Influence of Fermentation Water on Stable Isotopic D/H Ratios of Alcohol Obtained from Concentrated Grape Must
Abstract
1. Introduction
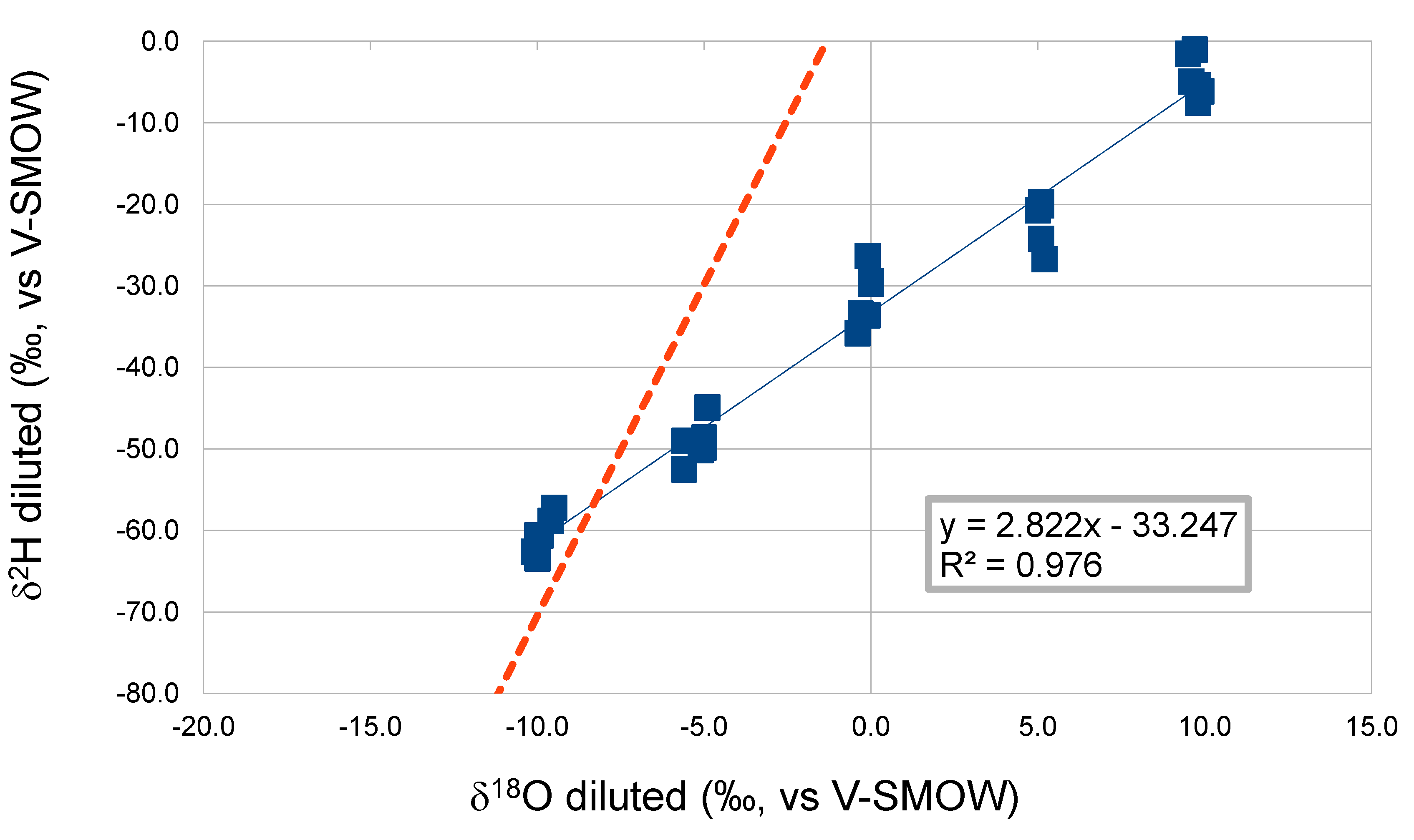
Rationale
- 0.19 refers to the percentage of hydrogen in the methyl site of ethanol that derives from the tap water used for fermentation, influencing the ratio (D/H)I
- 0.78 refers to the percentage of hydrogen in the methylene site of ethanol that derive from the tap water used for fermentation, influencing the ratio (D/H)II
2. Results and Discussion
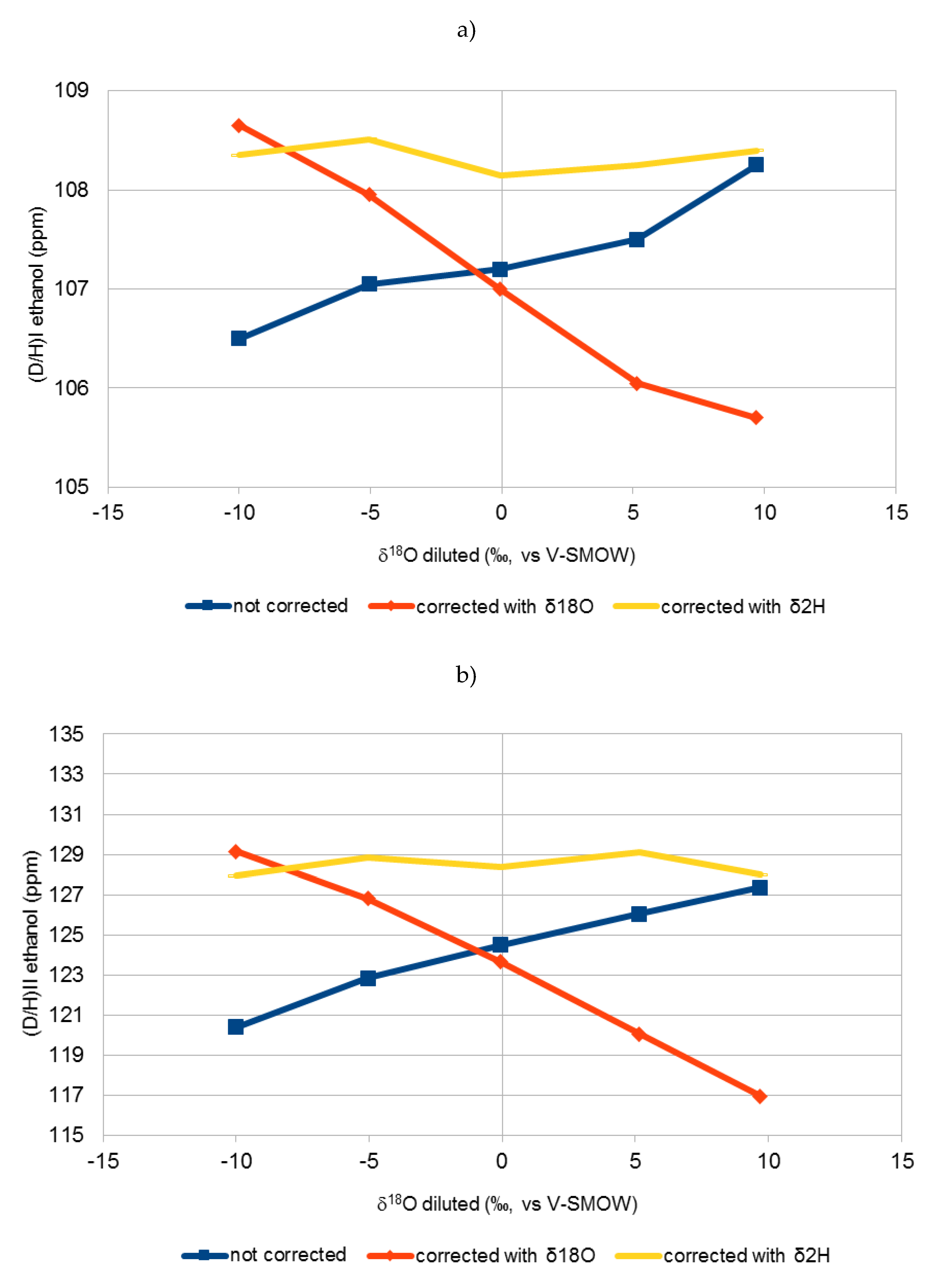
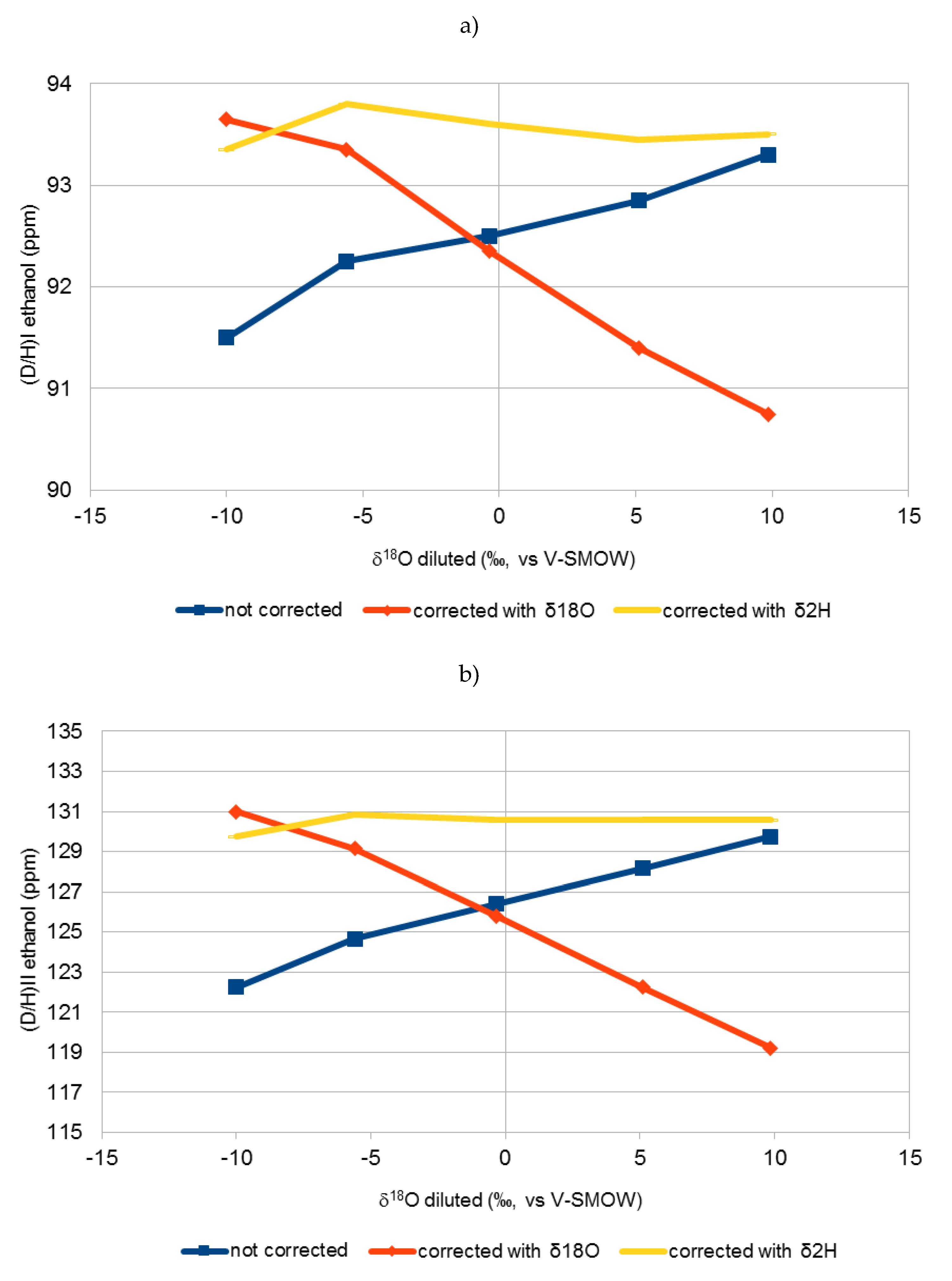
2.1. (D/H)I and (D/H)II Isotopic Ratios of Different Sugars
2.2. (D/H)I and (D/H)II Isotopic Ratios of Grape Must
3. Materials and Methods
3.1. Samples
3.2. Preparation of Water Samples for Dilution
3.3. Preparation of the Samples to Submit to Fermentation
3.4. Samples Fermentation and Distillation
3.5. Analysis of the Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Carter, J.F.; Chesson, L.A. Food Forensics: Stable Isotopes as a Guide to Authenticity and Origin; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781351647687. [Google Scholar]
- Calderone, G.; Guillou, C.; Naulet, N. Official methods based on stable isotope techniques for analysis of food. Ten years’ of European experience. Actual. Chim. 2003, 1, 22–24. [Google Scholar]
- Johnson, H.; Robinson, J. The World Atlas of Wine 8th Edition; Mitchell Beazley Publishers Limited: London, UK, 2019; ISBN 9781784726188. [Google Scholar]
- Teter, S.A.; Brandon Sutton, K.; Emme, B. Enzymatic processes and enzyme development in biorefining. In Advances in Biorefineries: Biomass and Waste Supply Chain Exploitation, 1st ed.; Waldron, K.W., Ed.; Woodhead Puplishing: Sawston, Cambridge, UK, 2014; pp. 199–233. ISBN 9780857097385. [Google Scholar]
- Singh, B.P. Isotopic composition of water in precipitation in a region or place. Appl. Radiat. Isot. 2013, 75, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A. Application of Environmental Isotopes in Hydrological Studies Along the River Nile Valley. Egypt. Isotopes in Hydrology, Marine Ecosystems and Climate Change Studies. In Proceedings of the International Symposium, Monaco, Germany, 27 March–1 April 2011. [Google Scholar]
- Martin, G.G.; Martin, Y.-L.; Naulet, N.; McManus, H.J.D. Application of 2H SNIF-NMR and13C SIRA-MS Analyses to Maple Syrup: Detection of Added Sugars. J. Agric. Food Chem. 1996, 44, 3206–3213. [Google Scholar] [CrossRef]
- Martin, G.G.; Hanote, V.; Lees, M.; Martin, M.Y. Interpretation of Combined 2H SNIF/NMR and 13C SIRA/MS Analyses of Fruit Juices to Detect Added Sugar. J. Aoac Int. 1996, 79, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, K.; Araguás-Araguás, L.; Gonfiantini, R. Isotopic Patterns in Modern Global Precipitation. In Climate Change in Continental Isotopic Records; Swart, P.K., Ed.; American Geophysical Union: Washington, DC, USA, 2013; pp. 1–36. [Google Scholar]
- Bauer-Christoph, C.; Wachter, H.; Christoph, N.; Rossmann, A.; Adam, L. Assignment of raw material and authentication of spirits by gas chromatography. hydrogen- and carbon-isotope ratio measurements. Z. Fur Lebensm. Unters. Forsch. 1997, 204, 445–452. [Google Scholar] [CrossRef]
- Horita, J.; Rozanski, K.; Cohen, S. Isotope effects in the evaporation of water: A status report of the Craig-Gordon model. Isot. Environ. Health Stud. 2008, 44, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Raco, B.; Dotsika, E.; Poutoukis, D.; Battaglini, R.; Chantzi, P. O–H–C isotope ratio determination in wine in order to be used as a fingerprint of its regional origin. Food Chem. 2015, 168, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, N.L.; Caldwell, E.A. Influence of weather on the stable isotopic ratios of wines: Tools for weather/climate reconstruction? J. Geophys. Res.: Atmos. 1999, 104, 2185–2194. [Google Scholar] [CrossRef]
- Longinelli, A.; Selmo, E. Isotopic composition of precipitation in Italy: A first overall map. J. Hydrol. 2003, 270, 75–88. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Cernusak, L.A.; Barnes, B. Heavy water fractionation during transpiration. Plant. Physiol. 2007, 143, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Duhr, A.; Hilkert, A.W. Application Note 30049: Automated H2/H2O Equilibration for δD Determination on Aqueous Samples Using Thermo Scientific GasBench II; Thermo Fisher Scientific: Bremen, Germany, 2004. [Google Scholar]
Sample Availability: Not available. |

| Cane Sugar | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | δ18O | δ2H | (D/H)I | (D/H)I | (D/H)I | (D/H)II | (D/H)II | (D/H)II |
| ID | Water Dluted Soltion | Water Dluted Soltion | Ethanol Not Corected | Ethanol Corrected with δ18O | Ethanol Corrected with δ2H | Ethanol Not Corected | Ethanol Corrected with δ18O | Ethanol Corrected with δ2H |
| (‰ vs. V-SMOW) | (‰, vs. V-SMOW) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | |
| 1a | −10.0 | −63.4 | 106.5 | 108.6 | 108.4 | 120.5 | 129.2 | 128.2 |
| 1b | −10.0 | −60.6 | 106.5 | 108.7 | 108.3 | 120.3 | 129.1 | 127.7 |
| 2a | −5.1 | −50.1 | 106.9 | 107.8 | 108.4 | 122.5 | 126.5 | 128.6 |
| 2b | −5.0 | −48.6 | 107.2 | 108.1 | 108.6 | 123.2 | 127.1 | 129.1 |
| 3a | −0.1 | −33.6 | 107.0 | 106.8 | 108.0 | 124.4 | 123.6 | 128.5 |
| 3b | 0.0 | −29.4 | 107.4 | 107.2 | 108.3 | 124.6 | 123.7 | 128.2 |
| 4a | 5.1 | −24.2 | 107.3 | 105.9 | 108.0 | 126.0 | 120.0 | 128.9 |
| 4b | 5.2 | −26.7 | 107.7 | 106.2 | 108.5 | 126.1 | 120.1 | 129.3 |
| 5a | 9.6 | −4.9 | 108.2 | 105.7 | 108.3 | 127.2 | 116.9 | 127.8 |
| 5b | 9.8 | −5.4 | 108.3 | 105.7 | 108.5 | 127.5 | 117.0 | 128.2 |
| Beet Sugar | ||||||||
| 6a | −9.9 | −60.6 | 91.2 | 93.3 | 93.0 | 122.1 | 130.8 | 129.5 |
| 6b | −10.1 | −62.6 | 91.8 | 94.0 | 93.7 | 122.4 | 131.2 | 130.0 |
| 7a | −5.6 | −49.0 | 92.3 | 93.4 | 93.8 | 124.5 | 129.0 | 130.5 |
| 7b | −5.6 | −52.5 | 92.2 | 93.3 | 93.8 | 124.8 | 129.3 | 131.2 |
| 8a | −0.3 | −33.4 | 92.8 | 92.7 | 93.8 | 126.6 | 125.9 | 130.6 |
| 8b | −0.4 | −35.8 | 92.2 | 92.0 | 93.4 | 126.2 | 125.7 | 130.6 |
| 9a | 5.1 | −20.1 | 92.8 | 91.4 | 93.4 | 128.1 | 122.2 | 130.5 |
| 9b | 5.1 | −19.8 | 92.9 | 91.4 | 93.5 | 128.2 | 122.3 | 130.6 |
| 10a | 9.8 | −7.5 | 92.8 | 90.3 | 93.0 | 128.9 | 118.4 | 129.8 |
| 10b | 9.9 | −6.1 | 93.8 | 91.2 | 94.0 | 130.6 | 120.0 | 131.3 |
| Grape Sugar | ||||||||
| 11a | −9.5 | −57.2 | 101.6 | 103.6 | 103.3 | 123.8 | 132.1 | 130.8 |
| 11b | −9.6 | −58.8 | 101.5 | 103.5 | 103.2 | 123.4 | 131.7 | 130.5 |
| 12a | −5.0 | −49.8 | 101.8 | 102.7 | 103.2 | 125.6 | 129.5 | 131.7 |
| 12b | −4.9 | −44.9 | 101.9 | 102.8 | 103.2 | 125.7 | 129.5 | 131.2 |
| 13a | −0.1 | −26.3 | 102.8 | 102.6 | 103.6 | 128.2 | 127.4 | 131.4 |
| 13b | 0.0 | −29.7 | 102.9 | 102.6 | 103.7 | 128.3 | 127.3 | 131.9 |
| 14a | 5.1 | −19.7 | 102.4 | 100.9 | 103.0 | 128.9 | 123.0 | 131.3 |
| 14b | 5.0 | −20.7 | 102.7 | 101.3 | 103.3 | 128.9 | 123.1 | 131.4 |
| 15a | 9.5 | −1.5 | 102.9 | 100.4 | 102.9 | 129.9 | 119.7 | 130.1 |
| 15b | 9.7 | −1.0 | 102.7 | 100.2 | 102.7 | 130.3 | 119.9 | 130.4 |
| Must 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | δ18O | δ2H | (D/H)I | (D/H)I | (D/H)I | (D/H)II | (D/H)II | (D/H)II |
| ID | Water | Water | Ethanol | Ethanol | Ethanol | Ethanol | Ethanol | Ethanol |
| Diluted Must | Diluted Must | Not Corected | Corrected with δ18O | Corrected with δ2H | Not Corected | Corrected with δ18O | Corrected with δ2H | |
| (‰ vs. V-SMOW) | (‰, vs. V-SMOW) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | (ppm) | |
| 16 | −7.3 | −48.0 | 99.3 | 100.3 | 100.7 | 123.4 | 129.5 | 129.2 |
| 17 | −3.0 | −36.6 | 99.5 | 100.0 | 100.6 | 124.8 | 126.8 | 129.2 |
| 18 | 2.0 | −21.4 | 99.9 | 99.2 | 100.5 | 126.5 | 123.5 | 129.1 |
| 19 | 5.0 | −10.1 | 100.3 | 98.7 | 100.6 | 128.0 | 121.7 | 129.2 |
| Must 2 | ||||||||
| 20 | −7.5 | −49.0 | 98.8 | 100.4 | 100.3 | 123.1 | 129.4 | 129.0 |
| 21 | −3.0 | −33.1 | 99.1 | 99.7 | 100.2 | 125.0 | 127.3 | 129.0 |
| 22 | 2.0 | −19.4 | 100.0 | 99.3 | 100.6 | 127.2 | 124.2 | 129.6 |
| 23 | 5.0 | −9.4 | 100.4 | 98.9 | 100.6 | 129.0 | 122.8 | 130.1 |
| Must 3 | ||||||||
| 24 | −7.2 | −45.0 | 100.6 | 102.1 | 101.9 | 123.9 | 129.9 | 129.3 |
| 25 | −3.0 | −32.3 | 101.1 | 101.6 | 102.0 | 125.7 | 127.7 | 129.6 |
| 26 | 2.0 | −18.0 | 101.1 | 100.3 | 101.6 | 126.7 | 123.3 | 128.9 |
| 27 | 5.0 | −5.9 | 101.7 | 100.5 | 101.9 | 128.3 | 123.1 | 129.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perini, M.; Failoni, A.; Simoni, M.; Tonon, A.; Camin, F. Influence of Fermentation Water on Stable Isotopic D/H Ratios of Alcohol Obtained from Concentrated Grape Must. Molecules 2020, 25, 3139. https://doi.org/10.3390/molecules25143139
Perini M, Failoni A, Simoni M, Tonon A, Camin F. Influence of Fermentation Water on Stable Isotopic D/H Ratios of Alcohol Obtained from Concentrated Grape Must. Molecules. 2020; 25(14):3139. https://doi.org/10.3390/molecules25143139
Chicago/Turabian StylePerini, Matteo, Andrea Failoni, Marco Simoni, Agostino Tonon, and Federica Camin. 2020. "Influence of Fermentation Water on Stable Isotopic D/H Ratios of Alcohol Obtained from Concentrated Grape Must" Molecules 25, no. 14: 3139. https://doi.org/10.3390/molecules25143139
APA StylePerini, M., Failoni, A., Simoni, M., Tonon, A., & Camin, F. (2020). Influence of Fermentation Water on Stable Isotopic D/H Ratios of Alcohol Obtained from Concentrated Grape Must. Molecules, 25(14), 3139. https://doi.org/10.3390/molecules25143139







