Self-Nanoemulsifying Drug Delivery Systems for Enhancing Solubility, Permeability, and Bioavailability of Sesamin
Abstract
1. Introduction
2. Results and Discussion
2.1. SNEDDS Characterization and Optimization
2.1.1. SSM Solubility Assessment
2.1.2. Dispersibility and Transmittance (%) of the SNEDDS Composition
2.2. Droplet Sizing and Morphological Characterization
2.3. Stability Study
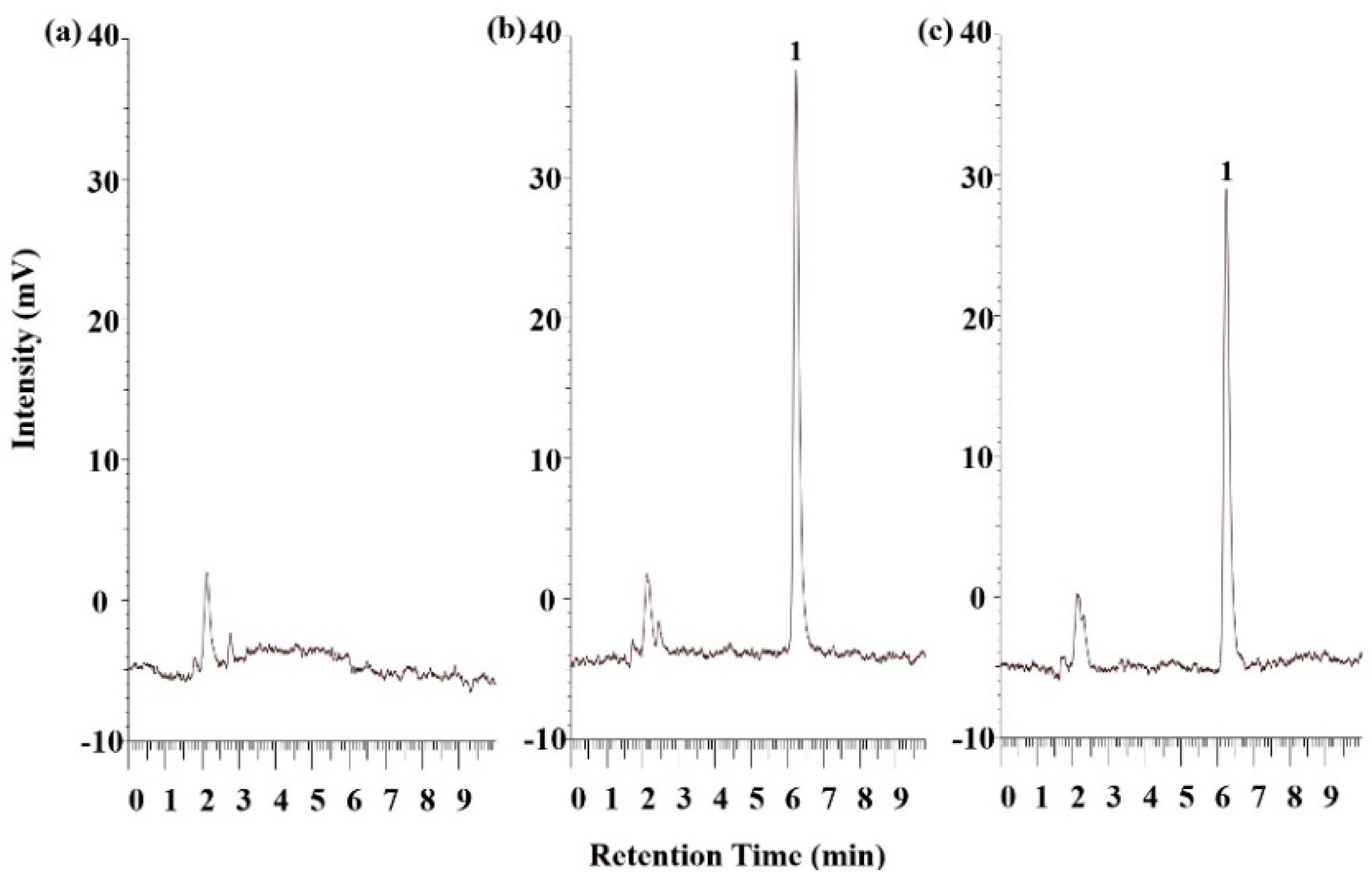
2.4. Permeability Evaluation
2.5. Pharmacokinetic Study
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Solubility Study
3.3. SSM SNEDDS Preparation
3.4. Dispersibility Test and Percentage Transmittance
3.5. Morphological Characterization and Droplet Sizing
3.5.1. Droplet Size and Distribution Analysis
3.5.2. TEM
3.6. Stability Study
3.7. Experimental Animal Preparation
3.8. Permeability Evaluation
3.9. Pharmacokinetic Study
3.10. Plasma Sample Preparation and HPLC Validation
3.11. Data Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, A.-Y.; Yun, C.-I.; Lee, J.-G.; Kim, Y.-J. Determination and daily intake estimation of lignans in sesame seeds and sesame oil products in Korea. Foods 2020, 9, 394. [Google Scholar] [CrossRef]
- Shimoyoshi, S.; Takemoto, D.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Tomono, S.; Unno, K.; Wakabayashi, K. Sesame lignans suppress age-related cognitive decline in senescence-accelerated mice. Nutrients 2019, 11, 1582. [Google Scholar] [CrossRef]
- Periasamy, S.; Liu, C.-T.; Chien, S.-P.; Chen, Y.-C.; Liu, M.-Y.; Information, P.E.K.F.C. Daily sesame oil supplementation mitigates ketoconazole-induced oxidative stress-mediated apoptosis and hepatic injury. J. Nutr. Biochem. 2016, 37, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Okumura, H.; Ono, Y.; Kitagawa, Y.; Rogi, T.; Shibata, H. Sesame lignans reduce LDL oxidative susceptibility by downregulating the platelet-activating factor acetylhydrolase. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2151–2161. [Google Scholar]
- Monteiro, É.M.; Chibli, L.A.; Yamamoto, C.H.; Pereira, M.C.; Vilela, F.M.; Rodarte, M.P.; de Oliveira Pinto, M.A.; Da Penha Henriques do Amaral, M.; Silvério, M.S.; de Matos Araújo, A.L.; et al. Antinociceptive and anti-inflammatory activities of the sesame oil and sesamin. Nutrients 2014, 6, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Yaguchi, Y.; Komura, T.; Nakadai, M.; Terao, K.; Kage-Nakadai, E.; Nishikawa, Y. Sesamin extends lifespan through pathways related to dietary restriction in Caenorhabditis elegans. Eur. J. Nutr. 2017, 57, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, D.; Yasutake, Y.; Tomimori, N.; Ono, Y.; Shibata, H.; Hayashi, J. Sesame lignans and Vitamin E supplementation improve subjective statuses and anti-oxidative capacity in healthy humans with feelings of daily fatigue. Glob. J. Heal. Sci. 2015, 7, 1–10. [Google Scholar] [CrossRef]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of dietary supplementation of astaxanthin and sesamin on daily fatigue: A randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Liangliang, C.; Yi, W.; Juncheng, H.; Qin, W.; Xiaoxiang, Z. Anti-aging effect of sesamin and its mechanism of action. Curr. Top. Nutraceut. Res. 2012, 10, 173. [Google Scholar]
- Sato, H.; Aoki, A.; Tabata, A.; Kadota, K.; Tozuka, Y.; Seto, Y.; Onoue, S. Development of sesamin-loaded solid dispersion with α-glycosylated stevia for improving physicochemical and nutraceutical properties. J. Funct. Foods 2017, 35, 325–331. [Google Scholar] [CrossRef]
- Tomimori, N.; Rogi, T.; Shibata, H. Absorption, distribution, metabolism, and excretion of [14C] sesamin in rats. Mol. Nutr. Food Res. 2017, 61, 1600844. [Google Scholar] [CrossRef]
- Iwamoto, K.; Matsumura, S.; Yoshioka, Y.; Yamamoto, A.; Makino, S.; Moriyama, T.; Zaima, N. Using turmeric oil as a solvent improves the distribution of sesamin-sesamolin in the serum and brain of mice. Lipids 2019, 54, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Sarmentocde, B.; Costa, P.C. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, T.; Kawish, M.; Maharjan, R.; Ghaffar, I.; Ali, H.S.; Imran, M.; Perveen, S.; Saifullah, S.; Simjee, S.U.; Shah, M. Design and development of permeation enhancer containing self-nanoemulsifying drug delivery system (SNEDDS) for ceftriaxone sodium improved oral pharmacokinetics. J. Mol. Liq. 2019, 289, 111098. [Google Scholar] [CrossRef]
- Gupta, S.; Chavhan, S.; Sawant, K.K. Self-nanoemulsifying drug delivery system for adefovir dipivoxil: Design, characterization, in vitro and ex vivo evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 145–155. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; El-Massik, M.; Abdallah, O.Y. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: Design and optimization. Int. J. Pharm. 2009, 380, 133–141. [Google Scholar] [CrossRef]
- Warner, K.; Mounts, T.L. Frying stability of soybean and canola oils with modified fatty acid compositions. J. Am. Oil Chem. Soc. 1993, 70, 983–988. [Google Scholar] [CrossRef]
- Holt, P.R. Medium chain triglycerides. A useful adjunct in nutritional therapy. Gastroenterology 1967, 53, 961–966. [Google Scholar] [CrossRef]
- Xi, J.; Chang, Q.; Chan, C.K.; Meng, Z.Y.; Wang, G.N.; Sun, J.B.; Wang, Y.T.; Tong, H.H.Y.; Zheng, Y. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech 2009, 10, 172–182. [Google Scholar] [CrossRef]
- Zeng, L.; Xin, X.; Zhang, Y. Development and characterization of promising Cremophor EL-stabilized o/w nanoemulsions containing short-chain alcohols as a cosurfactant. RSC Adv. 2017, 7, 19815–19827. [Google Scholar] [CrossRef]
- Kunieda, H.; Shigeta, K.; Suzuki, M. Phase behavior and formation of reverse vesicles in long-polyoxyethylene-chain nonionic surfactant systems. Langmuir 1999, 15, 3118–3122. [Google Scholar] [CrossRef]
- Amani, A.; York, P.; De Waard, H.; Anwar, J. Molecular dynamics simulation of a polysorbate 80 micelle in water. Soft Matter 2011, 7, 2900–2908. [Google Scholar] [CrossRef]
- Bumajdad, A.; Eastoe, J.; Nave, S.; Steytler, D.C.; Heenan, R.; Grillo, I. Compositions of mixed surfactant layers in microemulsions determined by small-angle neutron scattering. Langmuir 2003, 19, 2560–2567. [Google Scholar] [CrossRef]
- Miastkowska, M.; Lasoń, E.; Sikora, E.; Wolińska-Kennard, K. Preparation and characterization of water-based nano-perfumes. Nanomaterials 2018, 8, 981. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.-C.; Chang, C.-W.; Hsu, M.-C.; Wu, Y.-T. Self-nanoemulsifying drug delivery system for resveratrol: Enhanced oral bioavailability and reduced physical fatigue in rats. Int. J. Mol. Sci. 2017, 18, 1853. [Google Scholar] [CrossRef]
- Thiry, J.; Lebrun, P.; Vinassa, C.; Adam, M.; Netchacovitch, L.; Ziemons, E.; Hubert, P.; Krier, F.; Evrard, B. Continuous production of itraconazole-based solid dispersions by hot melt extrusion: Preformulation, optimization and design space determination. Int. J. Pharm. 2016, 515, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-X.; Kong, X.; Yang, J.-R.; Zhang, J.-X. Long-term intake of sesamin improves left ventricular remodelling in spontaneously hypertensive rats. Food Funct. 2013, 4, 453–460. [Google Scholar] [CrossRef]
- Chen, X.; Ying, X.; Chen, L.; Zhang, W.; Zhang, Y. Protective effects of sesamin on liver fibrosis through antioxidative and anti-inflammatory activities in rats. Immunopharmacol. Immunotoxicol. 2015, 37, 465–472. [Google Scholar] [CrossRef]
- Thuy, T.D.; Phan, N.N.; Wang, C.-Y.; Yu, H.-G.; Wang, S.-Y.; Huang, P.-L.; Yin, Y.; Lin, Y.-C. Novel therapeutic effects of sesamin on diabetes-induced cardiac dysfunction. Mol. Med. Rep. 2017, 15, 2949–2956. [Google Scholar] [CrossRef]
- Zimmerman, J.J.; Ferron, G.M.; Lim, H.K.; Parker, V. The effect of a high-fat meal on the oral bioavailability of the immunosuppressant sirolimus (rapamycin). J. Clin. Pharmacol 1999, 39, 1155–1161. [Google Scholar]
- Baloch, J.; Sohail, M.F.; Sarwar, H.S.; Kiani, M.H.; Khan, G.M.; Jahan, S.; Rafay, M.; Chaudhry, M.T.; Yasinzai, M.; Shahnaz, G. Self-nanoemulsifying drug delivery system (SNEDDS) for improved oral bioavailability of chlorpromazine: In vitro and in vivo evaluation. Medicina 2019, 55, 210. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, R.N.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef]
- Landete, J. Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Rich, K.M.; Bia, J.; Altice, F.L.; Feinberg, J. Integrated models of care for individuals with opioid use disorder: How do we prevent HIV and HCV? Curr. HIV/AIDS Rep. 2018, 15, 266–275. [Google Scholar] [CrossRef]
- Yen, C.-C.; Chen, Y.-C.; Wu, M.-T.; Wang, C.-C.; Wu, Y.-T. Nanoemulsion as a strategy for improving the oral bioavailability and anti-inflammatory activity of andrographolide. Int. J. Nanomed. 2018, 13, 669–680. [Google Scholar] [CrossRef]
- Badiali, L.; Cedernaes, J.; Olszewski, P.K.; Nylander, O.; Vergoni, A.V.; Schiöth, H.B. Adhesion GPCRs are widely expressed throughout the subsections of the gastrointestinal tract. BMC Gastroenterol. 2012, 12, 134. [Google Scholar] [CrossRef]
- Schwertner, H.A.; Rios, D.C. Analysis of Sesamin, Asarinin, and Sesamolin by HPLC with photodiode and fluorescent detection and by GC/MS: Application to sesame oil and serum samples. J. Am. Oil Chem. Soc. 2012, 89, 1943–1950. [Google Scholar] [CrossRef]
- Chang, C.-W.; Wang, C.; Wu, Y.-T.; Hsu, M.-C. Enhanced solubility, dissolution, and absorption of lycopene by a solid dispersion technique: The dripping pill delivery system. Powder Technol. 2016, 301, 641–648. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
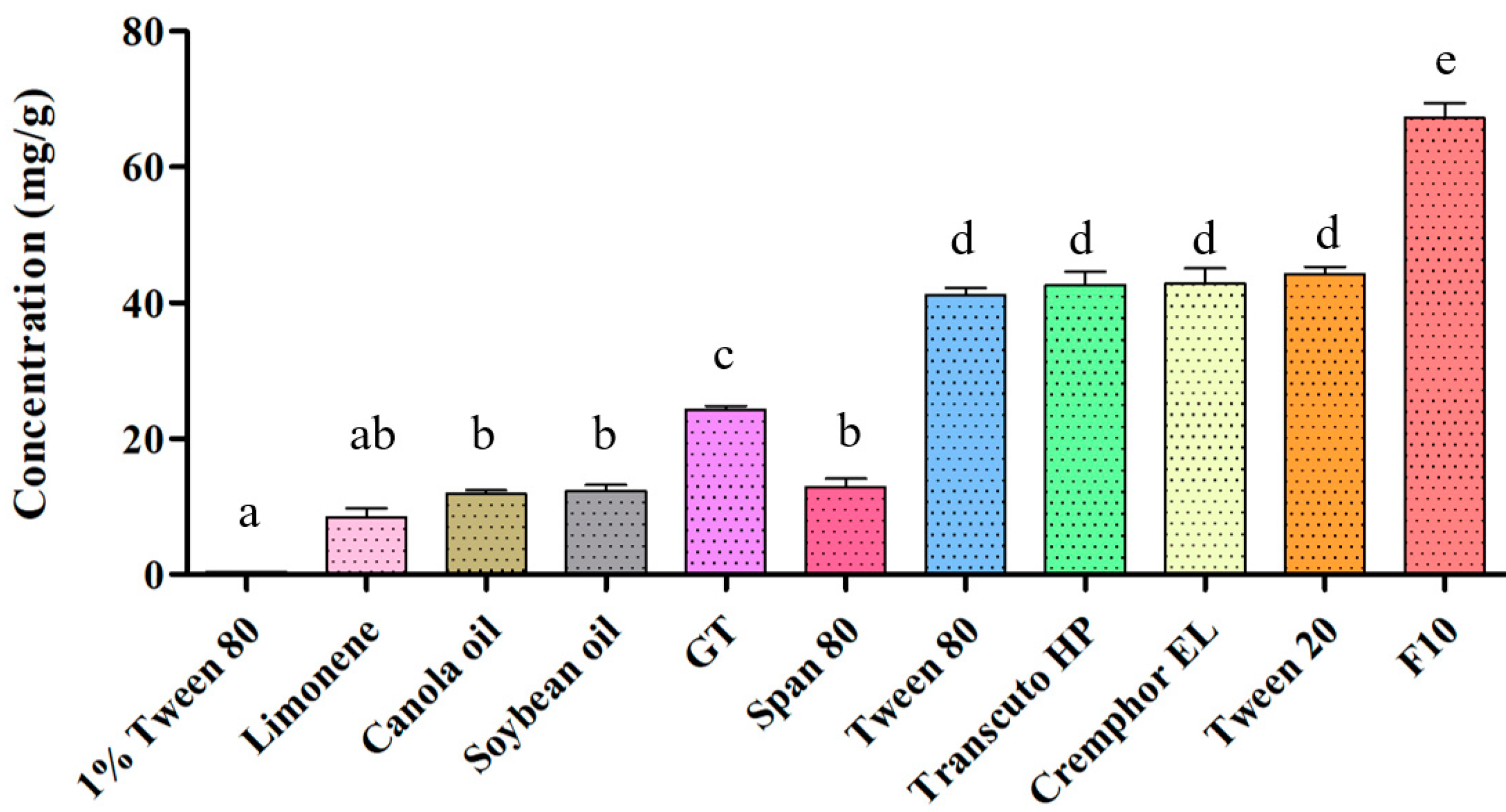
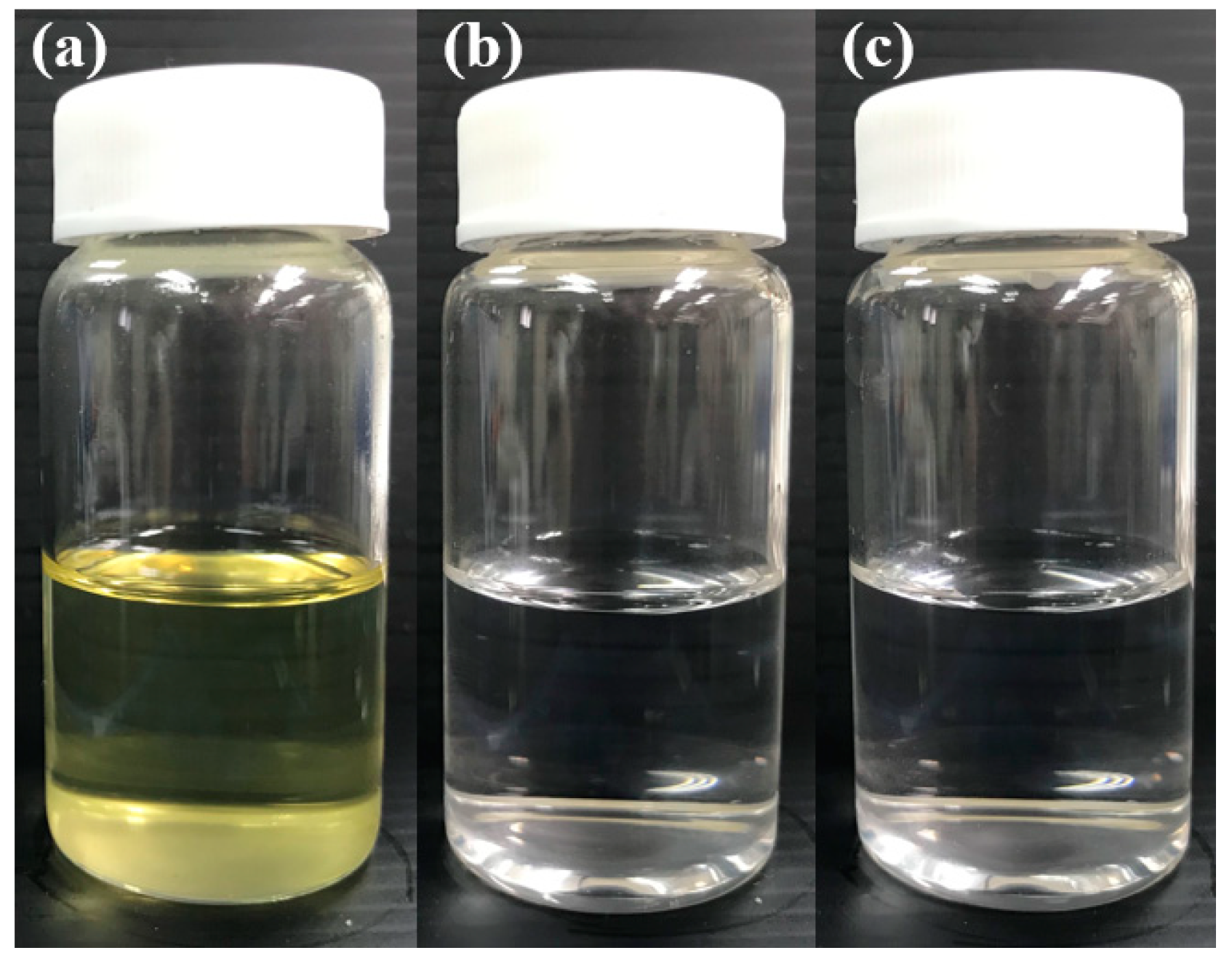
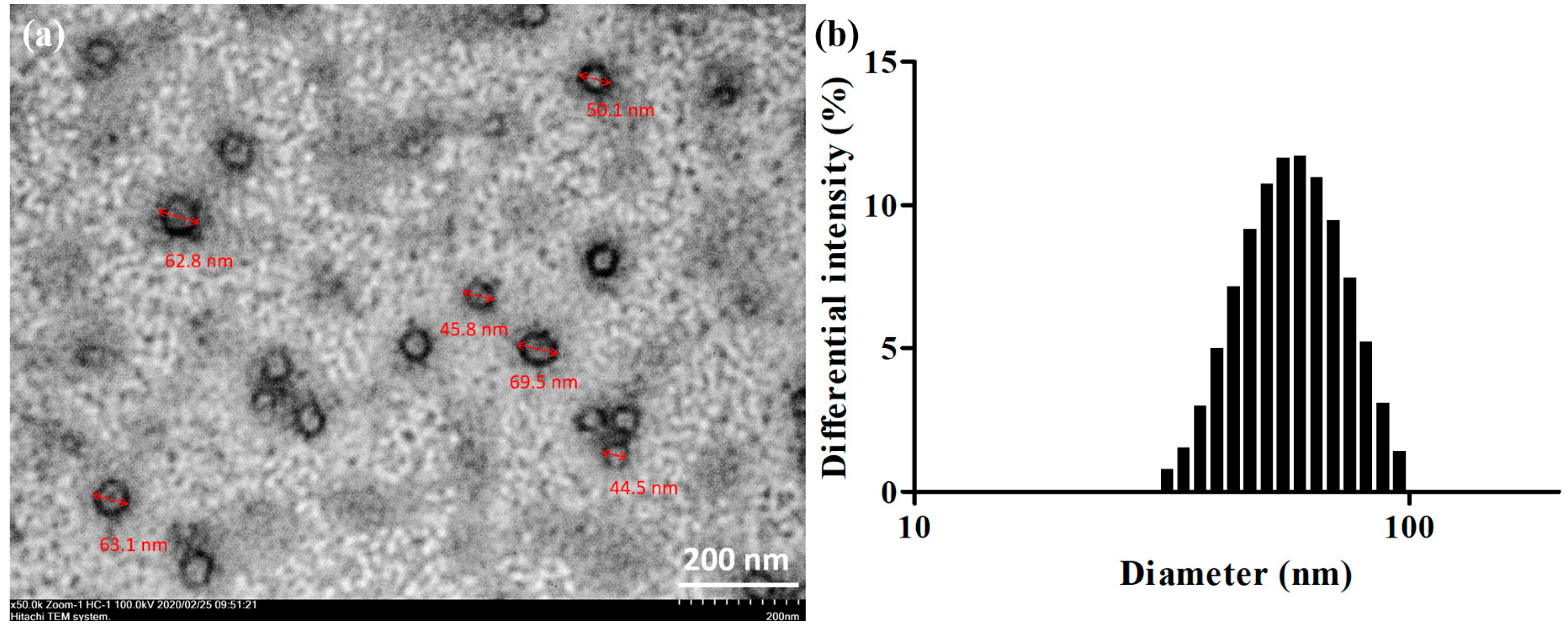
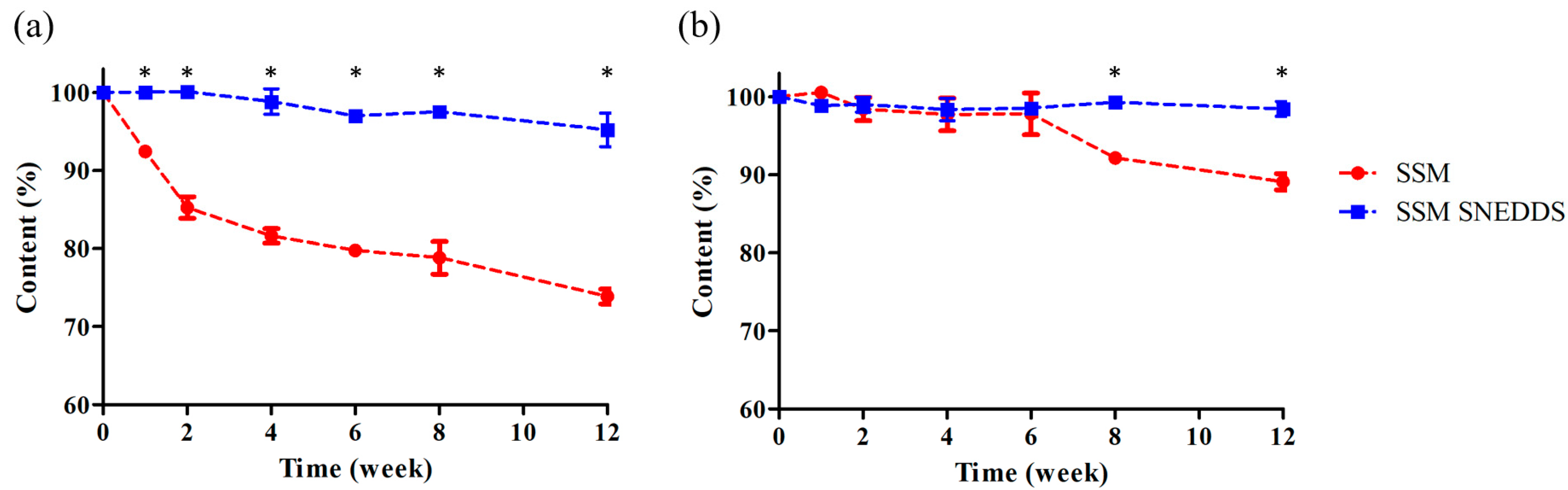


| Formulation | GT/Tween20 (w/w) | Dispersibility | Droplet Size (nm) | PDI | Transmittance (%) | |
|---|---|---|---|---|---|---|
| Water | 0.1 N HCl | |||||
| F1 | 1:1 | E | E | N/A | N/A | N/A |
| F2 | 1:3 | D | D | 156.5 ± 11.3 | 0.27 ± 0.01 | 1.2 |
| F3 | 1:5 | C | C | 166.1 ± 7.9 | 0.22 ± 0.05 | 0.1 |
| F4 | 1:7 | C | C | 107.1 ± 4.9 | 0.18 ± 0.02 | 57.6 |
| F5 | 1:9 | C | C | 90.7 ± 4.7 | 0.08 ± 0.06 | 70.2 |
| GT/Transcutol HP/Tween 20 (%, w/w/w) | ||||||
| F6 | 10:10:80 | B | B | 158.7 ± 60.1 | 0.26 ± 0.04 | 4.1 |
| F7 | 10:20:70 | B | B | 204.6 ± 126.5 | 0.23 ± 0.02 | 31.9 |
| F8 | 10:30:60 | B | B | 200.6 ± 94.9 | 0.19 ± 0.03 | 11.7 |
| F9 | 10:40:50 | B | B | 634.4 ± 130.1 | 0.28 ± 0.01 | 0.62 |
| GT/Cremophor EL/Tween 20 (%, w/w/w) | ||||||
| F10 | 10:10:80 | A | A | 66.4 ± 31.4 * | 0.05 ± 0.01 * | 91.6 |
| F11 | 10:20:70 | A | A | 142.9 ± 75.9 | 0.10 ± 0.01 | 92.5 |
| F12 | 10:30:60 | A | A | 174.3 ± 36.7 | 0.15 ± 0.01 | 94.9 |
| F13 | 10:40:50 | A | A | 242.5 ± 148.3 | 0.18 ± 0.01 | 95.7 |
| SSM Suspension (100 mg/kg, p.o.) | F10 (100 mg/kg, p.o.) | SSM Solution (1 mg/kg, i.v.) | |
|---|---|---|---|
| Cmax or C0 (ng/mL) | 25.6 ± 3.9 | 231.2 ± 15.3 * | 1336.5 ± 126.7 |
| t 1/2 (h) | 4.7 ± 3.1 | 10.5 ± 2.4 | 1.2 ± 0.5 |
| AUC0→t (h·ng/mL) | 131.9 ± 26.0 | 1697.9 ± 624.7 * | 385.9 ± 109.9 |
| Relative bioavailability (%) | N/A | 1287.3 | N/A |
| Absolute bioavailability (%) | 0.3 | 4.4 | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-Y.; Yen, C.-C.; Hsu, M.-C.; Wu, Y.-T. Self-Nanoemulsifying Drug Delivery Systems for Enhancing Solubility, Permeability, and Bioavailability of Sesamin. Molecules 2020, 25, 3119. https://doi.org/10.3390/molecules25143119
Wang C-Y, Yen C-C, Hsu M-C, Wu Y-T. Self-Nanoemulsifying Drug Delivery Systems for Enhancing Solubility, Permeability, and Bioavailability of Sesamin. Molecules. 2020; 25(14):3119. https://doi.org/10.3390/molecules25143119
Chicago/Turabian StyleWang, Chih-Yuan, Ching-Chi Yen, Mei-Chich Hsu, and Yu-Tse Wu. 2020. "Self-Nanoemulsifying Drug Delivery Systems for Enhancing Solubility, Permeability, and Bioavailability of Sesamin" Molecules 25, no. 14: 3119. https://doi.org/10.3390/molecules25143119
APA StyleWang, C.-Y., Yen, C.-C., Hsu, M.-C., & Wu, Y.-T. (2020). Self-Nanoemulsifying Drug Delivery Systems for Enhancing Solubility, Permeability, and Bioavailability of Sesamin. Molecules, 25(14), 3119. https://doi.org/10.3390/molecules25143119







