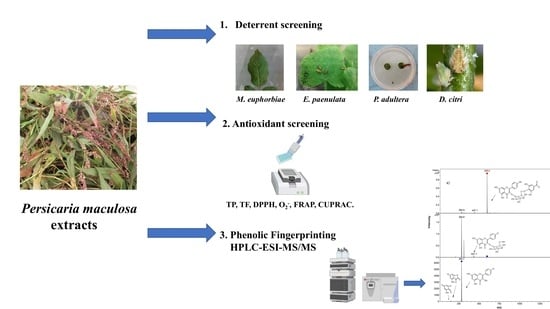
Phenolic Fingerprinting, Antioxidant, and Deterrent Potentials of Persicaria maculosa Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic (TP) and Total Flavonoid (TF) Content and Antioxidant Activity
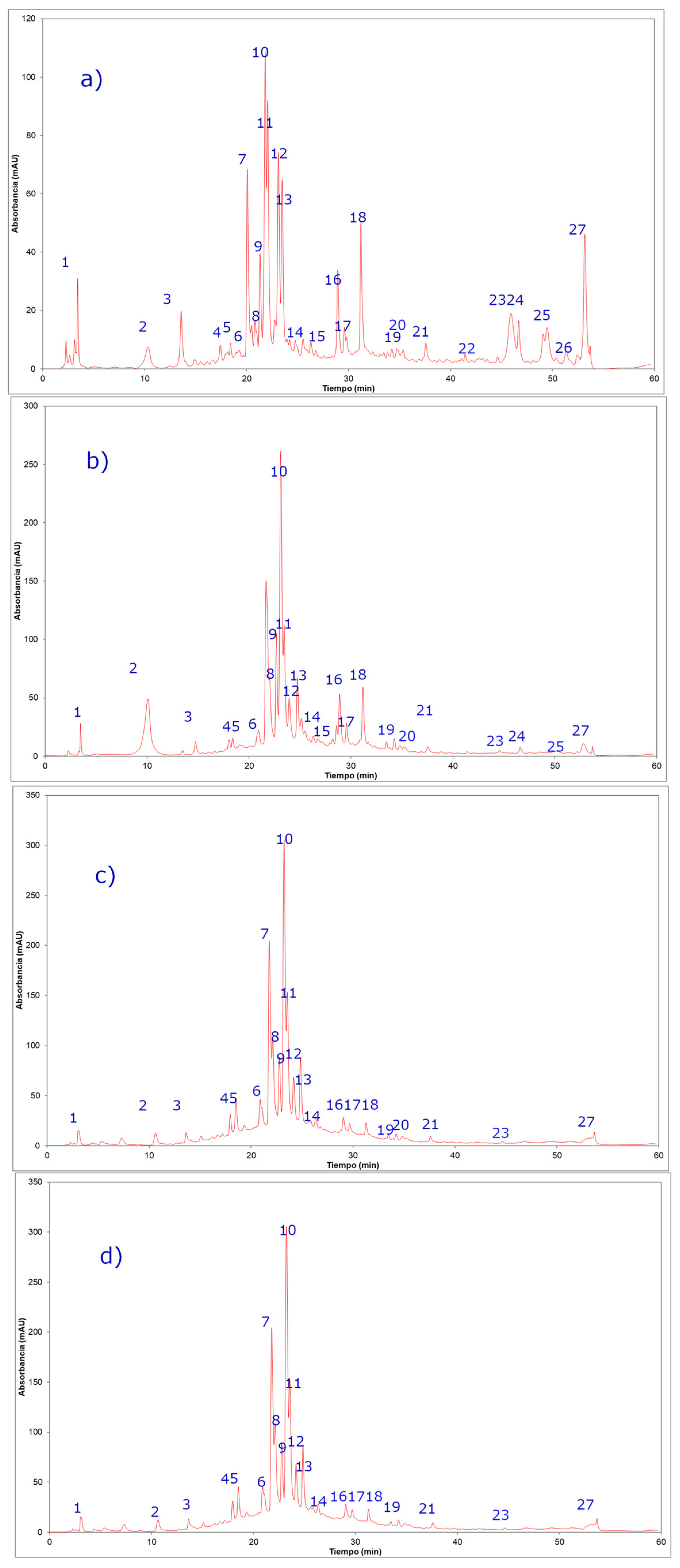
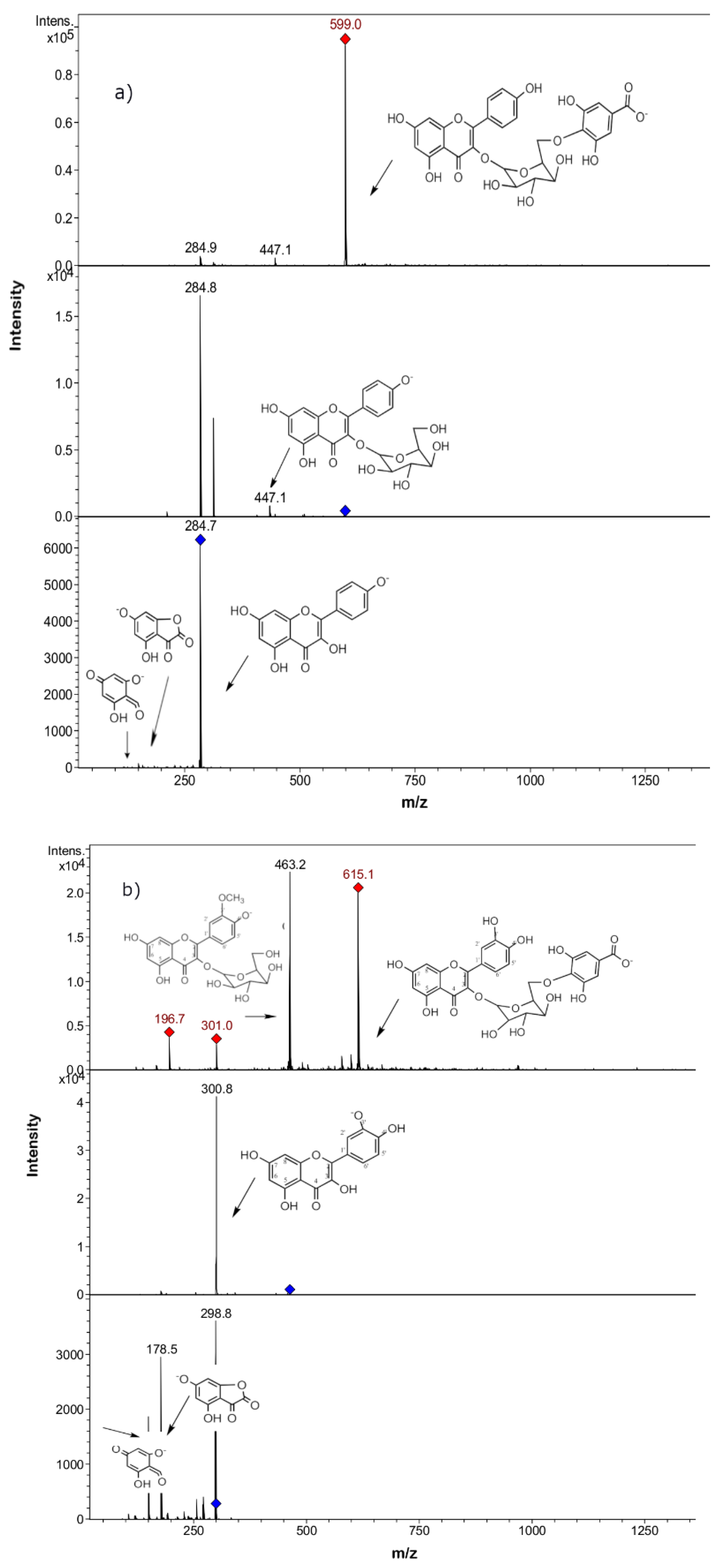
2.2. Phenolic Compounds Identification in Persicaria maculosa Extracts by HPLC-ESI-MS/MS
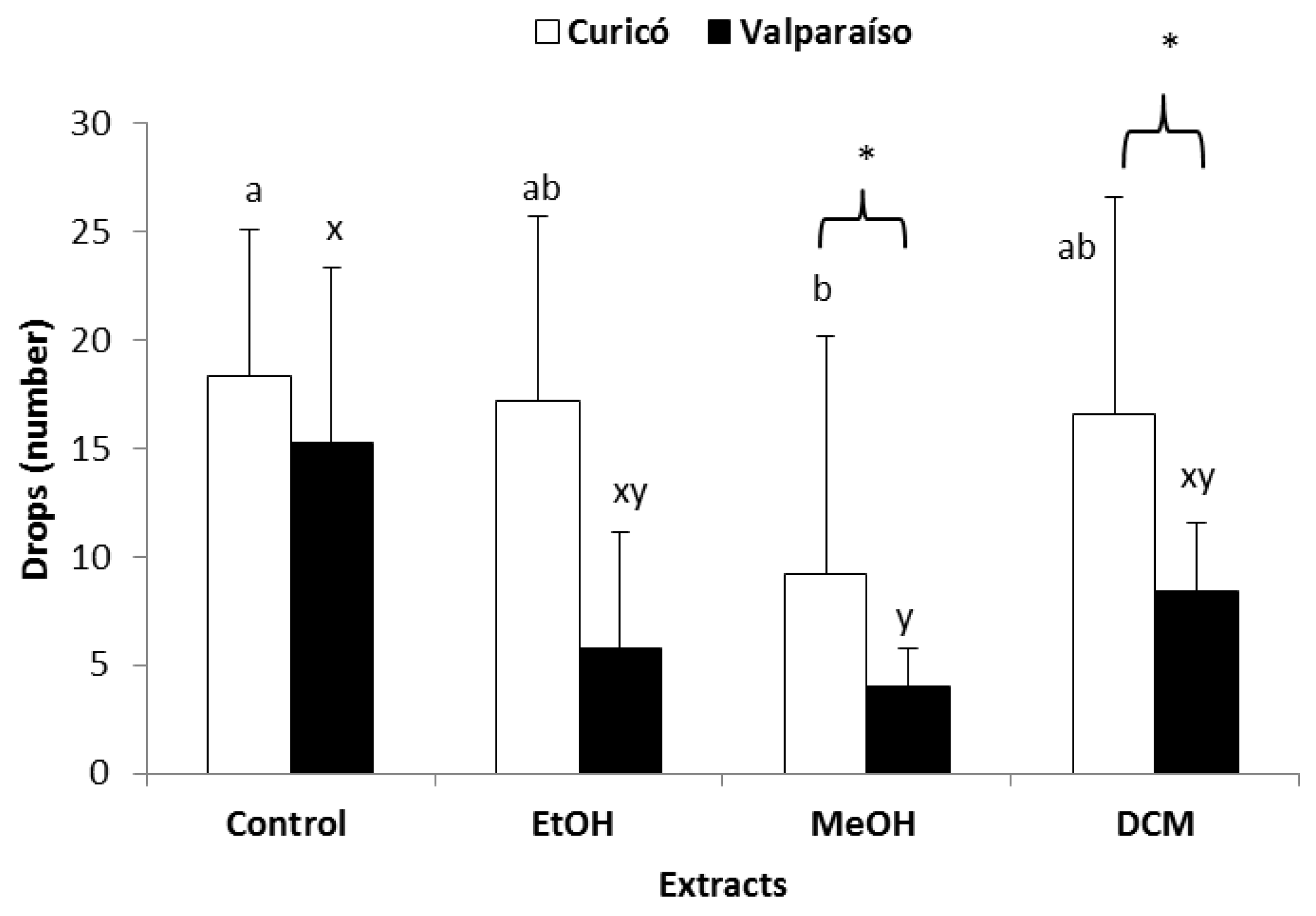
2.3. Antifeedant Activity
2.3.1. Antifeedant Activity against Chewing Insects (E. paenulata and P. adultera)
2.3.2. Antifeedant Activity against Sucking Insects (Diaphorina citri)
2.3.3. Anti-settling Activity against Sucking Insects (Macrosiphum euphorbiae)
3. Materials and Methods
3.1. Plant Material
3.2. Extracts Preparations
3.3. Total Phenolic (TP) and Total Flavonoid (TF) Content
3.4. Antioxidant Activity
3.5. HPLC-ESI-MS/MS Analysis
3.6. Insects
3.7. Antifeedant Bioassay (Choice Experiment)
3.8. Aphid Preference Bioassays (Choice Experiment)
3.9. Antifeedant Activity Evaluation on D. citri Adults (Choice Experiment)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oany, A.R.; Siddikey, A.A.; Hossain, M.U.; Islam, R.; Emran, A.-A. A preliminary evaluation of cytotoxicity, antihyperglycemic and antinociceptive activity of Polygonum hydropiper L. ethanolic leaf extract. Clin. Phytoscience 2017, 2, 2. [Google Scholar] [CrossRef]
- Narasimhulu, G.; Reddy, K.K.; Mohamed, J. The genus Polygonum (Polygonaceae): An ethnopharmacological and phytochemical perspectives - Review. Int. J. Pharm. Pharm. Sci. 2014, 6, 21–45. [Google Scholar]
- Duwiejua, M.; Zeitlin, I.J.; Gray, A.I.; Waterman, P.G. The anti-inflammatory compounds of Polygonum bistorta: Isolation and characterisation. Planta Med. 1999, 65, 371–374. [Google Scholar] [CrossRef]
- Shen, B.-B.; Yang, Y.-P.; Yasamin, S.; Liang, N.; Su, W.; Chen, S.-H.; Wang, X.-J.; Wang, W. Analysis of the Phytochemistry and Bioactivity of the Genus Polygonum of Polygonaceae. Digit. Chin. Med. 2018, 1, 19–36. [Google Scholar] [CrossRef]
- Sun, X.; Sneden, A. Neoflavonoids from Polygonum perfoliatum. Planta Med. 1999, 65, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Q.W.; Wang, L.; Zhang, X.Q.; Ye, W.C.; Wang, Y.T. Two new anthraquinone malonylglucosides from Polygonum cuspidatum. Nat. Prod. Res. 2012, 26, 1323–1327. [Google Scholar] [CrossRef]
- Murai, Y.; Kashimura, S.; Tamezawa, S.; Hashimoto, T.; Takaoka, S.; Asakawa, Y.; Kiguchi, K.; Murai, F.; Tagawa, M. Absolute configuration of (6S,9S)-roseoside from polygonum hydropiper. Planta Med. 2001, 67, 480–481. [Google Scholar] [CrossRef]
- Liu, X.Q.; Hua, H.M.; Liu, J.; Chen, F.K.; Wu, L.J. A new tannin-related compound from the rhizome of Polygonum bistorta L. J. Asian Nat. Prod. Res. 2006, 8, 299–302. [Google Scholar] [CrossRef]
- Wang, K.W.; Zhu, J.R.; Shen, L.Q. A new lignan with anti-tumour activity from Polygonum perfoliatum L. Nat. Prod. Res. 2013, 27, 568–573. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Zyzyńska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef]
- Jovanović, M.; Morić, I.; Nikolić, B.; Pavić, A.; Svirčev, E.; Šenerović, L.; Mitić-Ćulafić, D. Anti-Virulence Potential and in Vivo Toxicity of Persicaria maculosa and Bistorta officinalis Extracts. Molecules 2020, 25, 1811. [Google Scholar] [CrossRef] [PubMed]
- Datta, B.K.; Datta, S.K.; Rashid, M.A.; Nash, R.J.; Sarker, S.D. A sesquiterpene acid and flavonoids from Polygonum viscosum. Phytochemistry 2000, 54, 201–205. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. Bmc Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, F.; Feng, Z.; Yang, Y.; Jiang, J.; Li, L.; Zhang, P. Neuroprotective naphthalene and flavan derivatives from Polygonum cuspidatum. Phytochemistry 2015, 110, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ahmad, I.; Sadiq, A.; Ullah, F.; Ovais, M.; Khalil, A.T.; Devkota, H.P. Persicaria hydropiper (L.) Delarbre: A review on traditional uses, bioactive chemical constituents and pharmacological and toxicological activities. J. Ethnopharmacol. 2020, 251, 112516. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Jain, D.C.; Singh, S.C. Persistency of bioactive fractions of Indian plant, Polygonum hydropiper as an insect feeding deterrent. Phyther. Res. 1999, 13, 239–241. [Google Scholar] [CrossRef]
- Roy, S.; Gurusubramanian, G.; Nachimuthu, S.K. Anti-mite activity of Polygonum hydropiper L. (Polygonaceae) extracts against tea red spider mite, Oligonychus coffeae Nietner (Tetranychidae: Acarina). Int. J. Acarol. 2011, 37, 561–566. [Google Scholar] [CrossRef]
- Hussain, F.; Ahmad, B.; Hameed, I.; Dastagir, G.; Sanaullah, P.; Azam, S. Antibacterial, antifungal and insecticidal activities of some selected medicinal plants of polygonaceae. Afr. J. Biotechnol. 2010, 9, 5032–5036. [Google Scholar]
- Rahimi, V.; Hajizadeh, J.; Zibaee, A.; Sendi, J.J. Effect of Polygonum persicaria (Polygonales: Polygonaceae) Extracted Agglutinin on Life Table and Antioxidant Responses in Helicoverpa armigera (Lepidoptera: Noctuidae) Larvae. J. Econ. Entomol. 2018, 111, 662–671. [Google Scholar] [CrossRef]
- Kurkina, A.V.; Ryazanova, T.K.; Kurkin, V.A. Flavonoids from the Aerial Part of Polygonum persicaria. Chem. Nat. Compd. 2013, 49, 845–847. [Google Scholar] [CrossRef]
- Lajter, I.; Vasas, A.; Orvos, P.; Bánsághi, S.; Tálosi, L.; Jakab, G.; Béni, Z.; Háda, V.; Forgo, P.; Hohmann, J. Inhibition of G Protein-Activated Inwardly Rectifying K+ Channels by Extracts of Polygonum persicaria and Isolation of New Flavonoids from the Chloroform Extract of the Herb. Planta Med. 2013, 79, 1736–1741. [Google Scholar] [CrossRef]
- Hussein, S.; EL-Magly, U.; Tantawy, M.; Kawashty, S.; Saleh, N. Phenolics of selected species of Persicaria and Polygonum (Polygonaceae) in Egypt. Arab. J. Chem. 2017, 10, 76–81. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Direct identification of phenolic constituents in Boldo Folium (Peumus boldus Mol.) infusions by high-performance liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Shad, M.A.; Rauf, A. Optimization of extraction yield and antioxidant properties of Brassica oleracea Convar Capitata Var L. leaf extracts. Food Chem. 2018, 242, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean (Phaseolus vulgaris) seeds. Brazilian J. Pharm. Sci. 2020, 56. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Fan, P.; Terrier, L.; Hay, A.-E.; Marston, A.; Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar] [CrossRef]
- Noor Hashim, N.H.; Abas, F.; Shaari, K.; Lajis, N.H. LC–DAD–ESIMS/MS characterization of antioxidant and anticholinesterase constituents present in the active fraction from Persicaria hydropiper. Lwt Food Sci. Technol. 2012, 46, 468–476. [Google Scholar] [CrossRef]
- Peng, Z.F.; Strack, D.; Baumert, A.; Subramaniam, R.; Goh, N.K.; Chia, T.F.; Tan, S.N.; Chia, L.S. Antioxidant flavonoids from leaves of Polygonum hydropiper L. Phytochemistry 2003, 62, 219–228. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Boughalleb, F.; Mabrouk, M.; Tlili, N.; Potter, D.; Abdellaoui, R.; Nasri, N. Chemical analysis of the antioxidants from the aerial parts of wild Polygonum equisetiforme from Tunisia. Food Biosci. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Barros, L.; Boumehira, A.Z.; Bachari, K.; Heleno, S.A.; Alves, M.J.; Ferreira, I.C.F.R. Profiling polyphenol composition by HPLC-DAD-ESI/MSn and the antibacterial activity of infusion preparations obtained from four medicinal plants. Food Funct. 2018, 9, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; Kim, B.G.; Yoon, J.A.; Chong, Y.; Ahn, J.-H. Synthesis of flavonoid O-pentosides by Escherichia coli through engineering of nucleotide sugar pathways and glycosyltransferase. Appl. Env. Microbiol. 2014, 80, 2754–2762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, J.; Jiang, Q.; Wang, X.; Lu, Y.; Gong, L.; Chen, D. Simultaneous determination of three active components in rat plasma by UPLC-MS/MS: Application to pharmacokinetic study after oral administration of Herba Sarcandrae extract. Biomed. Chromatogr. 2017, 31, e3834. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Wilson, T.; Luthria, D.; Freeman, M.R.; Scott, R.M.; Bilenker, D.; Shah, S.; Somasundaram, S.; Vorsa, N. LC-MS–MS characterisation of curry leaf flavonols and antioxidant activity. Food Chem. 2011, 127, 80–85. [Google Scholar] [CrossRef]
- Sonibare, M.A.; Ayoola, I.O.; Gueye, B.; Abberton, M.T.; D’Souza, R.; Kuhnert, N. Leaves metabolomic profiling of Musa acuminata accessions using UPLC–QTOF–MS/MS and their antioxidant activity. J. Food Meas. Charact. 2018, 12, 1093–1106. [Google Scholar] [CrossRef]
- Saldanha, L.; Vilegas, W.; Dokkedal, A.; Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of Flavonoids and Phenolic Acids in Myrcia bella Cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS Combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef]
- Francescato, L.N.; Debenedetti, S.L.; Schwanz, T.G.; Bassani, V.L.; Henriques, A.T. Identification of phenolic compounds in Equisetum giganteum by LC–ESI-MS/MS and a new approach to total flavonoid quantification. Talanta 2013, 105, 192–203. [Google Scholar] [CrossRef]
- Pasini, F.; Verardo, V.; Caboni, M.F.; D’Antuono, L.F. Determination of glucosinolates and phenolic compounds in rocket salad by HPLC-DAD–MS: Evaluation of Eruca sativa Mill. and Diplotaxis tenuifolia L. genetic resources. Food Chem. 2012, 133, 1025–1033. [Google Scholar] [CrossRef]
- Koolen, H.H.F.; da Silva, F.M.A.; Gozzo, F.C.; de Souza, A.Q.L.; de Souza, A.D.L. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC-ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar] [CrossRef]
- Camarano, S.; Gonzalez, A.; Rossini, C. Biparental endowment of endogenous defensive alkaloids in Epilachna paenulata. J. Chem. Ecol. 2009, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, G.; Altesor, P.; McNeil, J.N.; González, A. Conspecific females promote calling behavior in the noctuid moth, Pseudaletia adultera. Entomol. Exp. Appl. 2016, 159, 362–369. [Google Scholar] [CrossRef]
- Beetge, L.; Krüger, K. Drought and heat waves associated with climate change affect performance of the potato aphid Macrosiphum euphorbiae. Sci. Rep. 2019, 9, 3645. [Google Scholar] [CrossRef] [PubMed]
- Keppanan, R.; Krutmuang, P.; Sivaperumal, S.; Hussain, M.; Bamisile, B.S.; Aguila, L.C.R.; Dash, C.K.; Wang, L. Synthesis of mycotoxin protein IF8 by the entomopathogenic fungus Isaria fumosorosea and its toxic effect against adult Diaphorina citri. Int. J. Biol. Macromol. 2019, 125, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Carpinella, M.C.; Defago, M.T.; Valladares, G.; Palacios, S.M. Antifeedant and insecticide properties of a limonoid from Melia azedarach (Meliaceae) with potential use for pest management. J. Agric. Food Chem. 2003, 51, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Inocente, E.A.; Nguyen, B.; Manwill, P.K.; Benatrehina, A.; Kweka, E.; Wu, S.; Cheng, X.; Rakotondraibe, L.H.; Piermarini, P.M. Insecticidal and Antifeedant Activities of Malagasy Medicinal Plant (Cinnamosma sp.) Extracts and Drimane-Type Sesquiterpenes against Aedes aegypti Mosquitoes. Insects 2019, 10, 373. [Google Scholar] [CrossRef]
- Mongkol, R.; Chavasiri, W. Antimicrobial, herbicidal and antifeedant activities of mansonone E from the heartwoods of Mansonia gagei Drumm. J. Integr. Agric. 2016, 15, 2795. [Google Scholar] [CrossRef]
- Pavunraj, M.; Baskar, K.; Paulkumar, K.; Janarthanan, S.; Rajendran, P. Antifeedant activity of crude extracts and fractions isolated from Catharanthus roseus leaf against spotted bollworm, Earias vittella. Phytoparasitica 2016, 44, 419–422. [Google Scholar] [CrossRef]
- Eman, R.E.; Abdelaziz, E.; Emad, M.A.; Ahmed, M.H.A. Antioxidant, antimicrobial and antifeedant activity of phenolic compounds accumulated in Hyoscyamus muticus L. Afr. J. Biotechnol. 2018, 17, 311–321. [Google Scholar] [CrossRef]
- Adebote, D.; Amupitan, J.; Oyewale, A.; Agbaji, A. Antifeedant Activity of Quercetin Isolated from the Stem Bark of Bobgunnia madagascariensis (Desv.) J.H.Kirkbr & Wiersema. (Caesalpiniaceae). Aust. J. Basic Appl. Sci. 2010, 4, 3342–3346. [Google Scholar]
- Ohmura, W.; Doi, S.; Aoyama, M.; Ohara, S. Antifeedant activity of flavonoids and related compounds against the subterranean termite Coptotermes formosanus Shiraki. J. Wood Sci. 2000, 46, 149–153. [Google Scholar] [CrossRef]
- Nenaah, G.E. Potential of using flavonoids, latex and extracts from Calotropis procera (Ait.) as grain protectants against two coleopteran pests of stored rice. Ind. Crop. Prod. 2013, 45, 327–334. [Google Scholar] [CrossRef]
- Morimoto, M.; Kumeda, S.; Komai, K. Insect antifeedant flavonoids from Gnaphalium affine D. Don. J. Agric. Food Chem. 2000, 48, 1888–1891. [Google Scholar] [CrossRef]
- Chen, W.S.; Yang, G.J.; Zhang, W.D.; Chen, H.S.; Qiao, C.Z. A new fatty ketone of Radix Polygoni Multiflori preparata. Zhongguo Zhong Yao Za Zhi 2000, 25, 476–477. [Google Scholar] [PubMed]
- Silva, G.; Lagunes, A.Y. Control de Sitophilus zeamais (Coleóptera: Curculionidae) con polvos vegetales solos y en mezcla con carbonato de calcio en maíz almacenado. Cienc. E Investig. Agrar. 2003, 30, 153–160. [Google Scholar] [CrossRef]
- Simmonds, M.S.J. Flavonoid-insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Morimoto, M.; Tanimoto, K.; Nakano, S.; Ozaki, T.; Nakano, A.; Komai, K. Insect Antifeedant Activity of Flavones and Chromones against Spodoptera litura. J. Agric. Food Chem. 2003, 51, 389–393. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Quispe, C.; Maria del Pilar, C.S.; Gonzalez, J.F.; Hüneke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC–DAD–MS/MSn. Food Res. Int. 2014, 62, 286–298. [Google Scholar]
- Simirgiotis, M.J.; Bórquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC–DAD–ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequén. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef]
- Cheel, J.; Theoduloz, C.; Rodríguez, J.A.; Caligari, P.D.S.; Schmeda-Hirschmann, G. Free radical scavenging activity and phenolic content in achenes and thalamus from Fragaria chiloensis ssp. chiloensis, F. vesca and F. x ananassa cv. Chandler. Food Chem. 2007, 102, 36–44. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Vieira, M.N.; Rodríguez-Werner, M.A.; Schmalfuss, E.; Winterhalter, P.; Schmeda-Hirschmann, G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in human AGS cells. J. Funct. Foods 2016, 26, 11–26. [Google Scholar]
- Díaz, M.; Castillo, L.; Díaz, C.E.; Álvarez, R.G.; González-Coloma, A.; Rossini, C. Differential deterrent activity of natural products isolated from Allophylus edulis (Sapindaceae). Adv. Biol. Chem. 2014, 4, 168–179. [Google Scholar] [CrossRef][Green Version]
- Altesor, P.; Garcia, A.; Font, E.; Rodriguez-Haralambides, A.; Vilaro, F.; Oesterheld, M.; Soler, R.; Gonzalez, A. Glycoalkaloids of wild and cultivated Solanum: Effects on specialist and generalist insect herbivores. J. Chem. Ecol. 2014, 40, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Boina, D.R.; Onagbola, E.O.; Salyani, M.; Stelinski, L.L. Antifeedant and sublethal effects of imidacloprid on Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2009, 65, 870–877. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the extracts and plants are available from the authors. |
| P. maculosa Extracts | Total Phenolics (g GAE/100 g Extract) | Total Flavonoids (g CE/100 g Extract) |
|---|---|---|
| EtOH extract from Curicó | 16.6 ± 0.2 a | 8.4± 0.1 a |
| EtOH extract from Valparaíso | 13.7 ± 0.5 b | 6.6 ± 0.2 b |
| MeOH extract from Curicó | 14.3 ± 0.9 b | 8.2 ± 0.2 a |
| MeOH extract from Valparaíso | 9.6 ± 0.2 c | 8.6 ± 0.2 a |
| P. persicaria Extracts | DPPH Scavenging SC50 (µg/mL) | O2− Scavenging SC50 (µg/mL) | FRAP (mmol TE/g Extract) | CUPRAC (mmol TE/g Extract) |
|---|---|---|---|---|
| EtOH extract from Curicó | 12.5 ± 0.2 a | 22.1 ± 0.6 a | 1.6 ± 0.1 a | 0.8 ± 0.1 a |
| EtOH extract from Valparaíso | 14.2 ± 0.2 a,b | 34.1 ± 3.2 b | 0.8 ± 0.1 b | 0.7 ± 0.1 a |
| MeOH extract from Curicó | 15.5 ± 1.1 b,c | 43.1 ± 1.6 c | 0.8 ± 0.1 b | 0.7 ± 0.1 a |
| MeOH extract from Valparaíso | 18.0 ± 1.6 c | 36.3 ± 1.1 b | 1.0 ± 0.1 b | 1.1 ± 0.1 b |
| Catechin # | 11.4 ± 1.6 | 8.7 ± 0.1 | 5.4 ± 0.1 | 13.4 ± 0.3 |
| Peak | UV Max | Rt (min) | [M − H]− Ion | Fragment Ions (m/z) | Tentative Identification | Persicaria maculosa Extracts * | |||
|---|---|---|---|---|---|---|---|---|---|
| C.E | V.E | C.M | V.M | ||||||
| 1 | 205 | 3.3 | 191 | 170, 152, 126, 110, 84 | Quinic acid b | + | + | + | + |
| 2 | 254–354 | 16.9 | 639 | 463(quercetin-3-O- hexose), 301, 179, 151 | Quercetin-3-O-hexosyl-glucuronide b | + | + | + | + |
| 3 | 254–354 | 19.6 | 625 | 463(quercetin-3-O- hexose moiety), 301, 179, 151 | Quercetin 3,4´-di-O-glucoside, (quercetin sophoroside) a | + | + | + | + |
| 4 | 254–354 | 20.3 | 479 | 317 (myricetyn), 179, 151 | Myricetin 3´-glucose b | + | + | + | + |
| 5 | 264–364 | 20.4 | 609 | 590, 429, 285 | Kaempferol-O-di-glucose a,b | + | + | + | + |
| 6 | 254–354 | 21.1 | 609 | 301(quercetin), 179, 151 | Quercetin-3-O-rutinose (rutin) a | + | + | + | + |
| 7 | 254–354 | 21.1 | 609 | 447 (quercetin rhamnose), 301 | Quercetin-O-rhamnosyl-hexose (Quercetin 3-O-neohesperidose) b | + | + | + | + |
| 8 | 264–364 | 21.4 | 579 | 285 (kaempferol), 179, 151 | Kaempferol-O-pentosyl glucose b | + | + | + | + |
| 9 | 254–354 | 21.7 | 463 | 301(quercetin), 179, 151 | Quercetin-O-glucose a | + | + | + | + |
| 10 | 264–364 | 22.3 | 593 | 285(kaempferol), 179,151 | Kaempferol-O-Rhamnosyl-glucose b | + | + | + | + |
| 11 | 254–354 | 23.1 | 433 | 301(quercetin), 179, 151 | Quercetin-O-pentose a,b | + | + | + | + |
| 12 | 254–354 | 23.3 | 477 | 301(quercetin), 179, 151 | Quercetin-O-glucuronide a | + | + | + | + |
| 13 | 264–364 | 23.3 | 447 | 285 (kaempferol), 179, 151 | Kaempferol-O-glucoside a,b | + | + | + | + |
| 14 | 254–354 | 23.5 | 447 | 301(quercetin), 271, 179, 151 | Quercetin-O-rhamnose b | + | + | + | + |
| 15 | 254–354 | 24.2 | 461 | 285(kaempferol), 179, 151 | Kaempferol-O-glucuronide b | + | + | + | + |
| 16 | 264–364 | 28.9 | 563 | 413, 285, 179, 151 | Kaempferol-O-rhamnosyl pentose b | + | + | + | + |
| 17 | 264–364 | 30.1 | 417 | 285(kaempferol), 179, 151 | Kaempferol-O-pentose a,b | + | + | + | + |
| 18 | 264–364 | 31.5 | 431 | 285(kaempferol), 179, 151 | Kaempferol-O-rhamnose a,b | + | + | + | + |
| 19 | 264–364 | 32.3 | 431 | 285(kaempferol), 179, 151 | Kaempferol-O-rhamnose a,b | + | + | + | + |
| 20 | 264–364 | 34.8 | 599 | 447 (kaempferol-3-O- hexose), 285 | Kaempferol-O-galloyl-hexose a,b | + | + | + | + |
| 21 | 254–354 | 30.1 | 615 | 463, 301, 179, 151 | Isorhamnetin-O-galloyl-hexose b | + | + | + | + |
| 22 | 264–364 | 41.6 | 609 | 323 (rutinose), 285, 179, 151 | Kaempferol-O-rutinose a,b | + | + | + | + |
| 23 | 264-364 | 45.2 | 609 | 323 (sophorose), 285, 179, 151 | Kaempferol-3-O-sophorose a,b | ND c | + | + | + |
| 24 | 254–354 | 48.1 | 639 | 463(quercetin-3-O-hexose), 315(isorhamnetin-3-O-hexose), 301, 179, 151 | Isorhamnetin-O-sophorose b | + | + | ND | ND |
| 25 | 254–354 | 50.2 | 301 | 194, 271, 179, 151 | Quercetin a,b | + | + | ND | ND |
| 26 | 254–354 | 50.5 | 315 | 300, 179, 151 | Isorhamnetin a,b | + | + | ND | ND |
| 27 | 264–364 | 53.4 | 285 | 179,151 | Kaempferol a,b | + | + | + | + |
| Insect Species | Extract | IFP (a) | Wilcoxon Signed-Rank Test Results | ||
|---|---|---|---|---|---|
| Curicó Extracts | Valparaíso Extracts | Curicó Extracts | Valparaíso Extracts | ||
| Pseudaletia adultera (b) | EtOH | 0.6 ± 0.2 * | 0.6 ± 0.2 * | W = −72; ns/r = 15; z = −2.03 P(1-tail) = 0.02; P(2-tail) = 0.04 | W = −72; ns/r = 15; z = −2.03 P(1-tail) = 0.02; P(2-tail) = 0.04 |
| MeOH | 0.5 ± 0.2 ** | 0.5 ± 0.2 ** | W = −56; ns/r = 15, z = −1.58 P(1-tail) = 0.05; P(2-tail) = 0.1 | W = −56; ns/r = 15; z = −1.58 P(1-tail) = 0.05; P(2-tail) = 0.1 | |
| DCM | 0.7 ± 0.1 * | 0.7 ± 0.2 * | W = −66; ns/r = 11; z = −2.91 P(1-tail) = 0.002; P(2-tail) = 0.004 | W = −88; ns/r = 15; z = −2.48 P(1-tail) = 0.007; P(2-tail) = 0.01 | |
| Epilachna paenulata (c)(d) | EtOH | 0.6 ± 0.3 ** | 1.0 ± 0.0 * | W = −33; ns/r = 10; z = −1.66 P(1-tail) = 0.05; P(2-tail) = 0.1 | W = −55; ns/r = 10; z = −2.78 P(1-tail) = 0.003; P(2-tail) = 0.005 |
| MeOH | 0.8 ± 0.2 * | 1.0 ± 0.0 * | W = −44; ns/r = 10; z = −2.22 P(1-tail) = 0.01; P(2-tail) = 0.03 | W = −55; ns/r = 10; z = −2.78 P(1-tail) = 0.003; P(2-tail) = 0.005 | |
| DCM | 0.8 ± 0.2 * | 1.0 ± 0.0 * | W = −44; ns/r = 10; z = −2.22 P(1-tail) = 0.01; P(2-tail) = 0.03 | W = −55; ns/r = 10; z = −2.78 P(1-tail) = 0.003; P(2-tail) = 0.005 | |
| Valparaíso Extracts | Curicó Extracts | |||||
|---|---|---|---|---|---|---|
| Type of Extract | % Settling on Treated Leaf | % settling on control leaf | PI (a) | % Settling on Treated Leaf | % Settling on Control Leaf | PI |
| EtOH | 34 ± 4 | 66 ± 4 | 0.32 ± 0.08 * | 44 ± 3 | 56 ± 3 | 0.12 ± 0.06 (NS) |
| MeOH | 31 ± 3 | 69 ± 3 | 0.37 ± 0.06 * | 41 ± 5 | 59 ± 5 | 0.18 ± 0.09 (NS) |
| DCM | 23 ± 4 | 77 ± 4 | 0.55 ± 0.08 * | 23 ± 5 | 79 ± 5 | 0.58 ± 0.09 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quesada-Romero, L.; Fernández-Galleguillos, C.; Bergmann, J.; Amorós, M.-E.; Jiménez-Aspee, F.; González, A.; Simirgiotis, M.; Rossini, C. Phenolic Fingerprinting, Antioxidant, and Deterrent Potentials of Persicaria maculosa Extracts. Molecules 2020, 25, 3054. https://doi.org/10.3390/molecules25133054
Quesada-Romero L, Fernández-Galleguillos C, Bergmann J, Amorós M-E, Jiménez-Aspee F, González A, Simirgiotis M, Rossini C. Phenolic Fingerprinting, Antioxidant, and Deterrent Potentials of Persicaria maculosa Extracts. Molecules. 2020; 25(13):3054. https://doi.org/10.3390/molecules25133054
Chicago/Turabian StyleQuesada-Romero, Luisa, Carlos Fernández-Galleguillos, Jan Bergmann, María-Eugenia Amorós, Felipe Jiménez-Aspee, Andrés González, Mario Simirgiotis, and Carmen Rossini. 2020. "Phenolic Fingerprinting, Antioxidant, and Deterrent Potentials of Persicaria maculosa Extracts" Molecules 25, no. 13: 3054. https://doi.org/10.3390/molecules25133054
APA StyleQuesada-Romero, L., Fernández-Galleguillos, C., Bergmann, J., Amorós, M.-E., Jiménez-Aspee, F., González, A., Simirgiotis, M., & Rossini, C. (2020). Phenolic Fingerprinting, Antioxidant, and Deterrent Potentials of Persicaria maculosa Extracts. Molecules, 25(13), 3054. https://doi.org/10.3390/molecules25133054