7-Acetoxycoumarin Inhibits LPS-Induced Inflammatory Cytokine Synthesis by IκBα Degradation and MAPK Activation in Macrophage Cells
Abstract
1. Introduction
2. Results
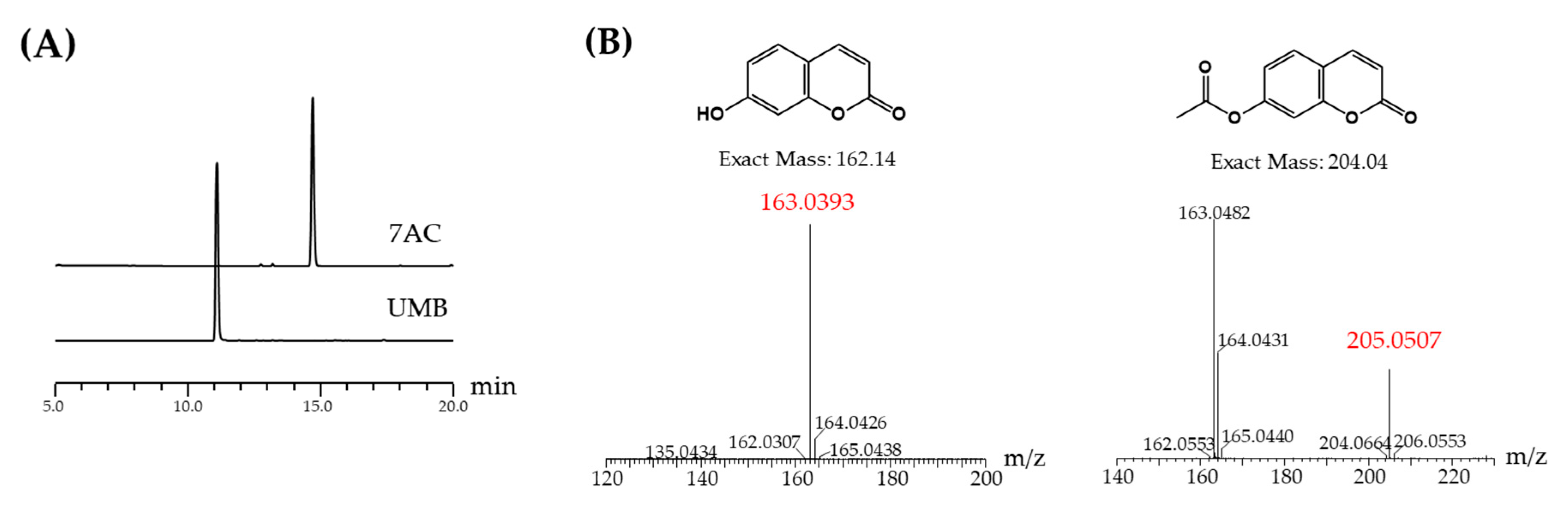
2.1. Acetylation of UMB
2.2. NMR Result
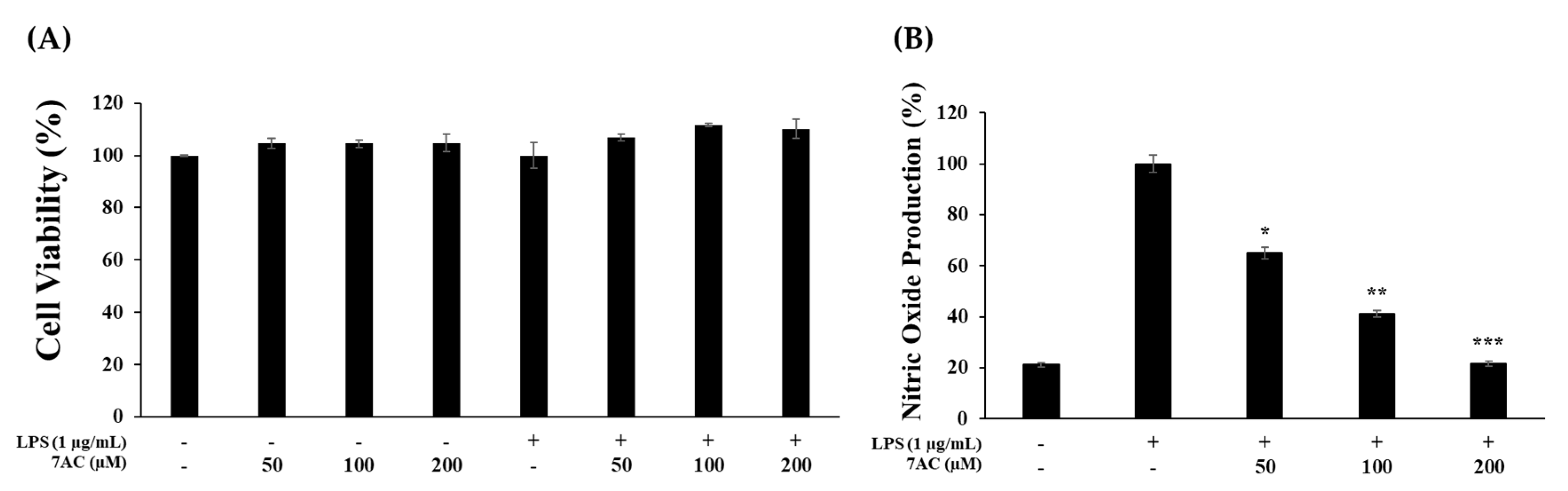
2.3. Cytotoxic Effects and NO Production of Compounds on RAW 264.7 Cells
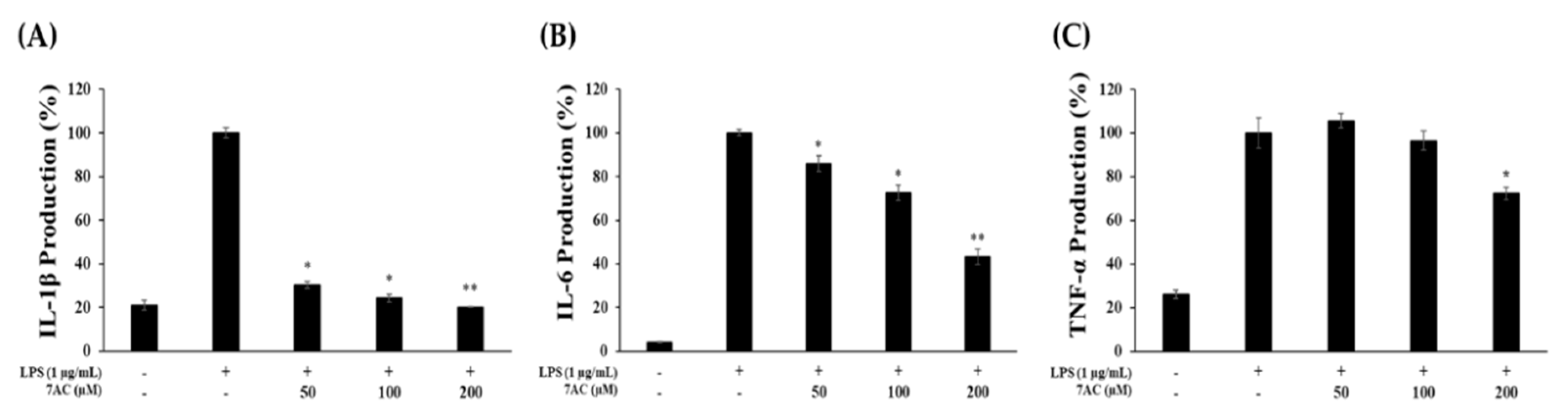
2.4. Production of Proinflammatory Cytokines
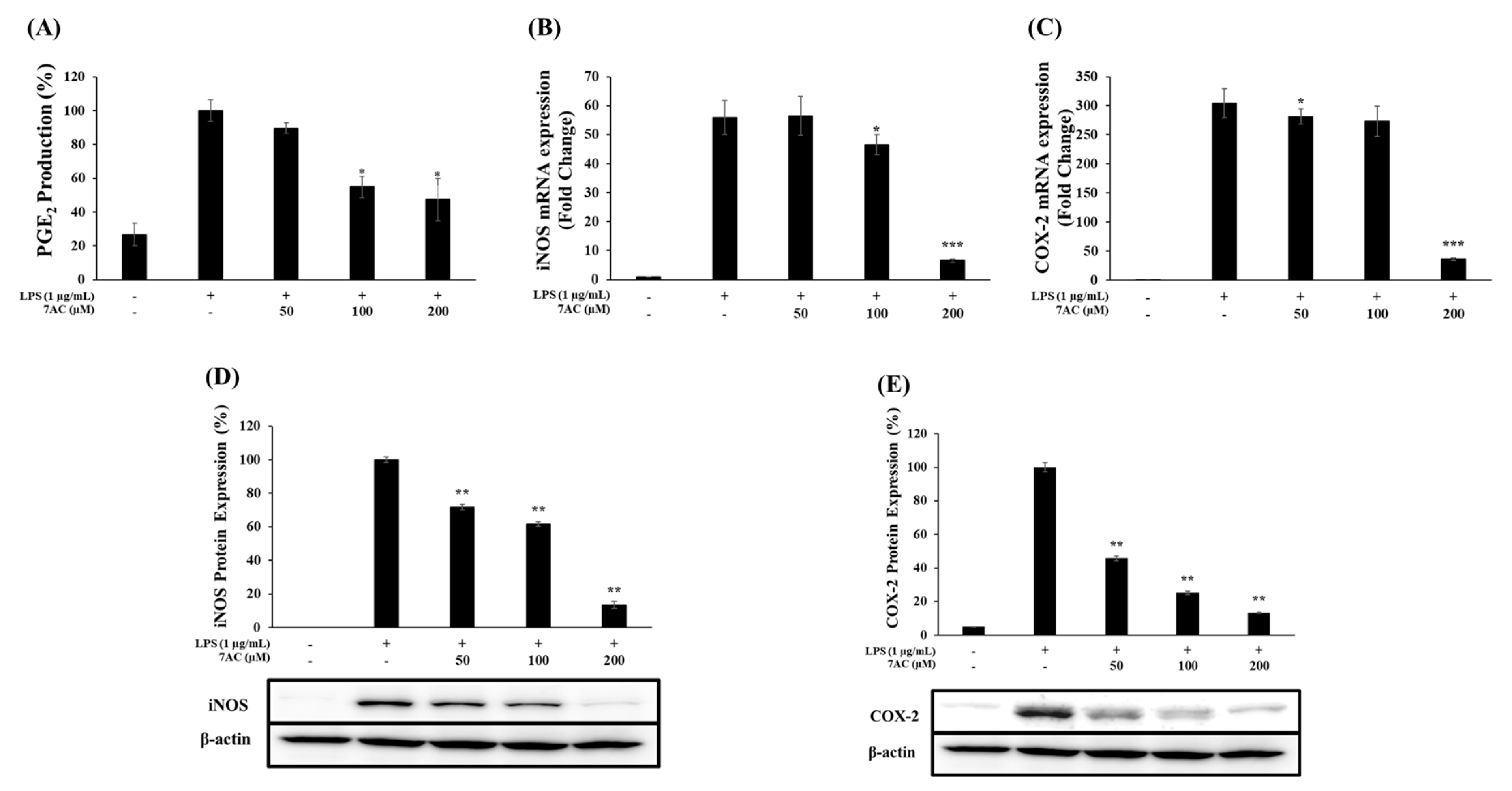
2.5. The Protein Expression and mRNA Levels of iNOS and COX-2
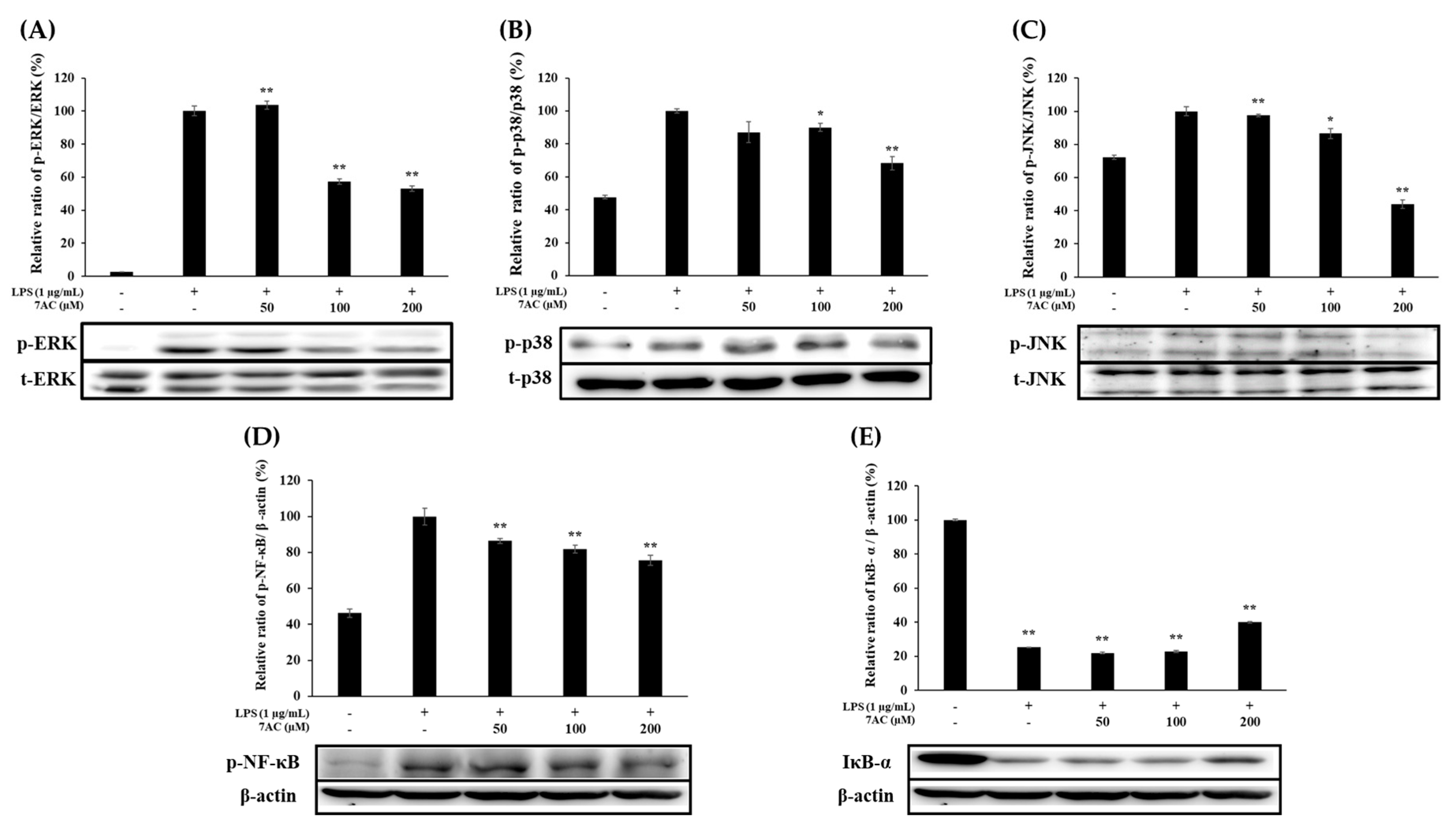
2.6. Expression of MAPK and NF-κB Pathways
3. Discussion
4. Materials and Methods
4.1. Acetylation of UMB
4.2. HPLC Analysis and Purification of 7-Acetoxycoumarin
4.3. LCMS and NMR Analysis 7-Acetoxycoumarin
4.4. Cell Culture and Viability Assay
4.5. Determination of NO and PGE2 Production
4.6. Determination of TNF-α, IL-1β and IL-6 Production
4.7. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR) Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Choi, H.J.; Eun, J.-S.; Park, Y.-R.; Kim, D.K.; Li, R.; Moon, W.S.; Park, J.M.; Kim, H.S.; Cho, N.-P.; Cho, S.-D.; et al. Ikarisoside A inhibits inducible nitric oxide synthase in lipopolysaccharide-stimulated RAW 264.7 cells via p38 kinase and nuclear factor-κB signaling pathways. Eur. J. Pharmacol. 2008, 601, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Mundandhara, S.; Devlin, R.B.; Madden, M.C. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicol. Appl. Pharmacol. 2005, 207, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Marcenaro, E.; Sivori, S.; Della Chiesa, M.; Vitale, M.; Moretta, L. Early liaisons between cells of the innate immune system in inflamed peripheral tissues. Trends Immunol. 2005, 26, 668–675. [Google Scholar] [CrossRef]
- Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.A.N.; Silva, R.H.M.; Queiroz, P.F.D.S.; Fernandes, C.V.; Garcia, J.B.S.; Ramos, R.; Da Rocha, C.Q.; Lima, S.T.D.J.R.M.; et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. Int. J. Mol. Sci. 2019, 20, 4512. [Google Scholar] [CrossRef]
- Luan, H.; Zhang, Q.; Wang, L.; Wang, C.; Zhang, M.; Xu, X.; Zhou, H.; Li, X.; Xu, Q.; He, F.; et al. OM85-BV Induced the Productions of IL-1β, IL-6, and TNF-α via TLR4- and TLR2-Mediated ERK1/2/NF-κB Pathway in RAW264.7 Cells. J. Interferon Cytokine Res. 2014, 34, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Kiemer, A.K.; Hartung, T.; Huber, C.; Vollmar, A.M. Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-κB pathway. J. Hepatol. 2003, 38, 289–297. [Google Scholar] [CrossRef]
- Lieb, K. Inhibition of LPS-induced iNOS and NO synthesis in primary rat microglial cells. Neurochem. Int. 2003, 42, 131–137. [Google Scholar] [CrossRef]
- Palmer, R.M.J.; Ashton, D.S.; Moncada, S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nat. 1988, 333, 664–666. [Google Scholar] [CrossRef]
- Abramson, S.B.; Amin, A.R.; Clancy, R.M.; Attur, M. The role of nitric oxide in tissue destruction. Best Pr. Res. Clin. Rheumatol. 2001, 15, 831–845. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Masferrer, J.L.; Zweifel, B.S.; Manning, P.T.; Hauser, S.D.; Leahy, K.M.; Smith, W.G.; Isakson, P.C.; Seibert, K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. USA 1994, 91, 3228–3232. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Zakim, D.; Weksler, B.B.; Dannenberg, A.J. Inhibition of cyclooxygenase: A novel approach to cancer prevention. Proc. Soc. Exp. Boil. Med. 1997, 216, 201–210. [Google Scholar] [CrossRef]
- Ben-Av, P.; Crofford, L.J.; Wilder, R.L.; Hla, T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: A potential mechanism for inflammatory angiogenesis. FEBS Lett. 1995, 372, 83–87. [Google Scholar] [CrossRef]
- Grilli, M.; Chiu, J.J.-S.; Lenardo, M.J. IMF-κB and Rel: Participants in a Multiform Transcriptional Regulatory System. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 1993; Volume 143, pp. 1–62. [Google Scholar]
- Pateras, I.; Giaginis, C.; Tsigris, C.; Patsouris, E.; Theocharis, S. NF-κB signaling at the crossroads of inflammation and atherogenesis: Searching for new therapeutic links. Expert Opin. Ther. Targets 2014, 18, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.-P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Maulik, N.; Sato, M.; Price, B.D.; Das, D.K. An essential role of NFkappaB in tyrosine kinase signaling of p38 MAP kinase regulation of myocardial adaptation to ischemia. FEBS Lett. 1998, 429, 365–369. [Google Scholar] [CrossRef]
- Bonizzi, G.; Karin, M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004, 25, 280–288. [Google Scholar] [CrossRef]
- Li, F.; Zhang, J.; Arfuso, F.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; Kumar, A.P.; Ahn, K.S.; Sethi, G. NF-κB in cancer therapy. Arch. Toxicol. 2015, 89, 711–731. [Google Scholar] [CrossRef] [PubMed]
- Pautz, A.; Art, J.; Hahn, S.; Nowag, S.; Voss, C.; Kleinert, H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 23, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Advance in studying early bolting of Umbelliferae medicinal plant. China J. Chin. Mater. Medica 2016, 41, 20–23. [CrossRef]
- Vijayalakshmi, A.; Sindhu, G. Dose responsive efficacy of umbelliferone on lipid peroxidation, anti-oxidant, and xenobiotic metabolism in DMBA-induced oral carcinogenesis. Biomed. Pharmacother. 2017, 88, 852–862. [Google Scholar] [CrossRef]
- Kumar, V.; Ahmed, D.; Verma, A.; Anwar, F.; Ali, M.; Mujeeb, M. Umbelliferone β-D-galactopyranoside from Aegle marmelos (L.) corr. an ethnomedicinal plant with antidiabetic, antihyperlipidemic and antioxidative activity. BMC Complement. Altern. Med. 2013, 13, 273. [Google Scholar] [CrossRef]
- Islam, N.; Choi, R.J.; Jin, S.E.; Kim, Y.S.; Ahn, B.R.; Zhao, D.; Jung, H.A.; Choi, J.S. Mechanism of anti-inflammatory activity of umbelliferone 6-carboxylic acid isolated from Angelica decursiva. J. Ethnopharmacol. 2012, 144, 175–181. [Google Scholar] [CrossRef]
- Kim, S.; Ko, H.; Son, S.; Shin, K.J.; Kim, D.J. A convenient total synthesis of (+)-decursinol from resorcinol. Tetrahedron Lett. 2001, 42, 7641–7643. [Google Scholar] [CrossRef]
- Murray, R.D.H.; Mendez, J.; Brown, S.A. Coumarin Activity in Plants and Bio Organism Aspects, 2nd ed.; John Wiley: Hoboken, NJ, USA, 1982; pp. 45–55. [Google Scholar]
- Ouyang, L.; Dan, Y.; Shao, Z.; Yang, S.; Yang, C.; Liu, G.; Zhou, W.; Duan, D. Effect of umbelliferone on adjuvant-induced arthritis in rats by MAPK/NF-κB pathway. Drug Des. Dev. Ther. 2019, 13, 1163–1170. [Google Scholar] [CrossRef]
- Lee, K.-S.; Kim, H.-J.; Kim, G.-H.; Shin, I.; Hong, J.-I. Fluorescent Chemodosimeter for Selective Detection of Cyanide in Water. Org. Lett. 2008, 10, 49–51. [Google Scholar] [CrossRef]
- Iqbal, P.F.; Bhat, A.R.; Azam, A. Antiamoebic coumarins from the root bark of Adina cordifolia and their new thiosemicarbazone derivatives. Eur. J. Med. Chem. 2009, 44, 2252–2259. [Google Scholar] [CrossRef]
- He, W.; Zhang, B.-L.; Zhou, S.; Sun, X.; Zhang, S.-Y. Facile Total Synthesis of Xanthotoxol. Synth. Commun. 2007, 37, 361–367. [Google Scholar] [CrossRef]
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.; Wallace, M.H.; Hawk, E.; Gordon, G.B.; Wakabayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T.; et al. The Effect of Celecoxib, a Cyclooxygenase-2 Inhibitor, in Familial Adenomatous Polyposis. N. Engl. J. Med. 2000, 342, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cuzzocrea, S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr. Med. Chem. 2009, 16, 3152–3167. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds aren’t available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, T.; Park, J.-S.; Sim, J.H.; Kim, S.-Y. 7-Acetoxycoumarin Inhibits LPS-Induced Inflammatory Cytokine Synthesis by IκBα Degradation and MAPK Activation in Macrophage Cells. Molecules 2020, 25, 3124. https://doi.org/10.3390/molecules25143124
Park T, Park J-S, Sim JH, Kim S-Y. 7-Acetoxycoumarin Inhibits LPS-Induced Inflammatory Cytokine Synthesis by IκBα Degradation and MAPK Activation in Macrophage Cells. Molecules. 2020; 25(14):3124. https://doi.org/10.3390/molecules25143124
Chicago/Turabian StylePark, Taejin, Jin-Soo Park, Ji Han Sim, and Seung-Young Kim. 2020. "7-Acetoxycoumarin Inhibits LPS-Induced Inflammatory Cytokine Synthesis by IκBα Degradation and MAPK Activation in Macrophage Cells" Molecules 25, no. 14: 3124. https://doi.org/10.3390/molecules25143124
APA StylePark, T., Park, J.-S., Sim, J. H., & Kim, S.-Y. (2020). 7-Acetoxycoumarin Inhibits LPS-Induced Inflammatory Cytokine Synthesis by IκBα Degradation and MAPK Activation in Macrophage Cells. Molecules, 25(14), 3124. https://doi.org/10.3390/molecules25143124