Increased Adsorption of Heavy Metal Ions in Multi-Walled Carbon Nanotubes with Improved Dispersion Stability
Abstract
1. Introduction
2. Results and Discussion
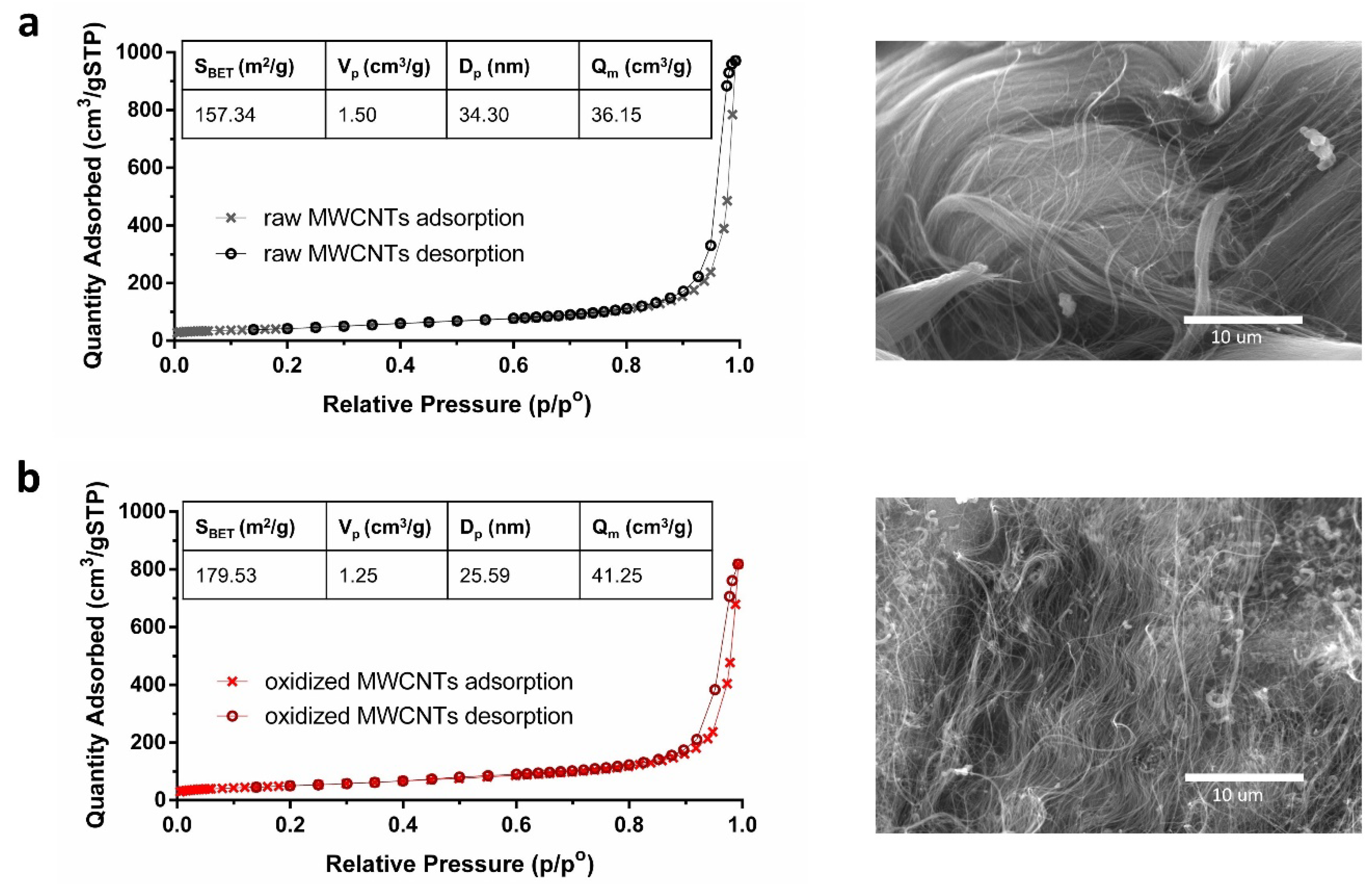
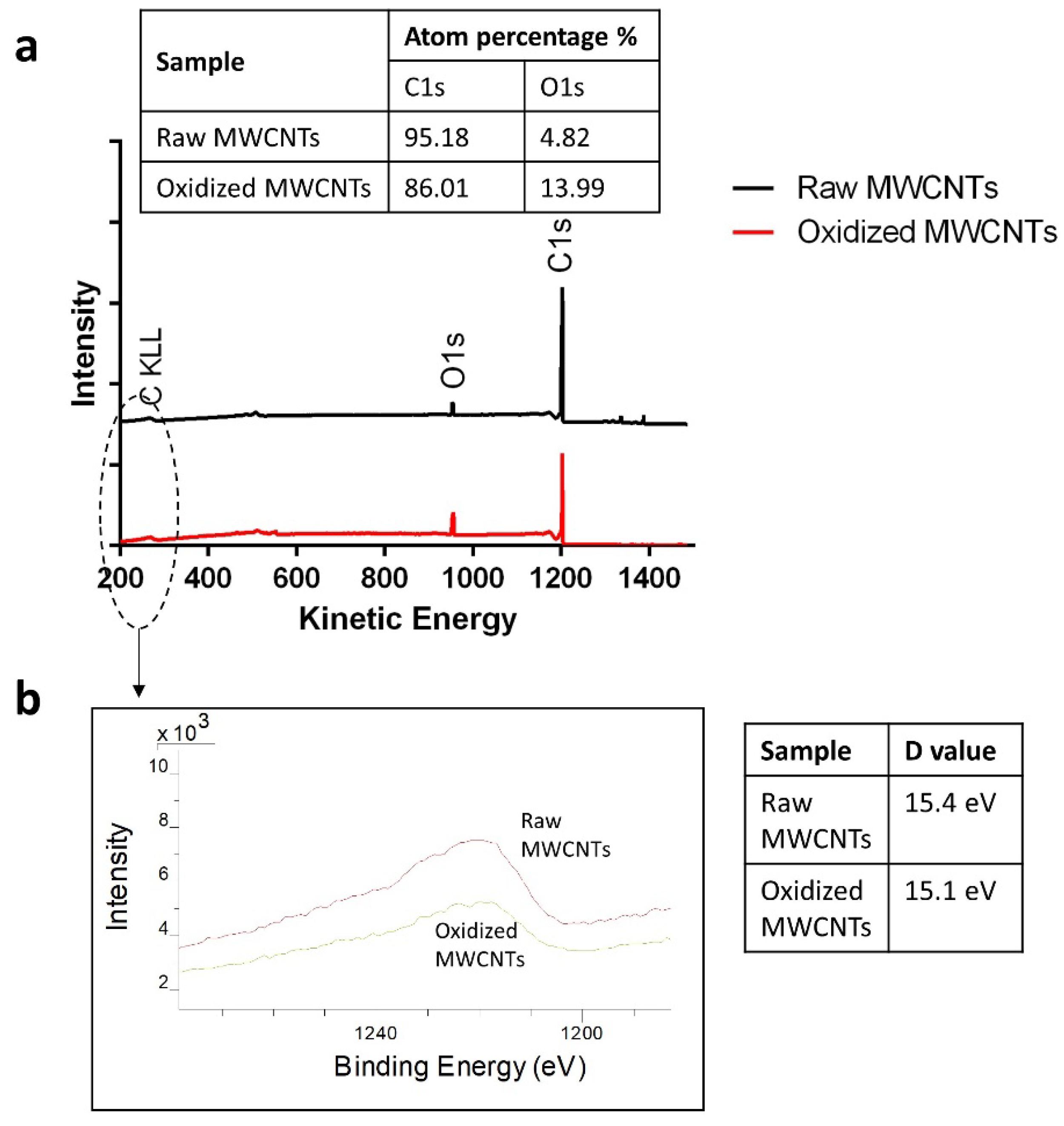
2.1. Characterization of MWCNTs
2.2. Qualitative Analysis of Dispersion
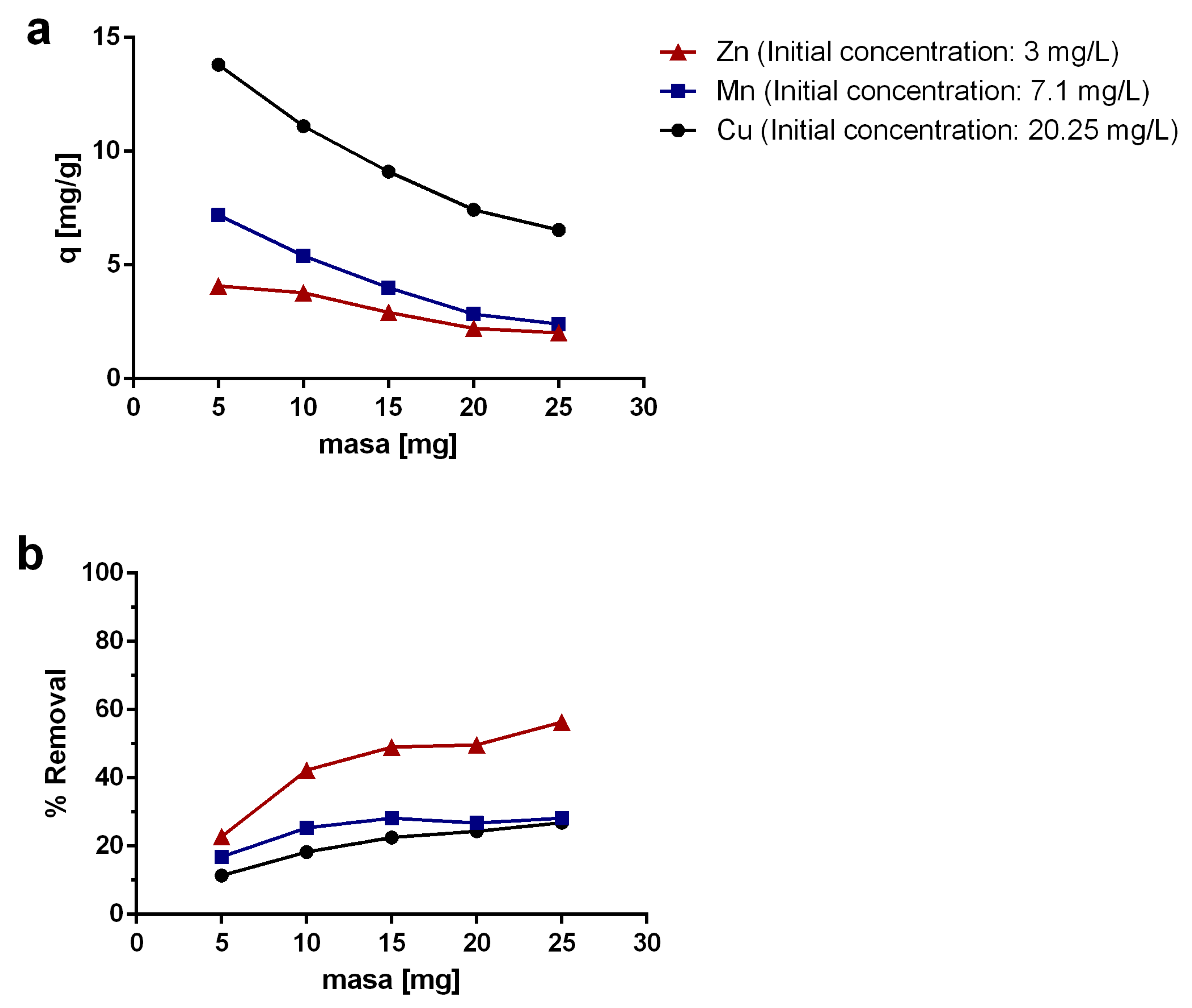
2.3. Effect of the Adsorbent Mass
2.4. Column Experiments
2.5. Kinetics Experiments
3. Materials and Methods
3.1. Characterization of MWCNTs
3.2. MWCNTs Oxidation
3.3. Qualitative Analysis of Dispersion
3.4. Adsorption Experiments
- Co: initial concentration of metal in aqueous solution (mg/L).
- Ce: equilibrium concentration of metal in aqueous solution (mg/L).
- V: total volume of solution (L).
- m: the mass of sorbent (g).
3.5. Effect of the Adsorbent Mass
3.6. Column Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahurpawar, M. Effects of heavy metals on human health. Int. J. Res. Granthaalayah 2015, 530, 1–7. [Google Scholar]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar] [PubMed]
- Ihsanullah, I.; Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Mohapatra, M.; Mohapatra, L.; Singh, P.; Anand, S.; Mishra, B. A comparative study on Pb(II), Cd(II), Cu(II), Co(II) adsorption from single and binary aqueous solutions on additive assisted nano-structured goethite. Int. J. Eng. Sci. Technol. 2011, 2, 89–103. [Google Scholar] [CrossRef]
- Oyarzun, R.; Lillo, J.; Higueras, P.; Oyarzún, J.; Maturana, H. Strong arsenic enrichment in sediments from the Elqui watershed, Northern Chile: Industrial (gold mining at El Indio-Tambo district) vs. geologic processes. J. Geochem. Explor. 2004, 84, 53–64. [Google Scholar] [CrossRef]
- Leiva, E.D.; Rámila, C.d.P.; Vargas, I.T.; Escauriaza, C.R.; Bonilla, C.A.; Pizarro, G.E.; Regan, J.M.; Pasten, P.A. Natural attenuation process via microbial oxidation of arsenic in a high Andean watershed. Sci. Total Environ. 2014, 466, 490–502. [Google Scholar] [CrossRef]
- Leiva, E.; Leiva-Aravena, E.; Rodríguez, C.; Serrano, J.; Vargas, I. Arsenic removal mediated by acidic pH neutralization and iron precipitation in microbial fuel cells. Sci. Total Environ. 2018, 645, 471–481. [Google Scholar] [CrossRef]
- Oyarzún, J.; Oyarzún, R. Sustainable development threats, inter-sector conflicts and environmental policy requirements in the arid, mining rich, northern Chile territory. Sustain. Dev. 2011, 19, 263–274. [Google Scholar] [CrossRef]
- Valdés-Pineda, R.; Pizarro, R.; García-Chevesich, P.; Valdés, J.B.; Olivares, C.; Vera, M.; Balocchi, F.; Pérez, F.; Vallejos, C.; Fuentes, R.; et al. Water governance in Chile: Availability, management and climate change. J. Hydrol. 2014, 519, 2538–2567. [Google Scholar] [CrossRef]
- Aitken, D.; Rivera, D.; Godoy-Faúndez, A.; Holzapfel, E.; Aitken, D.; Rivera, D.; Godoy-Faúndez, A.; Holzapfel, E. Water Scarcity and the Impact of the Mining and Agricultural Sectors in Chile. Sustainability 2016, 8, 128. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Total Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Obreque-Contreras, J.; Pérez-Flores, D.; Gutiérrez, P.; Chávez-Crooker, P. Acid Mine Drainage in Chile: An Opportunity to Apply Bioremediation Technology. J. Waste Water Treat. Anal. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Rodríguez, C.; Leiva-Aravena, E.; Serrano, J.; Leiva, E. Occurrence and Removal of Copper and Aluminum in a Stream Confluence Affected by Acid Mine Drainage. Water 2018, 10, 516. [Google Scholar] [CrossRef]
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Alvarez, P.; Li, Q.; Gardea-Torresdey, J.; Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. Nano 2016, 3, 1241–1253. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Ebert, L.B. Science of fullerenes and carbon nanotubes. Carbon N. Y. 1997, 3, 437–438. [Google Scholar] [CrossRef]
- Peng, X.; Jia, J.; Luan, Z. Oxidized carbon nanotubes for simultaneous removal of endrin and Cd(Π) from water and their separation from water. J. Chem. Technol. Biotechnol. 2009, 84, 275–278. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, B.; Wang, Y.; Morales, V.L.; Carpena, R.M.; Huang, Q.; Yang, L. Deposition and transport of functionalized carbon nanotubes in water-saturated sand columns. J. Hazard. Mater. 2012, 213, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Liu, J.; Yuan, X.; Dong, H.; Zeng, G.; Wu, H.; Wang, H.; Liu, J.; Hua, S.; Zhang, S.; et al. Facile synthesis of alumina-decorated multi-walled carbon nanotubes for simultaneous adsorption of cadmium ion and trichloroethylene. Chem. Eng. J. 2015, 273, 101–110. [Google Scholar] [CrossRef]
- Ihsanullah, I.; Al-Khaldi, F.A.; Abu-Sharkh, B.; Abulkibash, A.M.; Qureshi, M.I.; Laoui, T.; Atieh, M.A. Effect of acid modification on adsorption of hexavalent chromium (Cr(VI)) from aqueous solution by activated carbon and carbon nanotubes. Desalin. Water Treat. 2016, 57, 7232–7244. [Google Scholar] [CrossRef]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]
- Lu, C.; Liu, C. Removal of nickel(II) from aqueous solution by carbon nanotubes. J. Chem. Technol. Biotechnol. 2006, 81, 1932–1940. [Google Scholar] [CrossRef]
- Lu, C.; Chiu, H.; Liu, C. Removal of Zinc(II) from Aqueous Solution by Purified Carbon Nanotubes: Kinetics and Equilibrium Studies. Ind. Eng. Chem. Res. 2006, 45, 2850–2855. [Google Scholar] [CrossRef]
- Liu, X.; Wang, M.; Zhang, S.; Pan, B. Application potential of carbon nanotubes in water treatment: A review. J. Environ. Sci. 2013, 25, 1263–1280. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, B.; Morales, V.L.; Wu, L.; Wang, Y.; Muñoz-Carpena, R.; Cao, C.; Huang, Q.; Yang, L. Methods of using carbon nanotubes as filter media to remove aqueous heavy metals. Chem. Eng. J. 2012, 210, 557–563. [Google Scholar] [CrossRef]
- Stafiej, A.; Pyrzynska, K. Adsorption of heavy metal ions with carbon nanotubes. Sep. Purif. Technol. 2007, 58, 49–52. [Google Scholar] [CrossRef]
- Sharma, P.; Ahuja, P. Recent advances in carbon nanotube-based electronics. Mater. Res. Bull. 2008, 43, 2517–2526. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Ge, Y. Removal of lead ion and oil droplet from aqueous solution by lignin-grafted carbon nanotubes. Chem. Eng. J. 2017, 308, 809–817. [Google Scholar] [CrossRef]
- Sazali, N.; Harun, Z.; Sazali, N. A Review on Batch and Column Adsorption of Various Adsorbent towards the Removal of Heavy Metal. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 67, 66–88. [Google Scholar]
- Ouni, L.; Ramazani, A.; Taghavi Fardood, S. An overview of carbon nanotubes role in heavy metals removal from wastewater. Front. Chem. Sci. Eng. 2019, 13, 274–295. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S. Removal of heavy metals from wastewater using carbon nanotubes. Sep. Purif. Rev. 2014, 43, 311–338. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Deng, S.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.-W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004, 77, 126–134. [Google Scholar] [CrossRef]
- Wick, P.; Manser, P.; Limbach, L.K.; Dettlaff-Weglikowska, U.; Krumeich, F.; Roth, S.; Stark, W.J.; Bruinink, A. The degree and kind of agglomeration affect carbon nanotube cytotoxicity. Toxicol. Lett. 2007, 168, 121–131. [Google Scholar] [CrossRef]
- Rodríguez, C.; Leiva, E. Enhanced Heavy Metal Removal from Acid Mine Drainage Wastewater Using Double-Oxidized Multiwalled Carbon Nanotubes. Molecules 2019, 25, 111. [Google Scholar] [CrossRef] [PubMed]
- Kandah, M.I.; Meunier, J.L. Removal of nickel ions from water by multi-walled carbon nanotubes. J. Hazard. Mater. 2007, 146, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Yavari, R.; Huang, Y.D.; Ahmadi, S.J. Adsorption of cesium (I) from aqueous solution using oxidized multiwall carbon nanotubes. J. Radioanal. Nucl. Chem. 2011, 287, 393–401. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, S.; Luan, Z.; Ding, J.; Xu, C.; Wu, D. Adsorption of cadmium(II) from aqueous solution by surface oxidized carbon nanotubes. Carbon N. Y. 2003, 41, 1057–1062. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Gao, Z.; Bandosz, T.J.; Zhao, Z.; Han, M.; Qiu, J. Investigation of factors affecting adsorption of transition metals on oxidized carbon nanotubes. J. Hazard. Mater. 2009, 167, 357–365. [Google Scholar] [CrossRef]
- Martínez, M.T.; Callejas, M.A.; Benito, A.M.; Cochet, M.; Seeger, T.; Ansón, A.; Schreiber, J.; Gordon, C.; Marhic, C.; Chauvet, O.; et al. Sensitivity of single wall carbon nanotubes to oxidative processing: Structural modification, intercalation and functionalisation. Carbon N. Y. 2003, 41, 2247–2256. [Google Scholar] [CrossRef]
- Jung, A.; Graupner, R.; Ley, L.; Hirsch, A. Quantitative determination of oxidative defects on single walled carbon nanotubes. Phys. Status Solidi 2006, 243, 3217–3220. [Google Scholar] [CrossRef]
- Saleh, T.A. The influence of treatment temperature on the acidity of MWCNT oxidized by HNO 3 or a mixture of HNO 3/H 2 SO 4. Appl. Surf. Sci. 2011, 257, 7746–7751. [Google Scholar] [CrossRef]
- Xia, W.; Wang, Y.; Bergsträßer, R.; Kundu, S.; Muhler, M. Surface characterization of oxygen-functionalized multi-walled carbon nanotubes by high-resolution X-ray photoelectron spectroscopy and temperature-programmed desorption. Appl. Surf. Sci. 2007, 254, 247–250. [Google Scholar] [CrossRef]
- Liang, S.; Li, G.; Tian, R. Multi-walled carbon nanotubes functionalized with a ultrahigh fraction of carboxyl and hydroxyl groups by ultrasound-assisted oxidation. J. Mater. Sci. 2016, 51, 3513–3524. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon N. Y. 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Shin, Y.R.; Jeon, I.Y.; Baek, J.B. Stability of multi-walled carbon nanotubes in commonly used acidic media. Carbon N. Y. 2012, 50, 1465–1476. [Google Scholar] [CrossRef]
- Mezzi, A.; Kaciulis, S. Surface investigation of carbon films: From diamond to graphite. Surf. Interface Anal. 2010, 42, 1082–1084. [Google Scholar] [CrossRef]
- Lu, K.L.; Lago, R.M.; Chen, Y.K.; Green, M.L.H.; Harris, P.J.F.; Tsang, S.C. Mechanical damage of carbon nanotubes by ultrasound. Carbon N. Y. 1996, 34, 814–816. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128, 37–46. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Zhu, J.; Kim, J.D.; Peng, H.; Margrave, J.L.; Khabashesku, V.N.; Barrera, E.V. Improving the dispersion and integration of single-walled carbon nanotubes in epoxy composites through functionalization. Nano Lett. 2003, 3, 1107–1113. [Google Scholar] [CrossRef]
- Vaisman, L.; Marom, G.; Wagner, H.D. Dispersions of Surface-Modified Carbon Nanotubes in Water-Soluble and Water-Insoluble Polymers. Adv. Funct. Mater. 2006, 16, 357–363. [Google Scholar] [CrossRef]
- Brandao, S.D.F.; Andrada, D.; Mesquita, A.F.; Santos, A.P.; Gorgulho, H.F.; Paniago, R.; Pimenta, M.A.; Fantini, C.; Furtado, C.A. The influence of oxygen-containing functional groups on the dispersion of single-walled carbon nanotubes in amide solvents. J. Phys. Condens. Matter 2010, 22, 334222. [Google Scholar] [CrossRef]
- Sezer, N.; Koç, M. Stabilization of the aqueous dispersion of carbon nanotubes using different approaches. Therm. Sci. Eng. Prog. 2018, 8, 411–417. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Hong, C.K.; Shim, S.E. Measurement of the dispersion stability of pristine and surface-modified multiwalled carbon nanotubes in various nonpolar and polar solvents. Meas. Sci. Technol. 2007, 18, 3707. [Google Scholar] [CrossRef]
- Naseh, M.V.; Khodadadi, A.A.; Mortazavi, Y.; Sahraei, O.A.; Pourfayaz, F.; Sedghi, S.M. Functionalization of carbon nanotubes using nitric acid oxidation and DBD plasma. World Acad. Sci. Eng. Technol. 2009, 49, 177–179. [Google Scholar]
- Dehghani, M.H.; Mostofi, M.; Alimohammadi, M.; McKay, G.; Yetilmezsoy, K.; Albadarin, A.B.; Heibati, B.; AlGhouti, M.; Mubarak, N.M.; Sahu, J.N. High-performance removal of toxic phenol by single-walled and multi-walled carbon nanotubes: Kinetics, adsorption, mechanism and optimization studies. J. Ind. Eng. Chem. 2016, 35, 63–74. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Sheng, G.; Hu, J.; Tan, X.; Wang, X. Effect of surfactants on Pb(II) adsorption from aqueous solutions using oxidized multiwall carbon nanotubes. Chem. Eng. J. 2011, 166, 551–558. [Google Scholar] [CrossRef]
- Chen, S.J.; Qiu, C.Y.; Korayem, A.H.; Barati, M.R.; Duan, W.H. Agglomeration process of surfactant-dispersed carbon nanotubes in unstable dispersion: A two-stage agglomeration model and experimental evidence. Powder Technol. 2016, 301, 412–420. [Google Scholar] [CrossRef]
- Tiwari, M.D.; Sagar, G.H.; Bellare, J.R. Flow cytometry-based evaluation and enrichment of multiwalled carbon nanotube dispersions. Langmuir 2012, 28, 4939–4947. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Zimmerman, A.R.; Lee, X. Impregnation of multiwall carbon nanotubes in alginate beads dramatically enhances their adsorptive ability to aqueous methylene blue. Chem. Eng. Res. Des. 2018, 133, 235–242. [Google Scholar] [CrossRef]
- Khan, T.A.; Nazir, M.; Khan, E.A. Magnetically modified multiwalled carbon nanotubes for the adsorption of bismarck brown R and Cd(II) from aqueous solution: Batch and column studies. Desalin. Water Treat. 2016, 57, 19374–19390. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef]
- Warheit, D.B.; Laurence, B.R.; Reed, K.L.; Roach, D.H.; Reynolds, G.A.M.; Webb, T.R. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 2004, 77, 117–125. [Google Scholar] [CrossRef]
- Aschberger, K.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Hankin, S.M.; Peters, S.A.K.; Tran, C.L.; Christensen, F.M. Review of carbon nanotubes toxicity and exposure-appraisal of human health risk assessment based on open literature. Crit. Rev. Toxicol. 2010, 40, 759–790. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.C.; Chung, F.F.L.; Tan, Y.F.; Leong, C.O. Toxicity of single-walled carbon nanotubes. Arch. Toxicol. 2016, 90, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Helland, A.; Wick, P.; Koehler, A.; Schmid, K.; Som, C. Reviewing the environmental and human health knowledge base of carbon nanotubes. Environ. Health Perspect. 2007, 115, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Wang, S.; You, H.; Zhao, X. Understanding the toxicity of carbon nanotubes in the environment is crucial to the control of nanomaterials in producing and processing and the assessment of health risk for human: A review. Environ. Toxicol. Pharmacol. 2013, 36, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Di, Z.; Ding, J.; Wu, D.; Luan, Z.; Zhu, Y. Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Res. 2005, 39, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Liu, Y.; Guo, L.; Zeng, J.; Lu, H. Multiwalled carbon nanotubes as solid-phase extraction adsorbent for the preconcentration of trace metal ions and their determination by inductively coupled plasma atomic emission spectrometry. J. Anal. At. Spectrom. 2004, 19, 1489–1492. [Google Scholar] [CrossRef]
- Sprenger, D.; Anderson, O. Deconvolution of XPS spectra. Fresenius. J. Anal. Chem. 1991, 341, 116–120. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Sorption Capacity q (mg/g Sorbent) | |||
|---|---|---|---|
| Cu2+ | Mn2+ | Zn2+ | |
| Column experiments (MWCNTs on a support (filter), multimetallic water) | 35.2 ± 1.8 | 9.2 ± 0.8 | 4.4 ± 0.6 |
| Competition experiments (suspended MWCNTs, multimetallic water) | 5.0 ± 1.0 | 1.2 ± 0.2 | 0.1 ± 0.05 |
| Adsorption experiments (suspended MWCNTs, monometallic water) | 7.3 ± 0.6 | 2.8 ± 0.1 | 2.2 ± 0.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, C.; Briano, S.; Leiva, E. Increased Adsorption of Heavy Metal Ions in Multi-Walled Carbon Nanotubes with Improved Dispersion Stability. Molecules 2020, 25, 3106. https://doi.org/10.3390/molecules25143106
Rodríguez C, Briano S, Leiva E. Increased Adsorption of Heavy Metal Ions in Multi-Walled Carbon Nanotubes with Improved Dispersion Stability. Molecules. 2020; 25(14):3106. https://doi.org/10.3390/molecules25143106
Chicago/Turabian StyleRodríguez, Carolina, Sebastián Briano, and Eduardo Leiva. 2020. "Increased Adsorption of Heavy Metal Ions in Multi-Walled Carbon Nanotubes with Improved Dispersion Stability" Molecules 25, no. 14: 3106. https://doi.org/10.3390/molecules25143106
APA StyleRodríguez, C., Briano, S., & Leiva, E. (2020). Increased Adsorption of Heavy Metal Ions in Multi-Walled Carbon Nanotubes with Improved Dispersion Stability. Molecules, 25(14), 3106. https://doi.org/10.3390/molecules25143106






