Chemical Fingerprinting, Isolation and Characterization of Polyphenol Compounds from Heliotropium taltalense (Phil.) I.M. Johnst and Its Endothelium-Dependent Vascular Relaxation Effect in Rat Aorta
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Phenolic Compounds in H. taltalense Methanolic and Aqueous Extracts
2.1.1. Phenolic Acids
2.1.2. Organic Saturated Acids
2.1.3. Oxylipins
2.1.4. Benzoic Acid Derivatives
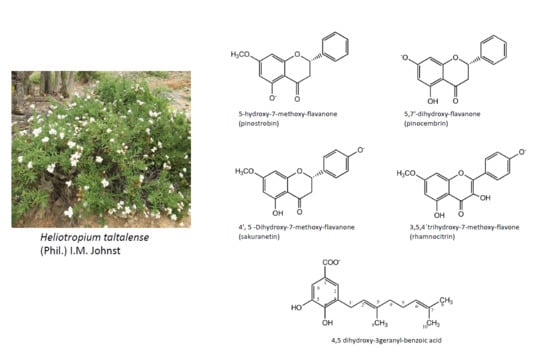
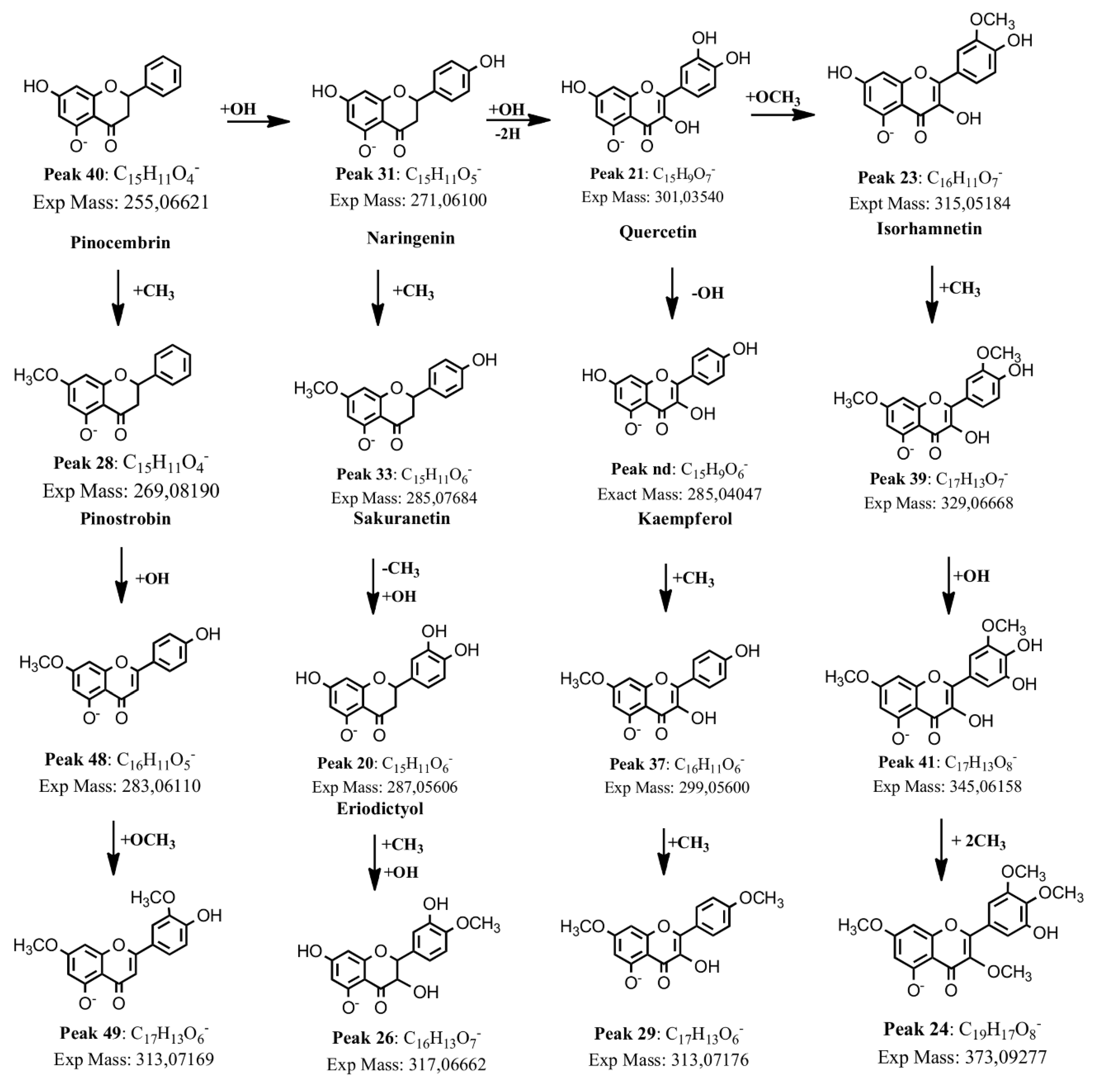
2.1.5. Flavonoids
2.2. Isolation and Identification of Major Compounds
2.3. Antioxidant Activity of H. taltalense
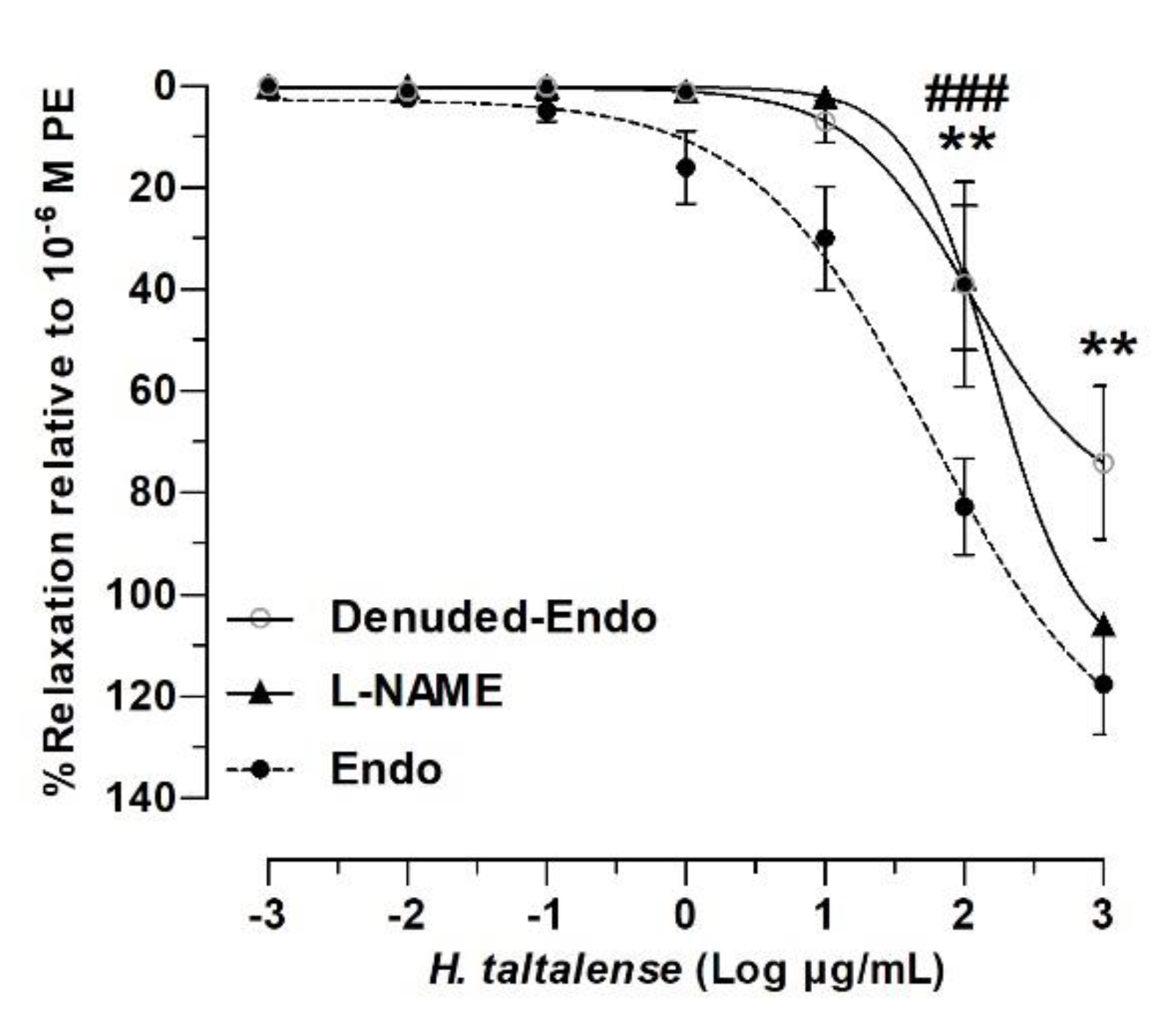
2.4. Role of the Endothelium in the Vascular Relaxation of H. taltalense
2.5. H. taltalense Decreases the Contractile Response to KCl and PE
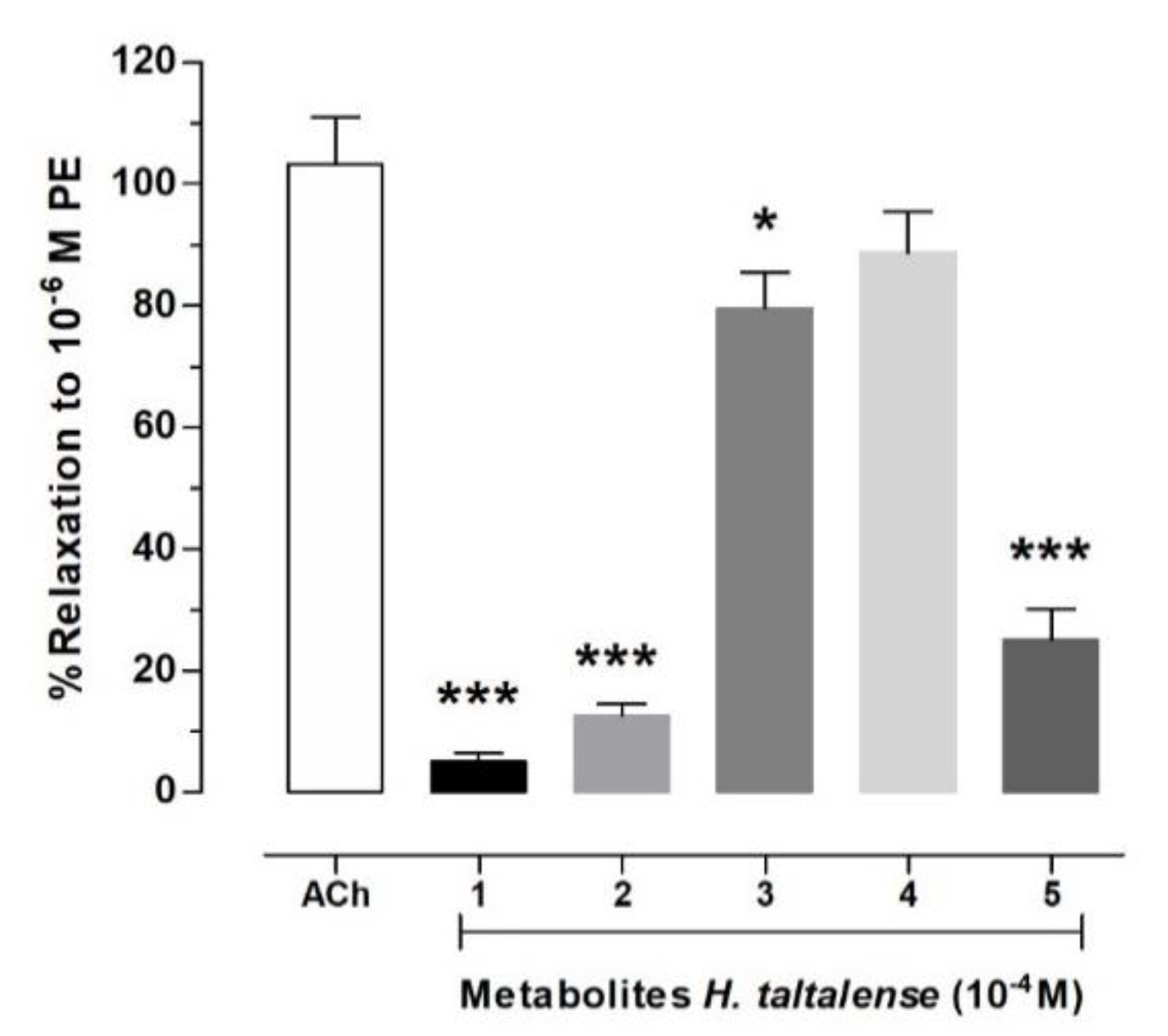
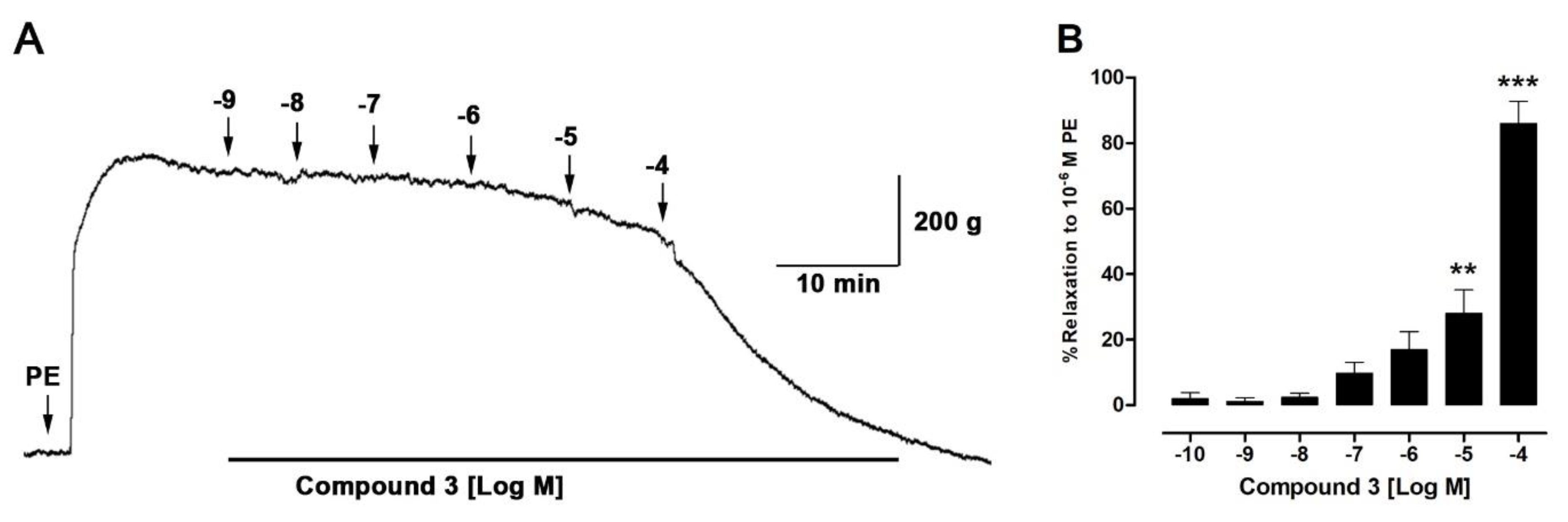
2.6. Isolated Compounds from H. taltalense Causes Relaxation in Rat Aorta
3. Materials and Methods
3.1. Chemicals and Plant Material
3.2. Plant Material
3.3. Extraction and Isolation
3.4. UHPLC-PDA-MS Instrument
3.5. LC Parameters
3.6. MS Parameters
3.7. Determination of Antioxidant Activity
3.8. Animals
3.9. Isolation of Rat Aorta and Vascular Reactivity Essays
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gutiérrez, G.G.; Lazo Salinas, L. Plantas Medicinales Silvestres de uso Tradicional en la Localidad de Paposo, Costa del Desierto de Atacama, II Región, Chile; Ministerio de Educación: Santiago, Chile, 1996. [Google Scholar]
- Mohsen Ibrahim, M. Hypertension in Developing Countries: A Major Challenge for the Future. Curr. Hypertens Rep. 2018, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Modak, B.; Rojas, M.; Torres, R. Chemical Analysis of the Resinous Exudate Isolated from Heliotropium taltalense and Evaluation of the Antioxidant Activity of the Phenolics Components and the Resin in Homogeneous and Heterogeneous Systems. Molecules 2009, 14, 1980–1989. [Google Scholar] [CrossRef]
- Torres, R.; Modak, B.; Villarroel, L.; Urzua, A.; Delle-Monache, F.; Sanchez-Ferrando, F. Flavonoides del exudado resinoso de Heliotropium sinuatum. Bol. Soc. Chil. Quím. 1996, 41, 195–197. [Google Scholar]
- Valenzuela, B.; Obreque, J.; Soto-Aguilera, S.; Maisey, K.; Imarai, M.; Modak, B. Key cytokines of adaptive immunity are differentially induced in rainbow trout kidney by a group of structurally related geranyl aromatic derivatives. Fish Shellfish Immunol. 2016, 49, 45–53. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Schmeda-Hirschmann, G.; Avendaño, M.; Sepúlveda, B.; Winterhalter, P. Fast high resolution Orbitrap MS fingerprinting of the resin of Heliotropium taltalense Phil. from the Atacama Desert. Ind. Crop. Prod. 2016, 85, 159–166. [Google Scholar] [CrossRef]
- Bourgou, S.; Tammar, S.; Salem, N.; Mkadmini, K.; Msaada, K. Phenolic Composition, Essential Oil, and Antioxidant Activity in the Aerial Part of Artemisia Herba-Alba from Several Provenances: A Comparative Study. Int. J. Food Prop. 2016, 19, 549–563. [Google Scholar] [CrossRef]
- Singh, G.; Passsari, A.K.; Leo, V.V.; Mishra, V.K.; Subbarayan, S.; Singh, B.P.; Kumar, B.; Kumar, S.; Gupta, V.K.; Lalhlenmawia, H.; et al. Evaluation of Phenolic Content Variability along with Antioxidant, Antimicrobial, and Cytotoxic Potential of Selected Traditional Medicinal Plants from India. Front. Plant. Sci. 2016, 7. [Google Scholar] [CrossRef]
- Tong, L.; Liu, H.; Xie, C.; Li, M. Quantitative analysis analysis of antibiotics in aquifer sediments by liquid chromatography coupled to high resolution mass spectrometry. J. Chromatogr. A 2016, 1452, 58–66. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; Rincon-Cervera, M.A.; Gonzalez-Fernandez, M.J.; Guil-Guerrero, J.L. Phytochemical Composition and Antitumor Activities of New Salad Greens: Rucola (Diplotaxis tenuifolia) and Corn Salad (Valerianella locusta). Plant. Foods Hum. Nutr. 2016, 71, 197–203. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B.X. Fast Detection of Phenolic Compounds in Extracts of Easter Pears (Pyrus communis) from the Atacama Desert by Ultrahigh-Performance Liquid Chromatography and Mass Spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef] [PubMed]
- Rameshrad, M.; Babaei, H.; Azarmi, Y.; Fouladia, D.F. Rat aorta as a pharmacological tool for in vitro and in vivo studies. Life Sci. 2016, 145, 190–204. [Google Scholar] [CrossRef]
- Paredes, A.; Palacios, J.; Quispe, C.; Nwokocha, C.R.; Morales, G.; Kuzmicic, J.; Cifuentes, F. Hydroalcoholic extract and pure compounds from Senecio nutans Sch. Bip (Compositae) induce vasodilation in rat aorta through endothelium-dependent and independent mechanisms. J. Ethnopharmacol. 2016, 192, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. [36] Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1990; Volume 186, pp. 343–355. [Google Scholar]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.A.; Martinez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef]
- Ajay, M.; Gilani, A.U.A.; Mustafa, M.R. Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. Life Sci. 2003, 74, 603–612. [Google Scholar] [CrossRef]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef]
- Machha, A.; Mustafa, M.R. Chronic treatment with flavonoids prevents endothelial dysfunction in spontaneously hypertensive rat aorta. J. Cardiovasc. Pharmacol. 2005, 46, 36–40. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Ramirez, J.E.; Schmeda Hirschmann, G.; Kennelly, E.J. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013, 54, 532–543. [Google Scholar] [CrossRef]
- Modak, B.; Torres, R.; De Saint Pierre, M.; Saud, K.; Armijo, A.; Caviedes, R.; Caviedes, P. In vitro antiproliferative activity of 3 H-spiro 1-benzofuran-2,1′cyclohexane derivatives. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011, 10, 281–288. [Google Scholar]
- Krueger, R.J. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; Dewick, P.M., Ed.; John Wiley & Sons: New York, NY, USA, 2002; ISBN 0-471-49640-5. [Google Scholar]
- Kranjc, E.; Albreht, A.; Vovk, I.; Makuc, D.; Plavec, J. Non-targeted chromatographic analyses of cuticular wax flavonoids from Physalis alkekengi L. J. Chromatogr. A 2016, 1437, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Kim, H.M.; Lee, J.S.; Lee, C.K.; Sezirahiga, J.; Woo, J.H.; Choi, J.H.; Jang, D.S. New Flavonol Glucuronides from the Flower Buds of Syzygium aromaticum (Clove). J. Agric. Food Chem. 2016, 64, 3048–3053. [Google Scholar] [CrossRef] [PubMed]
- Ayouni, K.; Berboucha-Rahmani, M.; Kim, H.K.; Atmani, D.; Verpoorte, R.; Choi, Y.H. Metabolomic tool to identify antioxidant compounds of Fraxinus angustifolia leaf and stem bark extracts. Ind. Crop. Prod. 2016, 88, 65–77. [Google Scholar] [CrossRef]
- Agrawal, P.K. Carbon-13 NMR of Flavonoids; Elsevier: Midland, MI, USA, 1989; p. 564. [Google Scholar]
- Smolarz, H.D.; Mendyk, E.; Bogucka-Kocka, A. Pinostrobin-An anti-Leukemic Flavonoid from Polygonum lapanthifolium L. ssp. Nodosum (Pers.) Dans. Zeitsch. Naturforsch. 2006, 61c, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.; Simirgiotis, M.J.; Brito, A.; Werner, M.R.; Bórquez, J.; Winterhalter, P.; Cárdenas, A. A non-centrosymmetric polymorph of 5-hydroxy-7-methoxy-2-phenylchroman-4-one. J. Chil. Chem. Soc. 2015, 60, 2864–2866. [Google Scholar] [CrossRef]
- Zhou, H.F.; Xie, C.H.; Jian, R.J.; Kang, J.; Li, Y.; Zhuang, C.L.; Yang, F.; Zhang, L.L.; Lai, L.; Wu, T.; et al. Biflavonoids from Caper (Capparis spinosa L.) Fruits and Their Effects in Inhibiting NF-kappa B Activation. J. Agric. Food Chem. 2011, 59, 3060–3065. [Google Scholar] [CrossRef]
- Brito, I.; Borquez, J.; Simirgiotis, M.; Cardenas, A.; Lopez-Rodriguez, M. 4′,5-Dihydroxy-7-methoxyflavanone dihydrate. Acta Crystallogr. Sect. E-Struct. Rep. Online 2012, 68. [Google Scholar] [CrossRef]
- Castaneda, H.G.T.; Dulcey, A.J.C.; Martinez, J.H.I. Flavonoid Glycosides from Siparuna gigantotepala Leaves and Their Antioxidant Activity. Chem. Pharm. Bull. 2016, 64, 502–506. [Google Scholar] [CrossRef][Green Version]
- Kleszczewski, T.; Buzun, L.; Lisowska, A.; Modzelewska, B. Potassium induced contraction of the internal thoracic artery in vitro is time related: The potential consequences in the analysis of the mechanism of the spasm after coronary artery bypass grafting and in the analysis of the results of in vitro studies. Heart Vessel. 2016, 31, 616–621. [Google Scholar] [CrossRef][Green Version]
- Lee, C.H.; Poburko, D.; Sahota, P.; Sandhu, J.; Ruehlmann, D.O.; van Breeman, C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J. Physiol. Lond. 2001, 534, 641–650. [Google Scholar] [CrossRef]
- Brozovich, F.V.; Nicholson, C.J.; Degen, C.V.; Gao, Y.Z.; Aggarwal, M.; Morgan, K.G. Mechanisms of Vascular Smooth Muscle Contraction and the Basis for Pharmacologic Treatment of Smooth Muscle Disorders. Pharmacol. Rev. 2016, 68, 476–532. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, F.; Palacios, J.; Bórquez, J.; Paredes, A.; Parra, C.; Bravo, A.; Simirgiotis, M.J. Fast Isolation of Flavonoids from the Endemic Species Nolana ramosissima I.M. Johnst and Its Endothelium-Independent Relaxation Effect in Rat Aorta. Molecules 2020, 25, 520. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.B.; Coelho, L.P.; Insuela, D.B.; Carvalho, V.F.; dos Santos, M.H.; Silva, P.M.; Martins, M.A. Mangiferin prevents guinea pig tracheal contraction via activation of the nitric oxide-cyclic GMP pathway. PLoS ONE 2013, 8, e71759. [Google Scholar] [CrossRef]
- Hammad, H.M.; Abdalla, S.S. Pharmacological effects of selected flavonoids on rat isolated ileum: Structure-activity relationship. Gen. Pharm. 1997, 28, 767–771. [Google Scholar] [CrossRef]
- Palacios, J.; Espinoza, F.; Munita, C.; Cifuentes, F.; Michea, L. Na+-K-2Cl− cotransporter is implicated in gender differences in the response of the rat aorta to phenylephrine. Br. J. Pharm. 2006, 148, 964–972. [Google Scholar] [CrossRef]
Sample Availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. |





| Extract | TPC | TFC | IC50 DPPH | IC50 ABTS | FRAP |
|---|---|---|---|---|---|
| Ht MeOH | 1283 ± 175 | 743 ± 51 | 400 ± 2 | 66 ± 1 | 4067 ± 262 |
| Ht Aq | 75 ± 2 | 261 ± 3 | 595 ± 3 | 40 ± 0 | 2503 ± 435 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrientos, R.E.; Simirgiotis, M.J.; Palacios, J.; Paredes, A.; Bórquez, J.; Bravo, A.; Cifuentes, F. Chemical Fingerprinting, Isolation and Characterization of Polyphenol Compounds from Heliotropium taltalense (Phil.) I.M. Johnst and Its Endothelium-Dependent Vascular Relaxation Effect in Rat Aorta. Molecules 2020, 25, 3105. https://doi.org/10.3390/molecules25143105
Barrientos RE, Simirgiotis MJ, Palacios J, Paredes A, Bórquez J, Bravo A, Cifuentes F. Chemical Fingerprinting, Isolation and Characterization of Polyphenol Compounds from Heliotropium taltalense (Phil.) I.M. Johnst and Its Endothelium-Dependent Vascular Relaxation Effect in Rat Aorta. Molecules. 2020; 25(14):3105. https://doi.org/10.3390/molecules25143105
Chicago/Turabian StyleBarrientos, Ruth E., Mario J. Simirgiotis, Javier Palacios, Adrián Paredes, Jorge Bórquez, Alejandra Bravo, and Fredi Cifuentes. 2020. "Chemical Fingerprinting, Isolation and Characterization of Polyphenol Compounds from Heliotropium taltalense (Phil.) I.M. Johnst and Its Endothelium-Dependent Vascular Relaxation Effect in Rat Aorta" Molecules 25, no. 14: 3105. https://doi.org/10.3390/molecules25143105
APA StyleBarrientos, R. E., Simirgiotis, M. J., Palacios, J., Paredes, A., Bórquez, J., Bravo, A., & Cifuentes, F. (2020). Chemical Fingerprinting, Isolation and Characterization of Polyphenol Compounds from Heliotropium taltalense (Phil.) I.M. Johnst and Its Endothelium-Dependent Vascular Relaxation Effect in Rat Aorta. Molecules, 25(14), 3105. https://doi.org/10.3390/molecules25143105