Validation of the 2H-SNIF NMR and IRMS Methods for Vinegar and Vinegar Analysis: An International Collaborative Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pre-Trial Study
2.2. Collaborative Study
3. Materials and Methods
3.1. Samples
3.2. Pilot Production of Vinegar and ABM
3.3. Participants
- Dr. Zedda Claudia, Agenzia delle Dogane, Laboratorio Chimico di Torino, Corso Sebastopoli 3, 10134 Torino, Italy
- Dr. Diana Costinel/Oana Botoran, Stable Isotope Laboratory, National Research and Development Institute for Cryogenic and Isotopic Technologies – ICSI Rm. Valcea, Uzinei Street No. 4, Post code 240050, Romania
- Dr. Freddy Thomas, Eurofins Analytics France, 9, Rue Pierre Adolphe Bobierre, B.P. 42301, F-44323 NANTES Cedex 3, France
- Dr. Rebecca Kokkinofta-Diogenou, State General Laboratory, 44 Kimonos str., 2081 Nicosia, Cyprus
- Mrs. Miriam Schmidt, Chelab Dr. Ara, Carl-Zeiss-Strasse 16, 30966 Hemmingen, Germany
- Dr. Armin Hermann, Institut für Lebensmittelchemie, Nikolaus-von-Weis-Straße 1, D-67346 Speyer, Germany
- Dr Matteo Perini/Federica Camin, Fondazione Edmund Mach, via Mach 1, 38010, San Michele all’Adige (TN), Italy
3.4. Methods
3.5. Preparation of Samples
3.5.1. Extraction and Purification of Acetic Acid
3.5.2. Liquid–Liquid Extraction
3.5.3. Purification of the Extract
3.5.4. Fermentation of the Aqueous Solution (for Balsamic Vinegar Only)
3.6. Analysis
3.6.1. Stable Isotope Analysis of Ethanol and of δ13C of Acetic Acid
3.6.2. Analysis of Vinegar and Balsamic Vinegar δ18O
3.6.3. Analysis of Acetic Acid (D/H)CH3 using SNIF-NMR
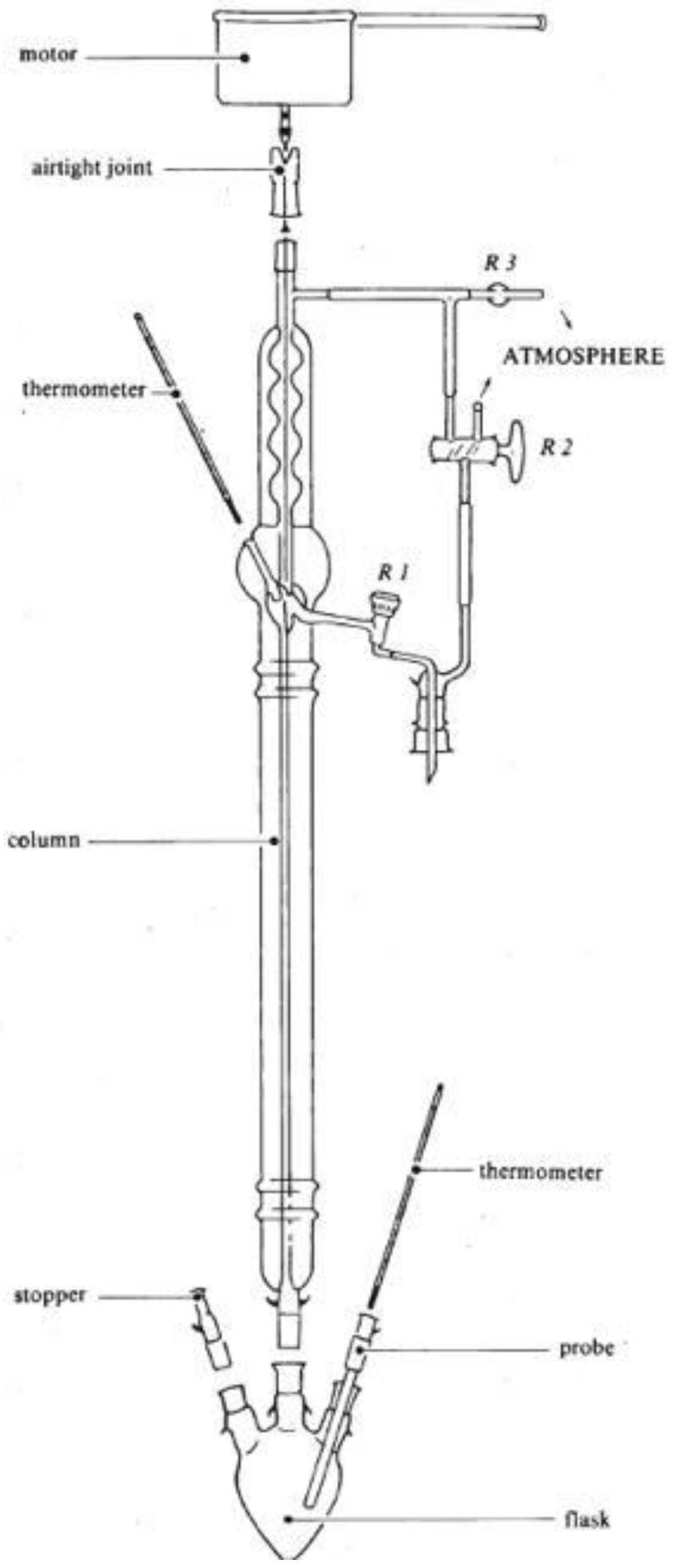
Preparation of the Acetic Acid Sample for NMR Measurement
Acquisition of the NMR Spectrum of Acetic Acid
- Probe temperature must be constant at 303 K;
- The sample should be rotated at 15 Hz;
- Acquisition time of 6.8 s at a spectral width of about 20 ppm;
- 90° pulse angle;
- Fix the offset 01 at 5.1 ppm;
- Quadratur detection mode;
- Set O2 to 2.5 ppm.
- Probe temperature and sample rotation must be the same as for 1H-NMR;
- Acquisition time of at least 4.1 s (the sum of AQ and D1 must be at least 11 s);
- Spectral width at least 16 ppm;
- Pulse angle of 30° or lower;
- D1 = 7 s (the sum of AQ and D1 must be at least 11 s);
- Quadratur detection mode;
- Set O1p to 5.1 ppm;
- The NS should be 8 at least;
- Use at least 8 dummy scans.
Calculation of Results of Acetic Acid
Quality Control of the Analyses
3.7. Statistical Evaluation of the Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Council Regulation. No 479/2008 of 29 April 2008 on the common organisation of the market in wine, amending Regulations (EC) No 1493/1999, (EC) No 1782/2003,(EC) No 1290/2005,(EC) No 3/2008 and repealing Regulations (EEC) No 2392/86 and (EC) No 1493/1999. Off. J. Europ. Union 2008, 148, 1–61. [Google Scholar]
- Perini, M.; Paolini, M.; Simoni, M.; Bontempo, L.; Vrhovsek, U.; Sacco, M.; Thomas, F.; Jamin, E.; Hermann, A.; Camin, F. Stable isotope ratio analysis for verifying the authenticity of balsamic and wine vinegar. J. Agric. Food Chem. 2014, 62, 8197–8203. [Google Scholar] [PubMed]
- Thompson, M.; Ellison, S.L.R.; Wood, R. The International Harmonized Protocol for the Proficiency Testing of Analytical Chemistry Laboratories. Pure Appl. Chem. 2009, 78. [Google Scholar] [CrossRef]
- Thomas, F.; Jamin, E. 2H NMR and 13C-IRMS analyses of acetic acid from vinegar, 18O-IRMS analysis of water in vinegar: International collaborative study report. Anal. Chim. Acta 2009, 649, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Fauhl, C.; Wittkowski, R. On-line1H-NMR to facilitate tube preparation in SNIF-NMR analysis. Zeitschrift fur Lebensmittel-Untersuchung und Forschung 1996, 203, 541–545. [Google Scholar] [CrossRef]
- Hermann, A. Determination of D/H isotope ratio in acetic acid from vinegars and pickled products by 2 H-NMR-spectroscopy. Europ. Food Res. Technol. 2001, 212, 683–686. [Google Scholar] [CrossRef]
Sample Availability: Not available |
| Participant | Lab A | Lab B | Lab C | Lab D | Lab E | Lab F | Lab G |
|---|---|---|---|---|---|---|---|
| weight TMU(g) | 1.0422 | 1.0428 | 1.0406 | 1.0428 | 1.0495 | 1.0431 | 1.0470 |
| mmol TMU | 8.9721 | 8.9773 | 8.9583 | 8.9773 | 9.0350 | 8.9799 | 9.0134 |
| weight of acetic acid solution (g) | 3.3806 | 3.3192 | 3.3698 | 3.3730 | 3.3707 | 3.3683 | 3.3575 |
| Step 1 | |||||||
| Interval of integration of TMU (Hz) | 1249–997 | 1489–1205 | 1200–1040 | Lorentzian calculation | Lorentian calculation | 1200–1041 | n.g. |
| Interval of integration of acetic acid (Hz) | 942–673 | 1157–904 | 1000–615 | Lorentzian calculation | Lorentian calculation | 973–570 | n.g. |
| NMR molar ratio TMU/acetic acid | 1.3334 | 1.3072 | 1.3297 | 1.3280 | 1.3187 | 1.3247 | 1.3166 |
| mmol of acetic acid | 47.854 | 46.940 | 47.648 | 47.687 | 47.658 | 47.582 | 47.468 |
| weight of pure acetic acid (g) | 2.874 | 2.819 | 2.861 | 2.864 | 2.862 | 2.857 | 2.850 |
| % w acetic acid | 85.00 | 84.92 | 84.91 | 84.90 | 84.90 | 84.83 | 84.90 |
| (D/H)CH3 mass using % w acetic acid | 122.80 | 121.32 | 122.16 | 121.80 | 122.74 | 122.53 | 121.40 |
| SD Mass | 0.20 | 0.91 | 0.60 | 0.90 | 0.69 | 2.34 | 0.40 |
| Step 2 | |||||||
| Interval of integration of TMU (Hz) | 1249–997 | 1699–1206 | 1245–990 | Lorentzian calculation | Lorentian calculation | 1300–1000 | n.g. |
| Interval of integration of acetic acid (Hz) | 942–673 | 1215–687 | 968–648 | Lorentzian calculation | Lorentian calculation | 927–636 | n.g. |
| 1H NMR molar ratio TMU/acetic acid | 1.3334 | 1.3078 | 1.3167 | 1.3242 | 1.3207 | 1.3095 | 1.2984 |
| mmol of AcOH | 47.854 | 46.961 | 47.182 | 47.549 | 47.728 | 47.036 | 46.812 |
| Weight of pure acetic acid (g) | 2.874 | 2.820 | 2.833 | 2.855 | 2.866 | 2.825 | 2.811 |
| % w acetic acid | 85.00 | 84.96 | 84.08 | 84.65 | 85.03 | 83.86 | 83.73 |
| (D/H)CH3 Proton | 122.57 | 121.29 | 122.10 | 122.44 | 123.09 | 124.06 | 122.70 |
| SD Proton | 0.20 | 0.91 | 0.70 | 0.95 | 0.69 | 2.37 | 0.40 |
| (D/H)CH3 Mass | 122.80 | 121.32 | 122.63 | 122.42 | 122.55 | 124.05 | 121.40 |
| SD Mass | 0.20 | 0.91 | 0.61 | 0.90 | 0.69 | 2.37 | 0.40 |
| (D/H)CH3 ppm ACETIC ACID | |||||||
| Sample Description | Wine 1 | Wine 2 | Apple Cider | ABM wine 1 | ABM wine 2 | ABM Apple | ABM Cane |
| number of valid results | 7 | 7 | 7 | 6 | 6 | 7 | 7 |
| number of replicates | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Mean | 104.94 | 103.46 | 100.84 | 104.73 | 103.33 | 100.51 | 109.1 |
| sr | 0.76 | 0.57 | 0.33 | 0.47 | 0.31 | 0.85 | 0.71 |
| sR | 1.13 | 1.07 | 1.49 | 0.63 | 0.61 | 1.61 | 0.91 |
| δ13C ‰ vs V-PDB ACETIC ACID | |||||||
| sample description | wine 1 | wine 2 | apple cider | ABM wine 1 | ABM wine 2 | ABM apple | ABM cane |
| number of valid results | 4 | 5 | 5 | 4 | 5 | 5 | 4 |
| number of replicates | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Mean | −26.46 | −27.55 | −27.64 | −26.15 | −27.38 | −27.22 | −14.30 |
| sr | 0.16 | 0.14 | 0.09 | 0.12 | 0.08 | 0.18 | 0.21 |
| sR | 0.19 | 0.31 | 0.24 | 0.16 | 0.24 | 0.40 | 0.21 |
| δ18O ‰ vs V-SMOW ACETIC ACID | |||||||
| sample description | wine 1 | wine 2 | apple cider | ABM wine 1 | ABM wine 2 | ABM apple | ABM cane |
| number of valid results | 4 | 4 | 3 | 3 | 3 | 4 | 4 |
| number of replicates | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Mean | 1.21 | 1.11 | −4.92 | 1.33 | 1.37 | −2.13 | −4.48 |
| sr | 0.09 | 0.08 | 0.04 | 0.15 | 0.11 | 0.13 | 0.13 |
| sR | 0.29 | 0.21 | 0.08 | 0.13 | 0.10 | 0.19 | 0.18 |
| (D/H)I ppm ETHANOL | |||||||
| sample description | ABM wine 1 | ABM wine 2 | ABM apple | ABM cane | |||
| number of valid results | 5 | 4 | 6 | 5 | |||
| number of replicates | 2 | 2 | 2 | 2 | |||
| Mean | 104.47 | 104.90 | 104.57 | 105.03 | |||
| sr | 0.19 | 0.05 | 0.21 | 0.30 | |||
| sR | 0.88 | 0.47 | 0.78 | 0.43 | |||
| (D/H)II ppm ETHANOL | |||||||
| sample description | ABM wine 1 | ABM wine 2 | ABM apple | ABM cane | |||
| number of valid results | 4 | 4 | 5 | 5 | |||
| number of replicates | 2 | 2 | 2 | 2 | |||
| Mean | 127.95 | 128.58 | 128.92 | 128.68 | |||
| sr | 1.08 | 0.93 | 1.28 | 1.28 | |||
| sR | 1.04 | 0.99 | 1.35 | 1.84 | |||
| δ13C ‰ vs V-PDB ETHANOL | |||||||
| sample description | ABM wine 1 | ABM wine 2 | ABM apple | ABM cane | |||
| number of valid results | 4 | 4 | 4 | 4 | |||
| number of replicates | 2 | 2 | 2 | 2 | |||
| Mean | −26.39 | −26.36 | −26.35 | −26.33 | |||
| sr | 0.08 | 0.08 | 0.05 | 0.13 | |||
| sR | 0.06 | 0.15 | 0.08 | 0.15 | |||
| Lab. | Instrument Model | Year Model | Producer | Standards Used | Deviation to the Protocol |
|---|---|---|---|---|---|
| Chelab Dr. V. Ara GmbH & Co. KG, GE | FT-NMR AVANCE III 400 | 2012 | BRUKER (a) | TMU STA-003m (b) | For purification of the acetic acid extract, Liebig condenser was used instead of vertical distillation column. |
| Landesuntersuchungsamt, Institut für Lebensmittelchemie und Arzneimittelprüfung, GE | FT-NMR AVANCE 400 | 2003 | BRUKER (a) | TMU STA-003m (b) | Use of TBME (tert. butyl methyl ether) instead of ether to extract ethanol |
| AGENZIA DELLE DOGANE, IT | AVANCE III 400 | 2008 | BRUKER (a) | working standard TMU calibrated against STA-003m (b) (D/H) value 128,39 | NO |
| ICSI Analytics Group, RO | SNIF-NMR Ascend 400/Avance III | 2011 | BRUKER (a) | TMU STA-003m (b) | NO |
| Eurofins Analytics, FR | FT-NMR AVANCE III 400 | 2014 | BRUKER (a) | TMU STA-003m (b) | Yes, Eurospec: automatic identification |
| State General Laboratory, CY | SNIF-NMR AVANCE III HD 400 | Magnet 2002 and console 2017 | BRUKER (a) | TMU STA-003m (b) | NO |
| FEM, IT | FT-NMR AVANCE III 400 | 2008 | BRUKER (a) | TMU STA-003m (b) | NO |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camin, F.; Simoni, M.; Hermann, A.; Thomas, F.; Perini, M. Validation of the 2H-SNIF NMR and IRMS Methods for Vinegar and Vinegar Analysis: An International Collaborative Study. Molecules 2020, 25, 2932. https://doi.org/10.3390/molecules25122932
Camin F, Simoni M, Hermann A, Thomas F, Perini M. Validation of the 2H-SNIF NMR and IRMS Methods for Vinegar and Vinegar Analysis: An International Collaborative Study. Molecules. 2020; 25(12):2932. https://doi.org/10.3390/molecules25122932
Chicago/Turabian StyleCamin, Federica, Marco Simoni, Armin Hermann, Freddy Thomas, and Matteo Perini. 2020. "Validation of the 2H-SNIF NMR and IRMS Methods for Vinegar and Vinegar Analysis: An International Collaborative Study" Molecules 25, no. 12: 2932. https://doi.org/10.3390/molecules25122932
APA StyleCamin, F., Simoni, M., Hermann, A., Thomas, F., & Perini, M. (2020). Validation of the 2H-SNIF NMR and IRMS Methods for Vinegar and Vinegar Analysis: An International Collaborative Study. Molecules, 25(12), 2932. https://doi.org/10.3390/molecules25122932







