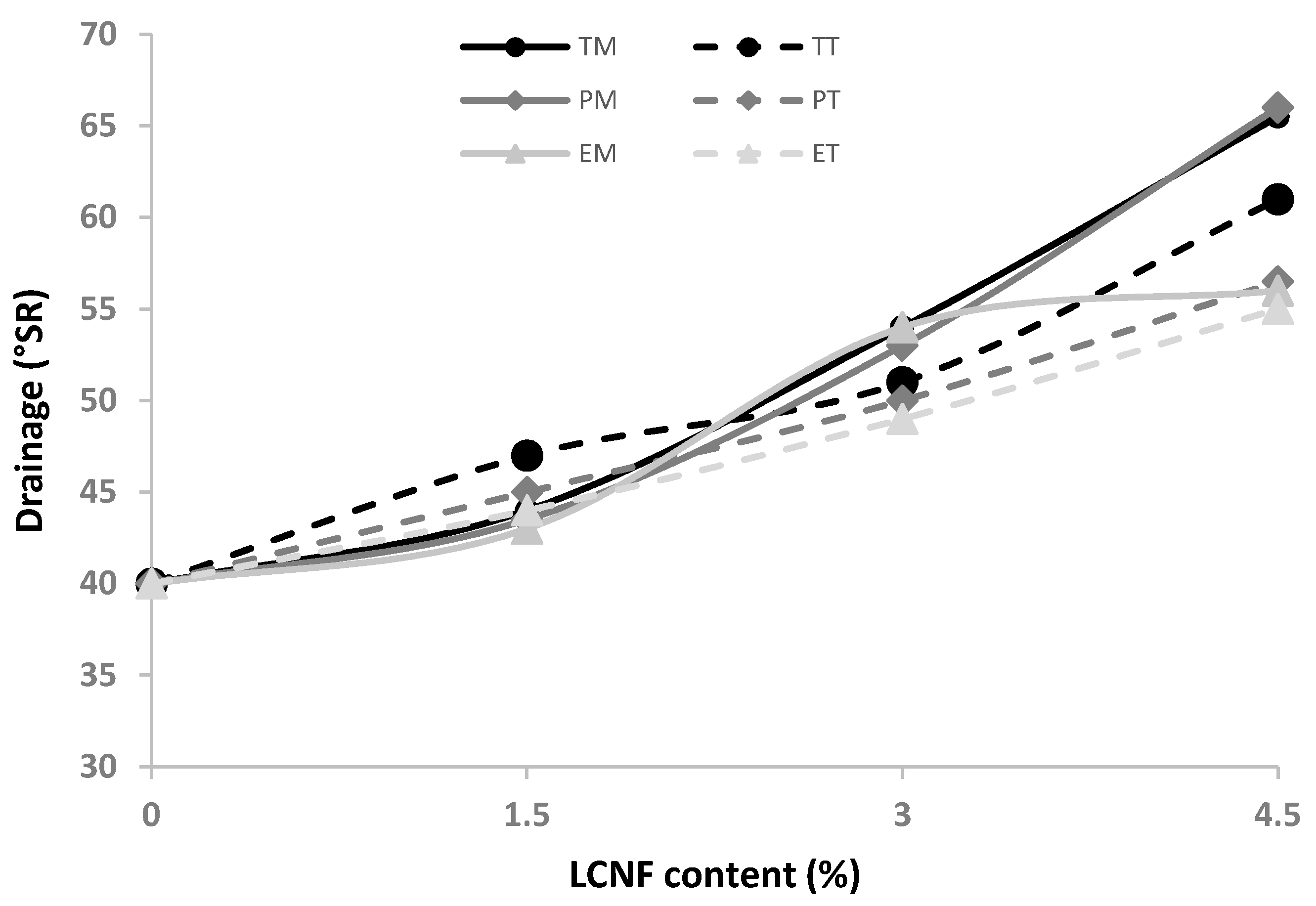
2.2. Lignocellulose Nanofiber Characterization
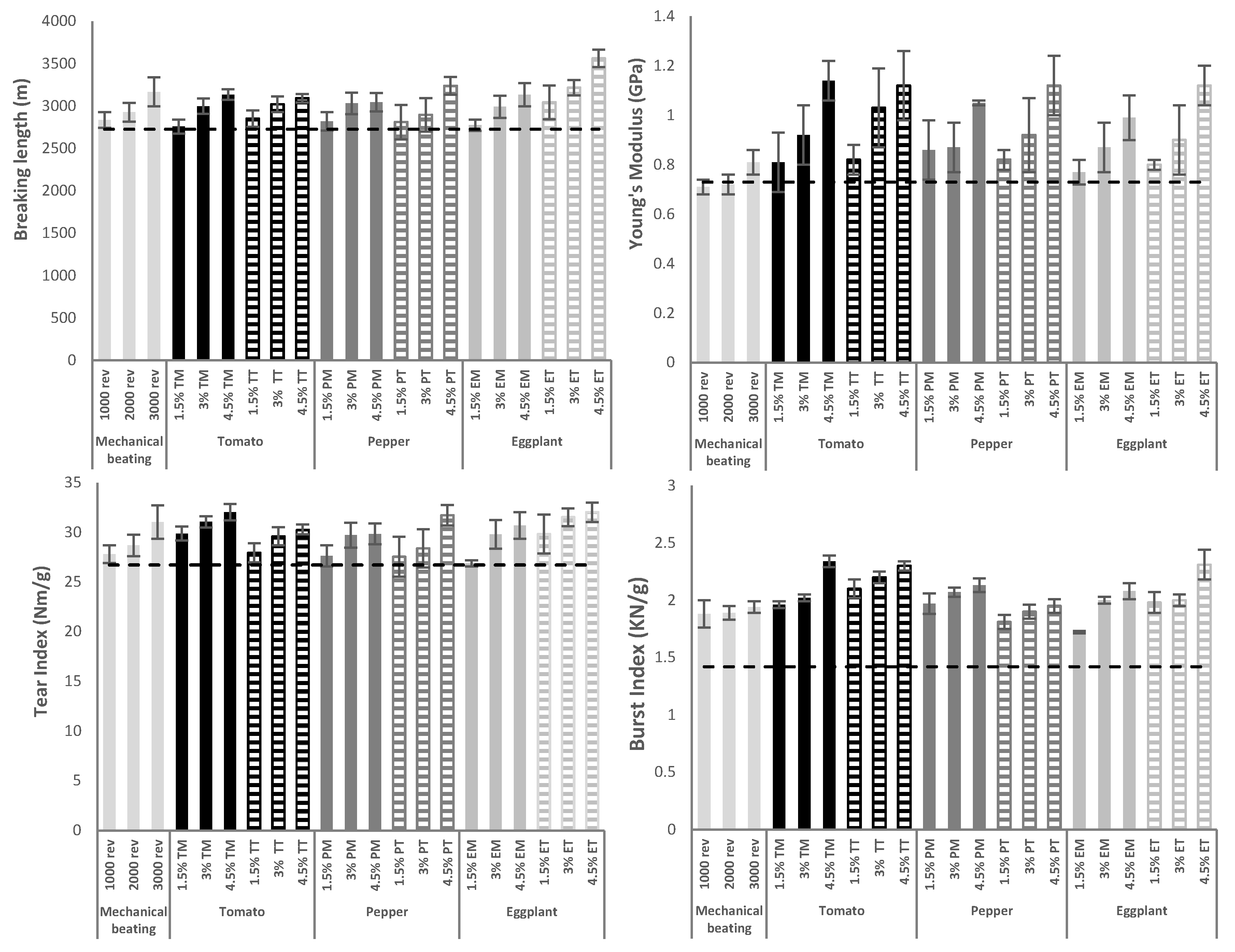
2.3. Lignocellulose Reinforcement on Recycled Paperboard
3. Materials and Methods
3.1. Materials
3.2. Soda Pulping
3.3. Pulp Characterization
3.4. Lignocellulose Nanofibers (LCNF) Production
3.4.1. Mechanical Pretreatment
3.4.2. TEMPO-Mediated Oxidation Pretreatment
3.4.3. High-Pressure Homogenization
3.5. LCNF Characterization
3.6. Polymerization Degree and Length
3.7. Spectroscopy Analysis
3.8. X-ray Diffraction (XRD) Analysis
3.9. Thermogravimetric Analysis
3.10. Reinforcement of Industrial Pulp
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- EEllen MacArthur Fundation, Towards a Circular Economy: Business rationale for an accelerated transition. 2015. Available online: https://www.ellenmacarthurfoundation.org/assets/downloads/TCE_Ellen-MacArthur-Foundation_9-Dec-2015.pdf. (accessed on 13 April 2020).
- Fundación COTEC, Situación y Evolución De La Economía Circular En España. 2017. Available online: http://cotec.es/media/informe-CotecISBN-1.pdf (accessed on 22 April 2020).
- European Commission, Innovating for Sustainable Growth: A Bioeconomy for Europe, (2012). Available online: https://ec.europa.eu/research/bioeconomy/index.cfm (accessed on 22 April 2020).
- Sheldon, R.A. Chemicals from renewable biomass: A renaissance in carbohydrate chemistry. Green Sustain. Chem. 2018, 14, 89–95. [Google Scholar] [CrossRef]
- Teja, K.; Sowlati, T. Biomass logistics: A review of important features, optimization modeling and the new trends. Renew. Sustain. Energy Rev. 2018, 94, 587–599. [Google Scholar]
- Pang, S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Sustainable Agriculture, Forestry and Fisheries in the Bioeconomy. A Challenge for Europe. 4th SCAR Foresight Exercise. 2015. Available online: http://ec.europa.eu/research/scar/pdf/feg4-draft-15_may_2015.pdf (accessed on 22 April 2020).
- George, M.; Chae, M.; Bressler, D.C. Composite materials with bast fibres: Structural, technical, and environmental properties. Prog. Mater. Sci. 2016, 83, 1–23. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; Tarrés, Q.; Pèlach, À.M.; Mutjé, P.; Fullana-I.-Palmer, P. Are Cellulose Nanofibres a Solution for a More Circular Economy of Paper Products? Environ. Sci. Technol. 2015, 49, 12206–12213. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Aguilar, M. Nanotecnología en el sector papelero: Mejoras en calidad y permanencia de las fibras de alto rendimiento y secundarias en una economía circular mediante el uso de nanofibras y el refino enzimático; University of Girona: Girona, Spain, 2015. [Google Scholar]
- Delgado-Aguilar, M.; González, I.; Pélach, M.A.; De La Fuente, E.; Negro, C. Improvement of deinked old newspaper/old magazine pulp suspensions by means of nanofibrillated cellulose addition. Cellulose 2015, 22, 789–802. [Google Scholar] [CrossRef]
- Anuario de Estadística. Ministerio de Agricultura, Pesca y Alimentación. 2018. Available online: https://www.mapa.gob.es/es/estadistica/temas/publicaciones/anuario-de-estadistica/default.aspx (accessed on 23 April 2020).
- Toledo, M.; Márquez, P.; Siles, J.A.; Chica, A.F.; Martín, M.A. Co-composting of sewage sludge and eggplant waste at full scale: Feasibility study to valorize eggplant waste and minimize the odoriferous impact of sewage sludge. J. Environ. Manag. 2019, 247, 205–213. [Google Scholar] [CrossRef]
- Hamraoui, K.; Gil, A.; El Bari, H.; Siles, J.A.; Chica, A.F.; Martín, M.A. Evaluation of hydrothermal pretreatment for biological treatment of lignocellulosic feedstock (pepper plant and eggplant). Waste Manag. 2020, 102, 76–84. [Google Scholar] [CrossRef]
- Guntekin, E.; Karakus, B. Feasibility of using eggplant (Solanum melongena) stalks in the production of experimental particleboard. Ind. Crop. Prod. 2008, 27, 354–358. [Google Scholar] [CrossRef]
- Covino, C.; Sorrentino, A.; di Pierro, P.; Roscigno, G.; PiaVece, A.; Masi, P. Lignocellulosic fibres from enzyme-treated tomato plantes: Characterisation and application in paperboard manufacturing. Int. J. Biol. Macromol. 2020, 161, 787–796. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; Otero, R.; Domínguez-Robles, J.; Rodríguez, A. A comparative study of the suitability of different cereal straws for lignocellulose nanofibres isolation. Int. J. Biol. Macromol. 2017, 103, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Chaker, A.; Alila, S.; Mutje, P.; Rei, M.; Sami, V. Key role of the hemicellulose content and the cell morphology on the nanofibrillation effectiveness of cellulose pulps. Cellulose 2013, 20, 2863–2875. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Alotaibi, M.D.; Alshammari, B.A.; Saba, N.; Alothman, O.Y.; Sanjay, M.R.; Almutairi, Z.; Jawaid, M. Characterization of natural fibre obtained from different parts of date palm tree (Phoenix dactylifera L.). Int. J. Biol Macromol. 2019, 135, 69–76. [Google Scholar] [CrossRef]
- Rajan, K.; Djioleu, A.; Kandhola, G.; Labbé, N.; Sakon, J.; Carrier, D.J.; Kim, J.W. Investigating the effects of hemicellulose pre-extraction on the production and characterization of loblolly pine nanocellulose. Cellulose 2020, 8, 3693–3706. [Google Scholar] [CrossRef]
- el Achaby, M.; el Miri, N.; Aboulkas, A.; Zahouily, M.; Bilal, E.; Barakat, A.; Solhy, A. Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse. Int. J. Biol. Macromol. 2017, 96, 340–352. [Google Scholar] [CrossRef]
- Rojo, E.; Peresin, M.S.; Sampson, W.W.; Hoeger, I.C.; Vartiainen, J.; Laine, J.; Rojas, O.J. Comprehensive elucidation of the effect of residual lignin on the physical, barrier, mechanical and surface properties of nanocellulose films. Green Chem. 2015, 17, 1853–1866. [Google Scholar] [CrossRef]
- Espinosa, E.; Sánchez, R.; González, Z.; Domínguez-Robles, J.; Ferrari, B.; Rodríguez, A. Rapidly growing vegetables as new sources for lignocellulose nanofibre isolation: Physicochemical, thermal and rheological characterisation. Carbohydr. Polym. 2017, 175, 27–37. [Google Scholar] [CrossRef]
- Tarrés, Q.; Pellicer, N.; Balea, A.; Merayo, N.; Negro, C.; Blanco, A.; Delgado-Aguilar, M.; Mutjé, P. Lignocellulosic micro/nanofibres from wood sawdust applied to recycled fibres for the production of paper bags. Int. J. Biol. Macromol. 2017, 105, 664–670. [Google Scholar] [CrossRef]
- Sánchez, R.; Espinosa, E.; Domínguez-robles, J.; Mauricio, J.; Rodríguez, A. Isolation and characterization of lignocellulose nanofibres from different wheat straw pulps. Int. J. Biol. Macromol. 2016, 92, 1025–1033. [Google Scholar] [CrossRef]
- Tarrés, Q.; Espinosa, E.; Domínguez-Robles, J.; Rodríguez, A.; Mutjé, P.; Delgado-Aguilar, M. The suitability of banana leaf residue as raw material for the production of high lignin content micro/nano fibres: From residue to value-added products. Ind. Crop. Prod. 2017, 99, 27–33. [Google Scholar] [CrossRef]
- Tarrés, Q.; Vanesa, N.; Evangelina, M.; Cristina, M.; Delgado-aguilar, M.; Mutjé, P. Lignocellulosic nanofibres from triticale straw: The influence of hemicelluloses and lignin in their production and properties. Carbohydr. Polym. 2017, 163, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Tarrés, Q.; Delgado-Aguilar, M.; Pèlach, M.A.; González, I.; Boufi, S.; Mutjé, P. Remarkable increase of paper strength by combining enzymatic cellulose nanofibres in bulk and TEMPO-oxidized nanofibres as coating. Cellulose 2016, 23, 3939–3950. [Google Scholar] [CrossRef]
- Serra, A.; González, I.; Oliver-Ortega, H.; Tarrès, Q.; Delgado-Aguilar, M.; Mutjé, P. Reducing the amount of catalyst in TEMPO-oxidized cellulose nanofibres: Effect on properties and cost. Polymers (Basel) 2017, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Tarrés, Q.; Boufi, S.; Mutjé, P.; Delgado-Aguilar, M. Enzymatically hydrolyzed and TEMPO-oxidized cellulose nanofibres for the production of nanopapers: Morphological, optical, thermal and mechanical properties. Cellulose 2017, 24, 3943–3954. [Google Scholar] [CrossRef]
- Sang, X.; Qin, C.; Tong, Z.; Kong, S.; Jia, Z. Mechanism and kinetics studies of carboxyl group formation on the surface of cellulose fibre in a TEMPO-mediated system. Cellulose 2017, 24, 2415–2425. [Google Scholar] [CrossRef]
- Yousefi, H.; Faezipour, M.; Hedjazi, S.; Mazhari, M. Comparative study of paper and nanopaper properties prepared from bacterial cellulose nanofibres and fibres/ground cellulose nanofibres of canola straw. Ind. Crop. Prod. 2013, 43, 732–737. [Google Scholar] [CrossRef]
- Tao, P.; Zhang, Y.; Wu, Z.; Liao, X.; Nie, S. Enzymatic pretreatment for cellulose nanofibrils isolation from bagasse pulp: Transition of cellulose crystal structure. Carbohydr. Polym. 2019, 214, 1–7. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibres from agricultural residues – Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, S.; Qin, C.; Wang, S. Removal of hexenuronic acid to reduce AOX formation in hot chlorine dioxide bleaching of bagasse pulp. Ind. Crops. Prod. 2019, 128, 338–345. [Google Scholar] [CrossRef]
- Boufi, S.; González, I.; Delgado-Aguilar, M.; Tarrès, Q.; Pèlach, M.À.; Mutjé, P. Nanofibrillated cellulose as an additive in papermaking process: A review. Carbohydr. Polym. 2016, 154, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Rol, F.; Bras, J.; Rodríguez, A. Production of lignocellulose nanofibres from wheat straw by different fibrillation methods. Comparison of its viability in cardboard recycling process. J. Clean. Prod. 2019, 239, 118083. [Google Scholar] [CrossRef]
- Espinosa, E.; Tarrés, Q.; Delgado-Aguilar, M.; González, I.; Mutjé, P.; Rodríguez, A. Suitability of wheat straw semichemical pulp for the fabrication of lignocellulosic nanofibres and their application to papermaking slurries. Cellulose 2015, 23, 837–852. [Google Scholar] [CrossRef]
- Lourenço, A.F.; Gamelas, J.A.F.; Sarmento, P.; Ferreira, P.J.T. Enzymatic nanocellulose in papermaking – The key role as filler flocculant and strengthening agent. Carbohydr. Polym. 2019, 224, 115200. [Google Scholar] [CrossRef]
- Vargas, F.; González, Z.; Sánchez, R.; Jiménez, L.; Rodríguez, A. Cellulosic pulps of cereal straws as raw material. BioResources 2012, 7, 4161–4170. [Google Scholar]
- Besbes, I.; Alila, S.; Boufi, S. Nanofibrillated cellulose from TEMPO-oxidized eucalyptus fibres: Effect of the carboxyl content. Carbohydr. Polym. 2011, 84, 975–983. [Google Scholar] [CrossRef]
- Carrasco, F.; Mutjé, P.; Pelach, M.A. Control of retention in paper-making by colloid titration and zeta potential techniques. Wood Sci. Technol. 1998, 32, 145–155. [Google Scholar] [CrossRef]
- Carrasco, F.; Mutjé, P.; Pelach, M.A. Refining of bleached cellulosic pulps: Characterization by application of the colloidal titration technique. Wood Sci. Technol. 1996, 30, 227–236. [Google Scholar] [CrossRef]
- Marx-Figini, M. The acid-catalyzed degradation of cellulose linters in distinct ranges of bacterial nano-cellulose reinforced fibre-cement composites. Constr. Build. Mater. 1987, 101, 958–964. [Google Scholar]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between length and degree of polymerization of TEMPO-oxidized cellulose nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| LCNF Sample | η (%) | CD (μeq/g) | CC (μmols/g) | σLCNF (m2/g) | Diameter (nm) | Length (nm) |
|---|---|---|---|---|---|---|
| TM-LCNF | 17.81 ± 2.59 | 298.39 ± 48.30 | 247.35 ± 6.14 | 24.86 | 112 | 5440 |
| PM-LCNF | 18.34 ± 3.37 | 166.46 ± 0.00 | 148.71 ± 1.66 | 8.64 | 278 | 4317 |
| EM-LCNF | 32.61 ± 3.48 | 248.30± 10.89 | 127.25 ± 3.99 | 58.95 | 42 | 5132 |
| TT-LCNF | 48.77 ± 1.30 | 707.86 ± 18.54 | 299.96 ± 48.76 | 198.65 | 12 | 2524 |
| PT-LCNF | 69.66 ± 6.11 | 513.37 ± 37.23 | 205.81 ± 5.86 | 148.78 | 17 | 1902 |
| ET-LCNF | 66.39 ± 1.52 | 563.38 ± 37.06 | 186.61 ± 63.78 | 183.48 | 14 | 2049 |
| Treatment | Sample | Thickness (μm) | Density (g/cm3) | Porosity (%) |
|---|---|---|---|---|
| Recycled paperboard | 150.3 ± 2.9 | 0.36 ± 0.01 | 75.55 ± 0.79 | |
| Mechanical beating | 1000 rev | 147.8 ± 6.5 | 0.37 ± 0.01 | 75.10 ± 1.01 |
| 2000 rev | 147.2 ± 8.0 | 0.38 ± 0.02 | 74.93 ± 1.49 | |
| 3000 rev | 146.8 ± 6.6 | 0.38 ± 0.01 | 74.75 ± 0.91 | |
| Tomato LCNF | 1.5% TM | 139.9 ± 4.2 | 0.39 ± 0.01 | 73.72 ± 0.93 |
| 3% TM | 136.6 ± 3.8 | 0.40 ± 0.02 | 73.06 ± 1.61 | |
| 4.5% TM | 134.9 ± 2.7 | 0.41 ± 0.02 | 72.72 ± 1.38 | |
| 1.5% TT | 142.4 ± 5.4 | 0.39 ± 0.01 | 74.53 ± 0.57 | |
| 3% TT | 142.6 ± 1.9 | 0.39 ± 0.03 | 74.23 ± 0.74 | |
| 4.5% TT | 138.4 ± 4.3 | 0.40 ± 0.02 | 73.38 ± 1.63 | |
| Pepper LCNF | 1.5% PM | 136.0 ± 4.9 | 0.40 ± 0.01 | 72.94 ± 1.34 |
| 3% PM | 134.2 ± 7.6 | 0.41 ± 0.02 | 72.82 ± 0.69 | |
| 4.5% PM | 133.1 ± 5.6 | 0.41 ± 0.02 | 72.78 ± 0.72 | |
| 1.5% PT | 147.5 ± 3.0 | 0.38 ± 0.02 | 75.08 ± 1.42 | |
| 3% PT | 147.8 ± 2.1 | 0.38 ± 0.03 | 74.86 ± 1.06 | |
| 4.5% PT | 147.4 ± 5.3 | 0.39 ± 0.01 | 74.04 ± 0.36 | |
| Eggplant LCNF | 1.5% EM | 146.0 ± 3.9 | 0.38 ± 0.01 | 74.91 ± 0.78 |
| 3% EM | 145.3 ± 4.5 | 0.39 ± 0.02 | 74.00 ± 1.83 | |
| 4.5% EM | 138.7 ± 9.2 | 0.40 ± 0.02 | 73.43 ± 1.54 | |
| 1.5% ET | 143.7 ± 8.1 | 0.38 ± 0.01 | 74.94 ± 0.83 | |
| 3% ET | 146.7 ± 4.4 | 0.38 ± 0.02 | 74.51 ± 1.23 | |
| 4.5% ET | 144.5 ± 6.1 | 0.39 ± 0.01 | 74.37 ± 0.66 |
| Raw Material | Pretreatment | Treatment | Codification |
|---|---|---|---|
| Tomato | Mechanical | High-pressure homogenization | TM-LCNF |
| TEMPO-mediated oxidation | TT-LCNF | ||
| Pepper | Mechanical | PM-LCNF | |
| TEMPO-mediated oxidation | PT-LCNF | ||
| Eggplant | Mechanical | EM-LCNF | |
| TEMPO-mediated oxidation | ET-LCNF |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bascón-Villegas, I.; Espinosa, E.; Sánchez, R.; Tarrés, Q.; Pérez-Rodríguez, F.; Rodríguez, A. Horticultural Plant Residues as New Source for Lignocellulose Nanofibers Isolation: Application on the Recycling Paperboard Process. Molecules 2020, 25, 3275. https://doi.org/10.3390/molecules25143275
Bascón-Villegas I, Espinosa E, Sánchez R, Tarrés Q, Pérez-Rodríguez F, Rodríguez A. Horticultural Plant Residues as New Source for Lignocellulose Nanofibers Isolation: Application on the Recycling Paperboard Process. Molecules. 2020; 25(14):3275. https://doi.org/10.3390/molecules25143275
Chicago/Turabian StyleBascón-Villegas, Isabel, Eduardo Espinosa, Rafael Sánchez, Quim Tarrés, Fernando Pérez-Rodríguez, and Alejandro Rodríguez. 2020. "Horticultural Plant Residues as New Source for Lignocellulose Nanofibers Isolation: Application on the Recycling Paperboard Process" Molecules 25, no. 14: 3275. https://doi.org/10.3390/molecules25143275
APA StyleBascón-Villegas, I., Espinosa, E., Sánchez, R., Tarrés, Q., Pérez-Rodríguez, F., & Rodríguez, A. (2020). Horticultural Plant Residues as New Source for Lignocellulose Nanofibers Isolation: Application on the Recycling Paperboard Process. Molecules, 25(14), 3275. https://doi.org/10.3390/molecules25143275










