In Vitro Antimycobacterial Activity and Physicochemical Characterization of Diaryl Ether Triclosan Analogues as Potential InhA Reductase Inhibitors
Abstract
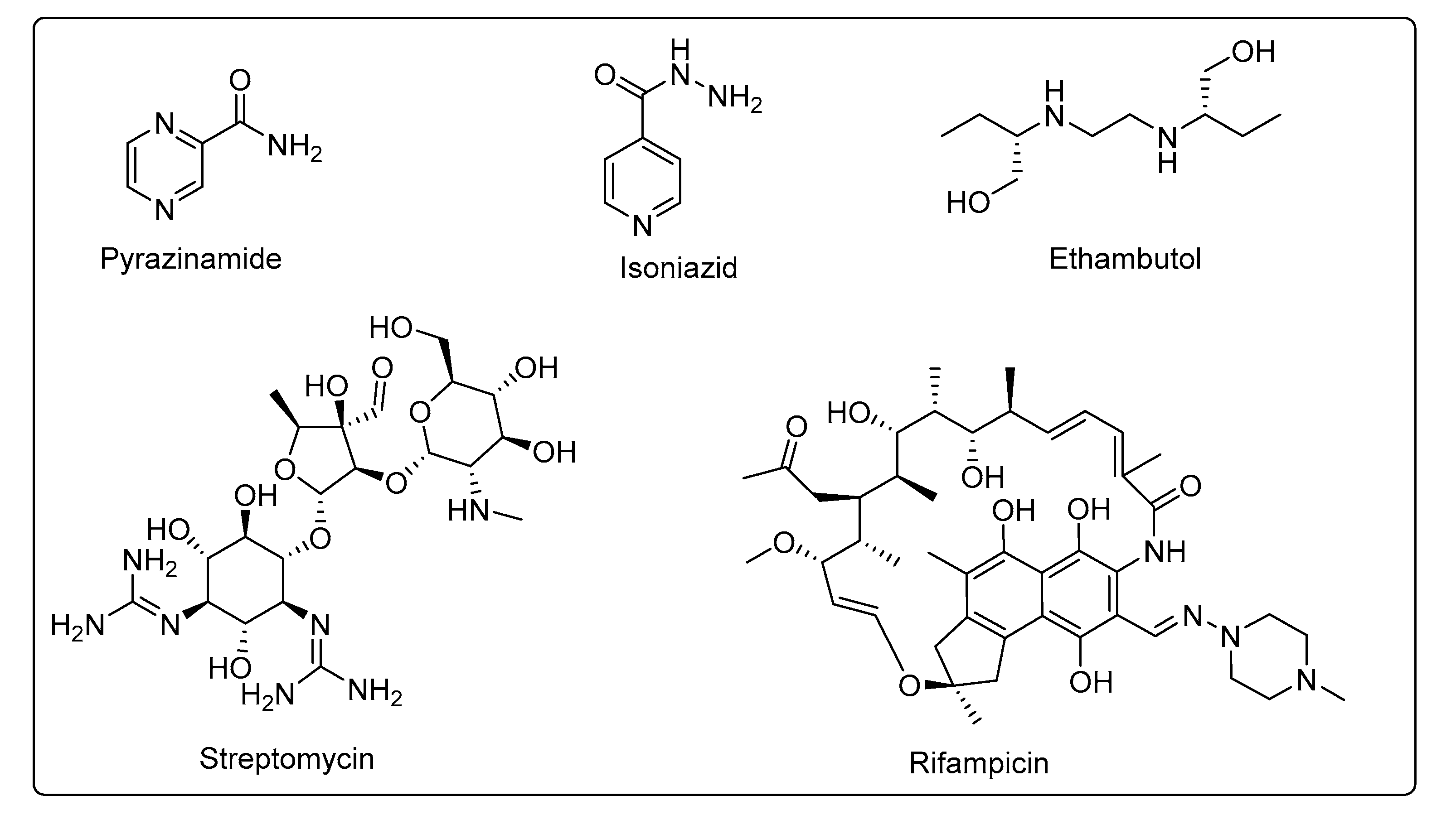
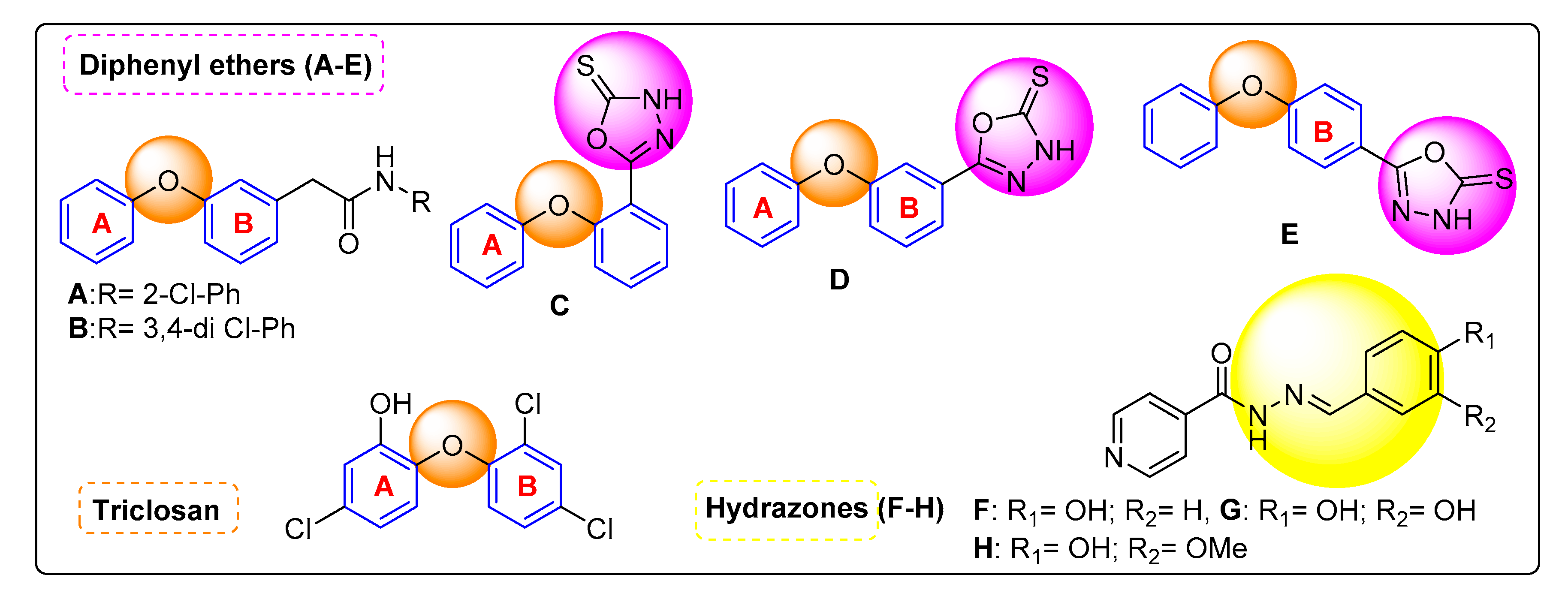
1. Introduction
2. Results and Discussion
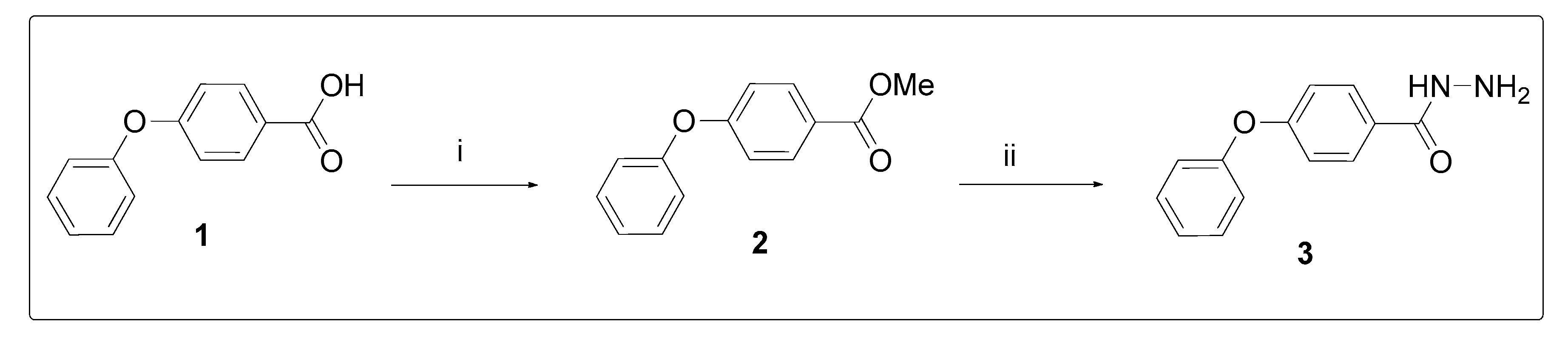
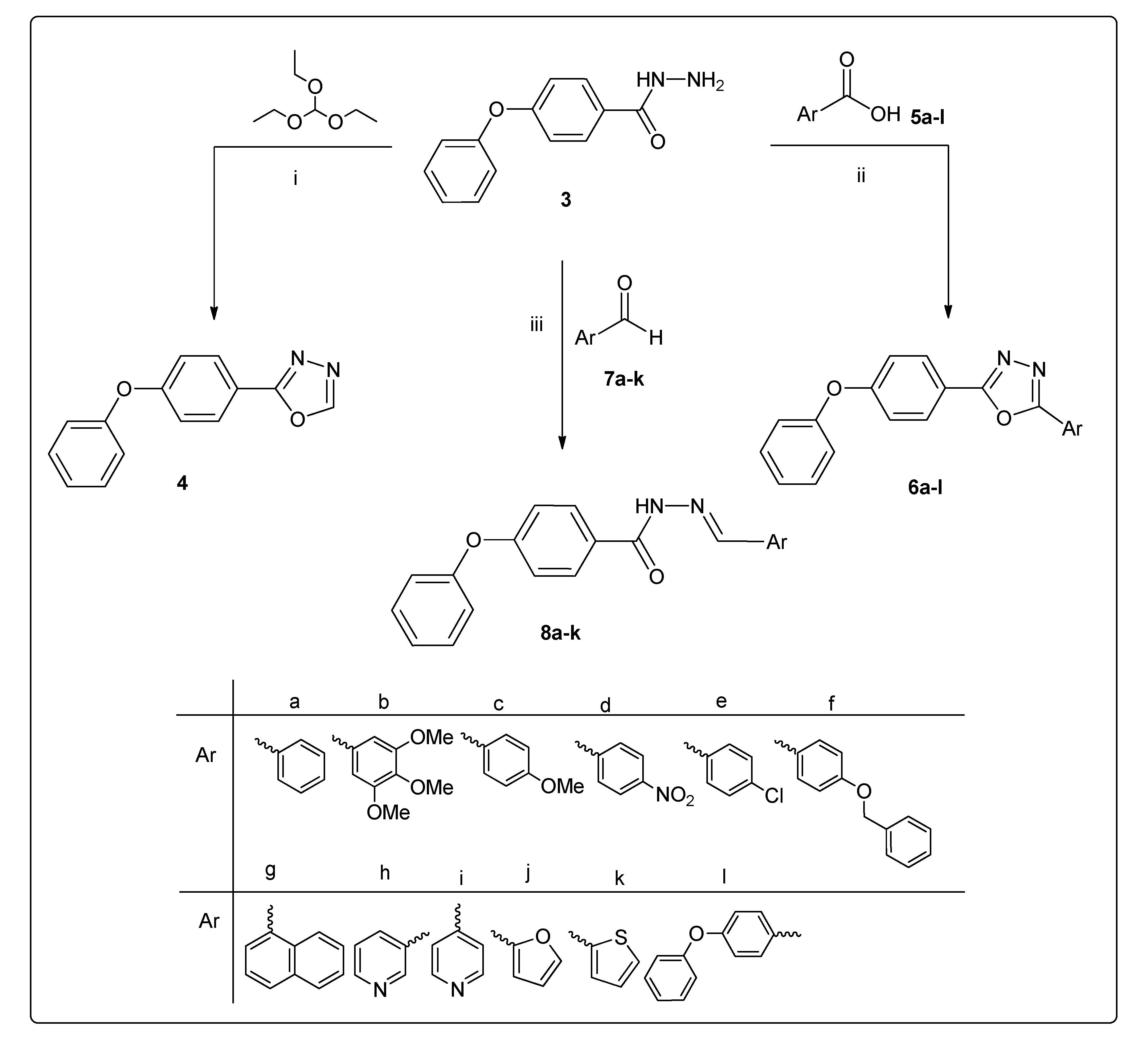
2.1. Chemistry
2.2. Biology
2.2.1. Anti-Tubercular Activity
2.2.2. In Vitro InhA Reductase Enzyme Inhibition
2.2.3. Cytotoxic Study
2.2.4. Molecular Docking
3. Material and Methods
3.1. Chemistry
3.2. Biology
3.2.1. In Vitro Anti-Tubercular Activity
3.2.2. In Vitro InhA Enzyme Inhibition
3.2.3. Cytotoxic Activity
3.2.4. Docking Methodology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report. 2015. Available online: www.who.int/tb/publications/global_report/gtbr15_main_text.pdf (accessed on 27 January 2020).
- World Health Organization. Global Tuberculosis Report. 2019. Available online: https://www.who.int/news-room/events/detail/2019/03/24/default-calendar/world-tb-day-2019 (accessed on 27 January 2020).
- Matteelli, A.; Roggi, A.; Carvalho, A.C. Extensively drug-resistant tuberculosis: Epidemiology and management. Clin. Epidemiol. 2014, 6, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Chollet, A.; Maveyraud, L.; Lherbet, C.; Bernardes-Genisson, V. An overview on crystal structures of InhA protein: Apo-form, in complex with its natural ligands and inhibitors, Eur. J. Med. Chem. 2018, 146, 318–343. [Google Scholar] [CrossRef]
- Ende, C.W.A.; Knudson, S.E.; Liu, N.; Childs, J.; Sullivan, T.J.; Boyne, M.; Xu, H.; Gegina, Y.; Knudson, D.L.; Johnson, F.; et al. Synthesis and in vitro antimycobacterial activity of B-ring modified diaryl ether InhA inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 3029–3033. [Google Scholar] [CrossRef] [PubMed]
- Perozzo, R.; Kuo, M.; Sidhu, A.B.S.; Valiyaveetti, J.T.; Bittman, R.; Jacobs, W.R., Jr.; Fidock, D.A.; Sacchettini, J.C.J. Structural Elucidation of the Specificity of the Antibacterial Agent Triclosan for Malarial Enoyl Acyl Carrier Protein Reductase. Biol. Chem. 2002, 277, 13106–13114. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ramya, T.N.C.; Surolia, A.; Surolia, N. Triclosan as a Systemic Antibacterial Agent in a Mouse Model of Acute Bacterial Challenge. Antimicrob. Agents Chemother. 2003, 47, 3859–3866. [Google Scholar] [CrossRef]
- Chhibber, M.; Kumar, G.; Parasuraman, P.; Ramya, T.N.; Surolia, N.; Surolia, A. Novel diphenyl ethers: Design, docking studies, synthesis and inhibition of enoyl ACP reductase of Plasmodium falciparum and Escherichia coli. Bioorg. Med. Chem. 2006, 14, 8086–8098. [Google Scholar] [CrossRef]
- Mishra, S.; Karmodiya, K.; Parasuraman, P.; Surolia, A.; Surolia, N. Design, synthesis, and application of novel triclosan prodrugs as potential antimalarial and antibacterial agents. Bioorg. Med. Chem. Lett. 2008, 16, 5536–5546. [Google Scholar] [CrossRef]
- Butler, M.S.; Cooper, M.A.J. Antibiotics in the clinical pipeline in 2011. J. Antibiot. 2011, 64, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Tonge, P.J.; Kisker, C.; Slayden, R.A. Development of Modern InhA Inhibitors to Combat Drug Resistant Strains of Mycobacterium tuberculosis. Curr. Top. Med. Chem. 2007, 7, 489–498. [Google Scholar] [CrossRef]
- Kini, S.G.; Bhat, A.R.; Bryant, B.; Williamson, J.S.; Dayan, F.E. Synthesis, antitubercular activity and docking study of novel cyclic azole substituted diphenyl ether derivatives. Eur. J. Med. Chem. 2009, 44, 492–500. [Google Scholar] [CrossRef]
- Kini, S.G.; Bhat, A.R.; Pan, Z.; Dayan, F.E. Synthesis and antitubercular activity of heterocycle substituted diphenyl ether derivatives. J. Enzyme Inhib. Med. Chem. 2010, 25, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, S.; Sullivan, T.J.; Johnson, F.; Novichenok, P.; Cui, G.; Simmerling, C.; Tonge, P.J. Inhibition of the Bacterial Enoyl Reductase FabI by Triclosan: A Structure−Reactivity Analysis of FabI Inhibition by Triclosan Analogues. J. Med. Chem. 2004, 47, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.J.; Yu, Y.T.; Shapiro, M.A.; Olson, E.; Rock, C.O. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 1998, 273, 30316–30320. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Wang, Z.L.; Yang, J.Z.; Yang, T.; Pi, W.Y.; Ang, W.; Lin, Y.N.; Liu, Y.Y.; Li, Z.C.; Luo, Y.F.; et al. Design, synthesis and evaluation of novel molecules with a diphenyl ether nucleus as potential antitubercular agents. Bioorg. Med. Chem. Lett. 2012, 22, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Vazquez, G.; Molina-Salinas, G.M.; Duarte-Fajardo, Z.V.; Vargas-Villarreal, J.; Estrada-Soto, S.; Gonzalez-Salazar, F.; Hernandez-Nunez, E.; Said-Fernandez, S. Synthesis and antimycobacterial activity of 4-(5-substituted-1,3,4-oxadiazol-2-yl)pyridines. Bioorg. Med. Chem. 2007, 15, 5502–5508. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.H.; Rastogi, R.C. Synthesis of some 2-[(5-aryl-1,3,4-oxadiazol-2-yl)amino]-1,3-heterazoles as potential pesticides. J. Agric. Food Chem. 1990, 38, 1068–1071. [Google Scholar] [CrossRef]
- Narayana, B.; Raj, K.K.V.; Ashalatha, B.V.; Kumari, N.S. Synthesis of Some New 2-(6-Methoxy-2-Naphthyl)-5-Aryl-1,3,4-Oxadiazoles as Possible Non-steroidal Anti-inflammatory and Analgesic Agents. Arch. Pharm. Chem. Life Sci. 2005, 338, 373–377. [Google Scholar] [CrossRef]
- Rasras, A.J.M.; Al-Tel, T.H.; Al-Aboudi, A.F.; Al-Qawasmeh, R.A. Synthesis and antimicrobial activity of cholic acid hydrazone analogues. Eur. J. Med. Chem. 2010, 45, 2307–2313. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Fleita, D.H.; Sakka, O.K. Novel Synthesis of Hydrazide-Hydrazone Derivatives and Their Utilization in the Synthesis of Coumarin, Pyridine, Thiazole and Thiophene Derivatives with Antitumor Activity. Molecules 2011, 16, 16–27. [Google Scholar] [CrossRef]
- Cascioferro, S.; Attanzio, A.; Di Sarno, V.; Musella, S.; Tesoriere, L.; Cirrincione, G.; Diana, P.; Parrino, B. New 1,2,4-Oxadiazole Nortopsentin Derivatives with Cytotoxic Activity. Mar. Drugs 2019, 17, 35. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, S.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef]
- Asif, M. Pharmacologically potentials of hydrazonone containing compounds: A promising scaffold. Int. J. Adv. Chem. 2014, 2, 85–103. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Guidetti, B.; Chamayou, A.; André-Barrès, C.; Madacki, J.; Korduláková, J.; Mori, G.; Orena, B.S.; Chiarelli, L.R.; Pasca, M.R.; et al. Mechanochemical Synthesis and Biological Evaluation of Novel Isoniazid Derivatives with Potent Antitubercular Activity. Molecules 2017, 22, 1457. [Google Scholar] [CrossRef]
- Freundlich, J.S.; Wang, F.; Vilcheze, C.; Gulten, G.; Langley, R.; Schiehser, G.A.; Jacobus, D.R.; Jacobs, W.R.; Sacchettini, J.C. Triclosan derivatives: Towards potent inhibitors of drug-sensitive and drug-resistant Mycobacterium tuberculosis. Chem. Med. Chem. 2009, 4, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Tatsumoto, S.; Ono, H.; Van Immerseel, F.; Raspoet, R.; Miyata, T. A novel antibiotic-delivery system by using ovotransferrin as targeting molecule. Eur. J. Pharm. Sci. 2015, 66, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Haga, N.; Takayanagi, H. Mechanisms of the Photochemical Rearrangement of Diphenyl Ethers. J. Org. Chem. 1996, 61, 735–745. [Google Scholar] [CrossRef]
- He, L.; Zhang, L.; Liu, X.; Li, X.; Zheng, M.; Li, H.; Yu, K.; Chen, K.; Shen, X.; Jiang, H.; et al. Discovering Potent Inhibitors Against the β-Hydroxyacyl-Acyl Carrier Protein Dehydratase (FabZ) of Helicobacter pylori: Structure-Based Design, Synthesis, Bioassay, and Crystal Structure Determination. J. Med. Chem. 2009, 52, 2465–2481. [Google Scholar] [CrossRef]
- Ibrahim, T.S.; Al-Mahmoudy, A.M.; Elagawany, M.; Ibrahim, M.A.; Panda, S.S. Synthesis and Antiviral Bioassay of New Diphenyl Ether-based Compounds. Chem. Biol. Drug Des. 2016, 88, 511–518. [Google Scholar] [CrossRef]
- Collins, L.; Franzblau, S.G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41, 1004–1009. [Google Scholar] [CrossRef]
- Franzblau, S.G.; Witzig, R.S.; McLaughlin, J.C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M.T.; Cook, M.B.; Quenzer, V.K.; Ferguson, R.M.; et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998, 36, 362–366. [Google Scholar] [CrossRef]
- Umaa, K.; Sudha Rani, S.; Sangeetha, G.; Maida Engels, S.E. Prediction of physiochemical parameters for anti-tubercular potentials of Mannich and Schiff bases. J. Chem. Pharm. Res. 2013, 5, 52–59. [Google Scholar]
- Lakshminarayana, S.B.; Huat, T.B.; Ho, P.C.; Manjunatha, U.H.; Dartois, V.; Dick, T.; Rao, S.P.S. Comprehensive physicochemical, pharmacokinetic and activity profiling of anti-TB agents. J. Antimicrob. Chemother. 2015, 70, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Wiener, H. Structural determination of Paraffin boiling point. J. Am. Chem. Soc. 1947, 69, 17–20. [Google Scholar] [CrossRef]
- Stec, J.; Vilchèze, C.; Lun, S.; Perryman, A.L.; Wang, X.; Freundlich, J.S.; Bishai, W.; Jacobs, W.R., Jr.; Kozikowski, A.P. Biological evaluation of potent triclosan-derived inhibitors of the enoyl-acyl carrier protein reductase InhA in drug-sensitive and drug-resistant strains of Mycobacterium tuberculosis. Chem. Med. Chem. 2014, 9, 2528–2537. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.A.; Youssif, B.G.M.; Shaykoon, M.S.A.; Abdelrahman, M.H.; Elsadek, B.E.M.; Aboraia, A.S.; Abuo-Rahma, G.E.A. Utilization of tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidinone as a cap moiety in design of novel histone deacetylase inhibitors. Bioorg. Chem. 2019, 91, 103127. [Google Scholar] [CrossRef] [PubMed]
- Youssif, B.G.M.; Mohamed, M.F.A.; Al-Sanea, M.M.; Moustafa, A.H.; Abdelhamid, A.A.; Gomaa, H.A.M. Novel aryl carboximidamides and 3-aryl-1,2,4-oxadiazoles analogues of naproxen as dual selective COX-2 / LOX inhibitors: Design, synthesis and Docking studies. Bioorg. Chem. 2019, 85, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G.; Yang, L. Cu(I)-catalyzed Synthesis of Diaryl Ether Using a Simple Hydrazone as Promoter. Chin. J. Chem. 2009, 27, 423–427. [Google Scholar] [CrossRef]
- Bar-Yuan, H.; Kun-Ming, Y.; Yun, C. Synthesis of electroluminescent copoly(aryl ether)s containing alternate 1,4-distyrylbenzene derivatives and aromatic 1,3,4-oxadiazole or 3,3″-terphenyldicarbonitrile segments. J. Polym. Sci. Pol. Chem. 2005, 43, 5009–5022. [Google Scholar]
- Sieniawska, E.; Sawicki, R.; Swatko-Ossor, M.; Napiorkowska, A.; Przekora, A.; Ginalska, G.; Augustynowicz-Kopec, E. The effect of combining natural terpenes and antituberculous agents against reference and clinical Mycobacterium tuberculosis strains. Molecules 2018, 23, 176. [Google Scholar] [CrossRef]
- Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Synthesis and antimycobacterial activity of thiazolidine-2,4-dione based derivatives with halogenbenzohydrazones and pyridinecarbohydrazones substituents. Eur. J. Med. Chem. 2020, 189, 112045. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 6a–l and 8a–k are available from the authors. |
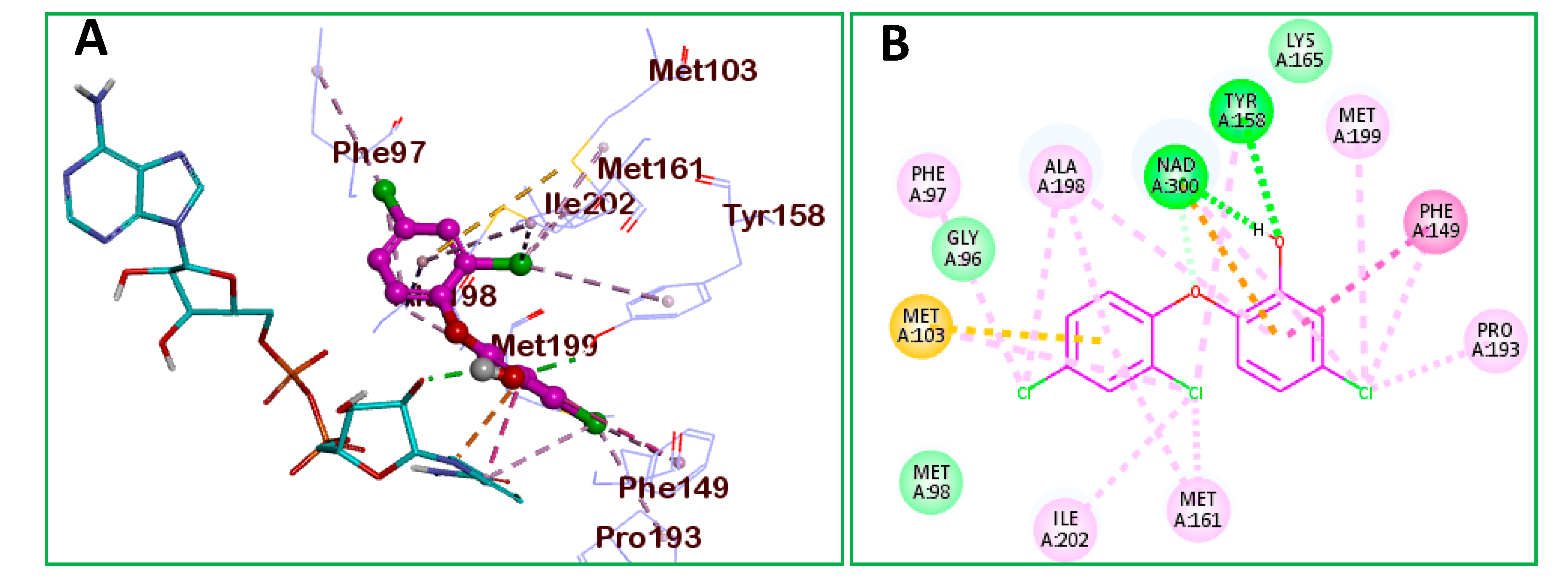

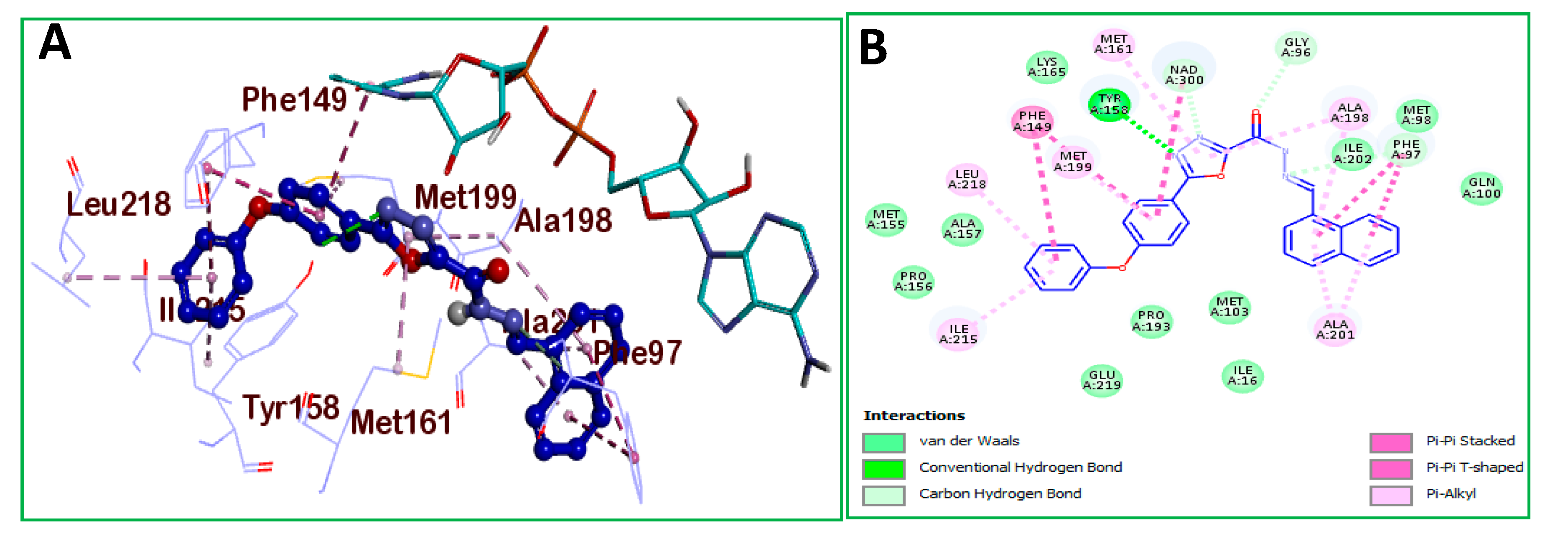
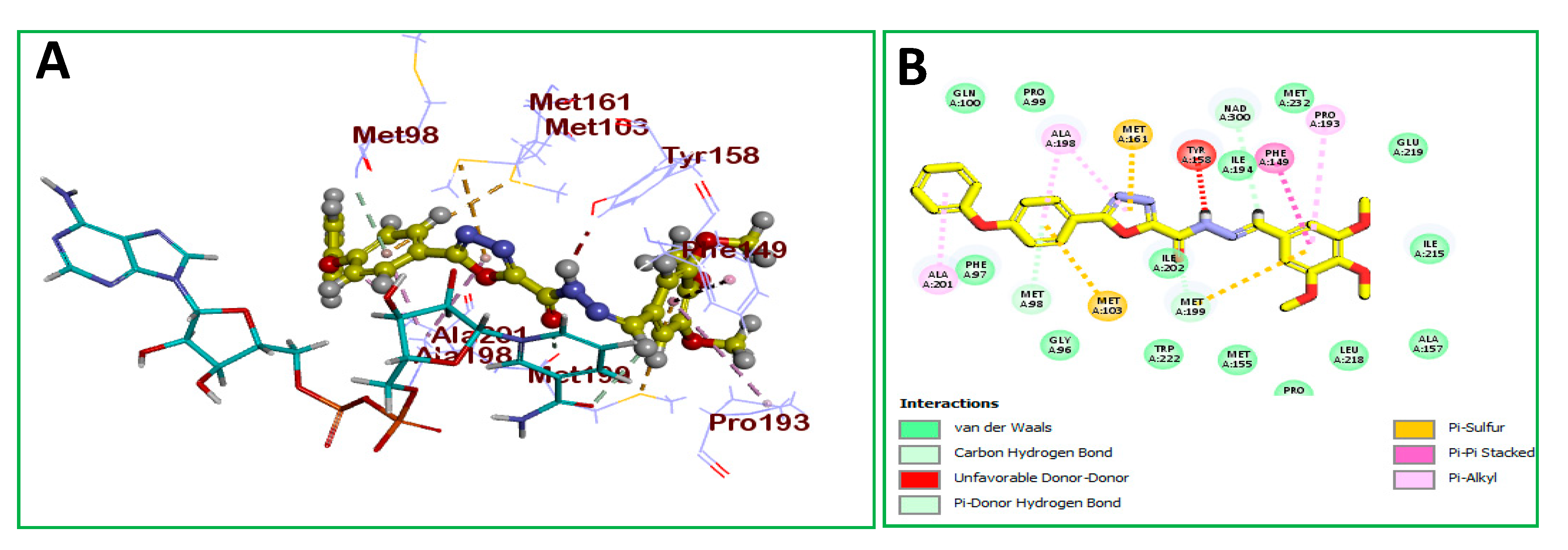
| Ar | No. | MIC (μg/mL) | IC50 (µM) | No. | MIC (μg/mL) | IC50 (µM) |
|---|---|---|---|---|---|---|
| ---------- | 3 | 0.86 | 3.43 | 4 | 0.61 | 3.28 |
 | 6a | 2.74 | >100 | 8a | 34.54 | 73.23 |
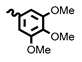 | 6b | 34.64 | >100 | 8b | 50.20 | >100 |
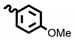 | 6c | 39.04 | >100 | 8c | 20.68 | 75.46 |
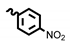 | 6d | 15.15 | 68.44 | 8d | 7.67 | 19.75 |
 | 6e | 15.89 | 64.55 | 8e | 2.89 | 7.46 |
 | 6f | 8.23 | 41.24 | 8f | 22.62 | 29.89 |
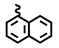 | 6g | 12.23 | 33.82 | 8g | 0.98 | 4.23 |
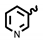 | 6h | 1.40 | 5.08 | 8h | 6.96 | 26.54 |
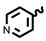 | 6i | 1.40 | 11.72 | 8i | 6.82 | 21.39 |
 | 6j | 11.12 | 51.42 | 8j | 6.35 | 18.37 |
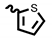 | 6k | 3.65 | 78.65 | 8k | 1.69 | 5.61 |
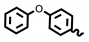 | 6l | 1.17 | 5.63 | INH | 0.20 | ND |
| ---------- | -------- | ---------- | ---------- | Triclosan | 10.00 | 1.10 |
| Comp. No. | MIC (μg/mL) | Molecular Mass | Balaban Index | Molecular Topology: Wiener Index | Topological Diameter Bond (s) | Molar Refractivity, cm3/mol | Clog p | Heat of Formation [kJ/mol] | Gibbs Energy [kJ/mol] Energy | H Bond Donor | H Bond Acceptor | Lipinski’s Rule |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 0.86 | 228.250 | 118,297 | 586 | 11 | 6.589 | 2.231 | −60.70 | 176.14 | 2 | 3 | Yes |
| 4 | 0.61 | 238.250 | 121,561 | 682 | 11 | 6.656 | 2.753 | 115.57 | 339.72 | 0 | 4 | Yes |
| 6a | 2.74 | 314.340 | 400,250 | 1561 | 15 | 9.167 | 4.806 | 216.79 | 493.02 | 0 | 4 | Yes |
| 6b | 34.64 | 404.422 | 110,779 | 2828 | 17 | 11.018 | 4.427 | −276.20 | 174.39 | 0 | 7 | Yes |
| 6c | 39.04 | 344.370 | 59,322 | 1990 | 17 | 9.784 | 4.679 | 52.46 | 386.81 | 0 | 5 | Yes |
| 6d | 15.15 | 359.340 | 71,066 | 2219 | 17 | 9.778 | ND | ND | ND | 0 | 5 | Yes |
| 6e | 15.89 | 348.770 | 488,162 | 1763 | 16 | 9.659 | 5.364 | 189.58 | 471.46 | 0 | 4 | No |
| 6f | 8.23 | 420.470 | 142,971 | 3769 | 21 | 12.295 | 6.412 | 165.15 | 549.74 | 0 | 5 | No |
| 6g | 12.23 | 364.404 | 706,482 | 2391 | 17 | 10.855 | 5.803 | 313.83 | 623.72 | 0 | 4 | No |
| 6h | 1.40 | 315.332 | 400,250 | 1561 | 15 | 8.956 | 3.468 | 270.22 | 561.65 | 0 | 5 | No |
| 6i | 1.40 | 315.332 | 400,250 | 1561 | 15 | 8.956 | 3.468 | 270.22 | 561.65 | 0 | 5 | Yes |
| 6j | 11.12 | 304.305 | 326,596 | 1380 | 14 | 8.381 | 3.421 | 74.450 | 372.20 | 0 | 5 | Yes |
| 6k | 3.65 | 320.062 | 32,659 | 1380 | 14 | 8.976 | 4.788 | 251.71 | 498.18 | 0 | 4 | Yes |
| 6l | 1.17 | 406.440 | 120,686 | 3377 | 20 | 11.831 | 6.343 | 185.97 | 541.32 | 0 | 5 | No |
| 8a | 34.54 | 384.390 | 104,520 | 2849 | 19 | 10.994 | 4.734 | 145.52 | ND | 1 | 6 | Yes |
| 8b | 50.20 | 474.470 | 250,732 | 4753 | 21 | 12.845 | 4.354 | −347.47 | ND | 1 | 9 | Yes |
| 8c | 20.68 | 414.420 | 145,706 | 3497 | 21 | 11.611 | 4.607 | −18.810 | ND | 1 | 7 | Yes |
| 8d | 7.67 | 414.420 | 145,706 | 3497 | 21 | 11.610 | ND | −18.81 | ND | 1 | 7 | Yes |
| 8e | 2.89 | 418.840 | 12,360 | 3158 | 20 | 11.485 | 5.292 | 118.31 | ND | 1 | 6 | Yes |
| 8f | 22.62 | 490.519 | 301,096 | 6023 | 25 | 14.122 | 6.339 | 93.88 | ND | 1 | 7 | No |
| 8g | 1.40 | 434.450 | 161,770 | 4015 | 20 | 12.682 | 5.730 | 242.56 | ND | 1 | 6 | No |
| 8h | 6.96 | 385.380 | 1,045,201 | 2849 | 19 | 10783 | 3.396 | 198.95 | ND | 1 | 7 | No |
| 8i | 6.82 | 385.383 | 1,045,201 | 2849 | 19 | 10.783 | 3.396 | 198.95 | ND | 1 | 7 | Yes |
| 8j | 6.35 | 374.356 | 880,346 | 2566 | 18 | 10.208 | 3.349 | 3.180 | ND | 1 | 7 | Yes |
| 8k | 1.69 | 390.417 | 88,034 | 2566 | 18 | 10.803 | 4.715 | 180.44 | ND | 1 | 6 | Yes |
| R | - | 0.308 | −0.179 | 0.315 | 0.260 | −0.100 | 0.152 | −0.683 | −0.602 | −0.370 | −0.360 | - |
| Compound | M. tuberculosis | Vero Cell Line | SI (IC50/MIC) |
|---|---|---|---|
| MIC, µg/mL | IC50 µg/mL ± SEM | ||
| 3 | 0.86 | 80.7 ± 1.31 | 93.83 |
| 4 | 0.61 | 99.4 ± 1.79 | 162.95 |
| 6h | 1.40 | 91.4 ± 1.55 | 65.29 |
| 6i | 1.40 | 95.5 ± 1.36 | 68.21 |
| 6l | 1.17 | 92.6 ± 1.80 | 79.15 |
| 8g | 0.98 | 72.2 ± 1.87 | 73.67 |
| 6k | 1.69 | 68.6 ± 1.19 | 40.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, T.S.; Taher, E.S.; Samir, E.; M. Malebari, A.; Khayyat, A.N.; Mohamed, M.F.A.; Bokhtia, R.M.; AlAwadh, M.A.; Seliem, I.A.; Asfour, H.Z.; et al. In Vitro Antimycobacterial Activity and Physicochemical Characterization of Diaryl Ether Triclosan Analogues as Potential InhA Reductase Inhibitors. Molecules 2020, 25, 3125. https://doi.org/10.3390/molecules25143125
Ibrahim TS, Taher ES, Samir E, M. Malebari A, Khayyat AN, Mohamed MFA, Bokhtia RM, AlAwadh MA, Seliem IA, Asfour HZ, et al. In Vitro Antimycobacterial Activity and Physicochemical Characterization of Diaryl Ether Triclosan Analogues as Potential InhA Reductase Inhibitors. Molecules. 2020; 25(14):3125. https://doi.org/10.3390/molecules25143125
Chicago/Turabian StyleIbrahim, Tarek S., Ehab S. Taher, Ebtihal Samir, Azizah M. Malebari, Ahdab N. Khayyat, Mamdouh F. A. Mohamed, Riham M. Bokhtia, Mohammed A. AlAwadh, Israa A. Seliem, Hani Z. Asfour, and et al. 2020. "In Vitro Antimycobacterial Activity and Physicochemical Characterization of Diaryl Ether Triclosan Analogues as Potential InhA Reductase Inhibitors" Molecules 25, no. 14: 3125. https://doi.org/10.3390/molecules25143125
APA StyleIbrahim, T. S., Taher, E. S., Samir, E., M. Malebari, A., Khayyat, A. N., Mohamed, M. F. A., Bokhtia, R. M., AlAwadh, M. A., Seliem, I. A., Asfour, H. Z., Alhakamy, N. A., Panda, S. S., & AL-Mahmoudy, A. M. M. (2020). In Vitro Antimycobacterial Activity and Physicochemical Characterization of Diaryl Ether Triclosan Analogues as Potential InhA Reductase Inhibitors. Molecules, 25(14), 3125. https://doi.org/10.3390/molecules25143125








