Bio-Based Hydrogels Composed of Humic Matter and Pectins of Different Degree of Methyl-Esterification
Abstract
1. Introduction
2. Results and Discussion
2.1. 13C CPMAS NMR Spectroscopy
2.2. Morphology of Hydrogels by SEM and MRI Analyses
2.3. Rheological Characterization of Hydrogels
2.4. Phloroglucinol Release Study
3. Materials and Methods
3.1. Materials
3.2. Composts and Extraction of Humic Substances
3.3. Depolymerization of Lignocellulose Residues
3.4. Preparation of Hydrogels
3.5. Characterization of Hydrogels
3.5.1. Solid-state NMR Spectroscopy
3.5.2. Magnetic Resonance Imaging (MRI) Experiments
3.5.3. Scanning Electron Microscopy (SEM)
3.5.4. Rheological Measurements
3.5.5. Release of Phloroglucinol from Hydrogels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and Pectin-Based Composite Materials: Beyond Food Texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed]
- Rascón-Chu, A.; Díaz-Baca, J.A.; Carvajal-Millán, E.; López-Franco, Y.; Lizardi-Mendoza, J. New Use for an “Old” Polysaccharide: Pectin-Based Composite Materials. In Handbook of Sustainable Polymers: Structure and Chemistry; Thakur, V.K., Thakur, M.K., Eds.; Pan Stanford Publishing Pte. Ltd.: Singapore, 2016; pp. 72–107. [Google Scholar]
- Sundar, A.; Rubila, S.; Jayabalan, R.; Ranganathan, T.V. A Review on Pectin: Chemistry due to General Properties of Pectin and its Pharmaceutical Uses. Sci. Rep. 2012, 1, 1–4. [Google Scholar]
- Liu, L.; Fishman, M.L.; Hicks, K.B. Pectin in Controlled Drug Delivery—A Review. Cellulose 2007, 14, 15–24. [Google Scholar] [CrossRef]
- Luppi, B.; Bigucci, F.; Abruzzo, A.; Corace, G.; Cerchiara, T.; Zecchi, V. Freeze-Dried Chitosan/Pectin Nasal Inserts for Antipsychotic Drug Delivery. Eur. J. Pharm. Biopharm. 2010, 75, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Sriamornsak, P. Application of Pectin in Oral Drug Delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ahuja, M.; Kaur, H. Thiolated Pectin Nanoparticles: Preparation, Characterization and ex vivo Corneal Permeation Study. Carbohydr. Polym. 2012, 87, 1606–1610. [Google Scholar] [CrossRef]
- Munarin, F.; Tanzi, M.C.; Petrini, P. Advances in Biomedical Applications of Pectin Gels. Int. J. Biol. Macromol. 2012, 51, 681–689. [Google Scholar] [CrossRef]
- Rascón-Chu, A.; Martínez-López, A.; Carvajal-Millán, E.; Ponce de León-Renova, N.; Márquez-Escalante, J.; Romo-Chacón, A. Pectin from Low Quality “Golden Delicious”Apples: Composition and Gelling Capability. Food Chem. 2009, 116, 101–113. [Google Scholar] [CrossRef]
- Masmoudi, M.; Besbes, S.; Abbes, F.; Robert, C.; Paquot, M.; Blecker, C.; Attia, H. Pectin Extraction from Lemon By-Product with Acidified Date Juice: Effect of Extraction Conditions on Chemical Composition of Pectins. Food Bioprocess Technol. 2012, 5, 687–695. [Google Scholar] [CrossRef]
- Mohnen, D. Biosynthesis of Pectins and Galactomannans. In Comprehensive Natural Products Chemistry; Barton, D., Nakanishi, K., Meth-Cohn, O., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1999; Volume 3, pp. 497–527. [Google Scholar]
- Liu, J.; Willför, S.; Xu, C. A Review of Bioactive Plant Polysaccharides: Biological Activities, Functionalization, and Biomedical Applications. Bioact. Carbohydr. Dietary Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An Emerging New Bioactive Food Polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Mukhiddinov, Z.K.; Khalikov, D.K.; Abdusamiev, F.T.; Avloev, C.C. Isolation and Structural Characterization of a Pectin Homo and Ramnogalacturonan. Talanta 2000, 53, 171–176. [Google Scholar] [CrossRef]
- Sriamornsak, P. Chemistry of Pectin and its Pharmaceutical Uses: A Review. Univ. Int. J. 2003, 206–223. [Google Scholar]
- Voragen, F.; Beldman, G.; Schols, H. Chemistry and Enzymology of Pectins. In Advanced Dietary Fibre Technology; McCleary, B.V., Prosky, L., Eds.; Blackwell Science Ltd.: Oxford, UK, 2001; pp. 379–398. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and Uses of Pectin—A Review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Kastner, H.; Einhorn-Stoll, U.; Senge, B. Structure formation in sugar containing pectin gels: Influence of Ca2+ on the gelation of low-methoxylated pectin at acidic pH. Food Hydrocoll. 2012, 27, 42–49. [Google Scholar] [CrossRef]
- Axelos, M.A.V.; Thibault, J.F. The Chemistry of Low-Methoxyl Pectin Gelation. In The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 109–118. [Google Scholar]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.; Thom, D. Biological Interactions between Polysaccharides and Divalent Cations: The egg-Box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Braccini, I.; Pérez, S. Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromolecules 2001, 2, 1089–1096. [Google Scholar] [CrossRef]
- Sharma, R.; Naresh, L.; Dhuldhoya, N.; Merchant, S.; Merchant, U. An Overview on Pectins. Times Food Process. J. 2006, 44–51. [Google Scholar]
- Nuzzo, A.; Mazzei, P.; Drosos, M.; Piccolo, A. Novel Humo-Pectic Hydrogels for Controlled Release of Agroproducts. ACS Sustain. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Savy, D.; Cozzolino, V.; Nebbioso, A.; Drosos, M.; Nuzzo, A.; Mazzei, P.; Piccolo, A. Humic-like bioactivity on emergence and early growth of maize (Zea mays L.) of water-soluble lignins isolated from biomass for energy. Plant Soil 2016, 402, 221–233. [Google Scholar] [CrossRef]
- Spaccini, R.; Cozzolino, V.; Di Meo, V.; Savy, D.; Drosos, M.; Piccolo, A. Bioactivity of humic substances and water extracts from compost made by ligno-cellulose wastes from biorefinery. Sci. Total Environ. 2019, 646, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Baigorri, R.; Urrutia, O.; Erro, J.; Aparicio-Tejo, P.M.; Yvin, J.C.; García-Mina, J.M. Improving the short-term efficiency of rock phosphate-based fertilizers in pastures by using edaphic biostimulants. Chem. Biol. Technol. Agric. 2016, 3, 5. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar]
- Piccolo, A.; Conte, P.; Cozzolino, A. Chromatographic and spectrophotometric properties of dissolved humic substances compared with macromolecular polymers. Soil Sci. 2001, 166, 174–185. [Google Scholar] [CrossRef]
- Nuzzo, A.; De Martino, A.; Di Meo, V.; Piccolo, A. Potential alteration of iron–humate complexes by plant root exudates and microbial siderophores. Chem. Biol. Technol. Agric. 2018, 5, 19. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Brus, J. 13C CP/MAS NMR spectra of pectins: A peak-fitting analysis in the C-6 region. Czech J. Food Sci. 2003, 21, 1–12. [Google Scholar] [CrossRef]
- Spaccini, R.; Todisco, D.; Drosos, M.; Nebbioso, A.; Piccolo, A. Decomposition of biodegradable plastic polymer in a real on-farm composting process. Chem. Biol. Technol. Agric. 2016, 3, 4. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances. Soil Sci. 2001, 166, 810–833. [Google Scholar] [CrossRef]
- Nuzzo, A.; Sánchez, A.; Fontaine, B.; Piccolo, A. Conformational changes of dissolved humic and fulvic superstructures with progressive iron complexation. J. Geochem. Explor. 2013, 129, 1–5. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Wang, B.; Yan, S.; Zhang, K.; Ding, J.; Yin, J. Self-healing supramolecular self-assembled hydrogels based on poly(L-glutamic acid). Biomacromolecules 2015, 16, 3508–3518. [Google Scholar] [CrossRef]
- Klucakova, M.; Smilek, J.; Sedlacek, P. How humic acids affect the rheological and transport properties of hydrogels. Molecules 2019, 24, 1545. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Peppas, N.A. Solute and penetrant diffusion in swellable polymers. III. Drug release from glassy poly(HEMA-co-NVP) copolymers. J. Control. Release 1984, 1, 89–98. [Google Scholar] [CrossRef]
- Serra, L.; Doménech, J.; Peppas, N.A. Drug transport mechanisms and release kinetics from molecularly designed poly(acrylic acid-g-ethylene glycol) hydrogels. Biomaterials 2006, 27, 5440–5451. [Google Scholar] [CrossRef]
- Savy, D.; Nebbioso, A.; Mazzei, P.; Drosos, M.; Piccolo, A. Molecular composition of water-soluble lignins separated from different non-food biomasses. Fuel Process. Technol. 2015, 131, 175–181. [Google Scholar] [CrossRef]
- Piccolo, A.; Conte, P.; Spaccini, R.; Mbagwu, J.S.C. Influence of land use on the humic substances of some tropical soils of Nigeria. Eur. J. Soil Sci. 2005, 56, 343–352. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
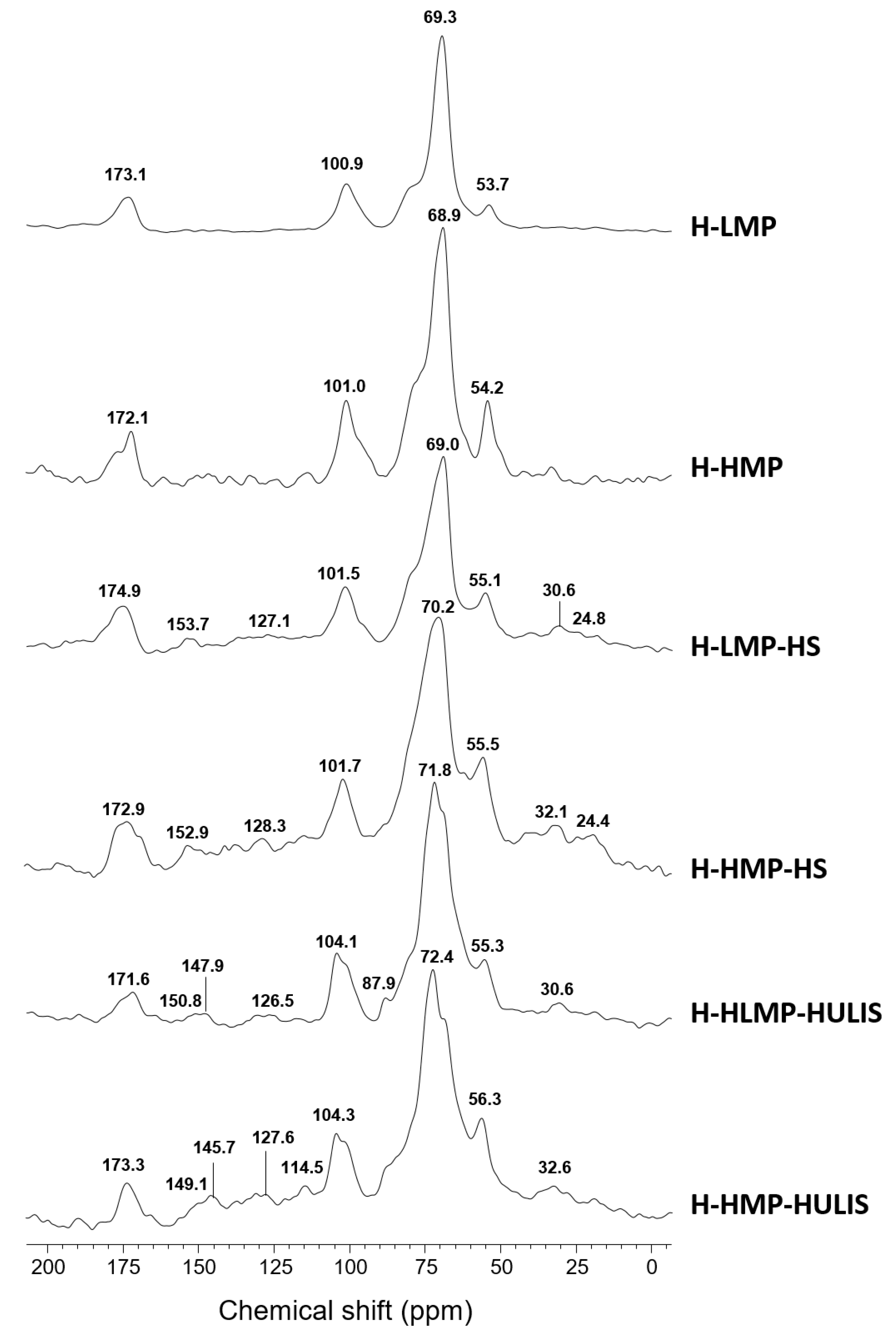


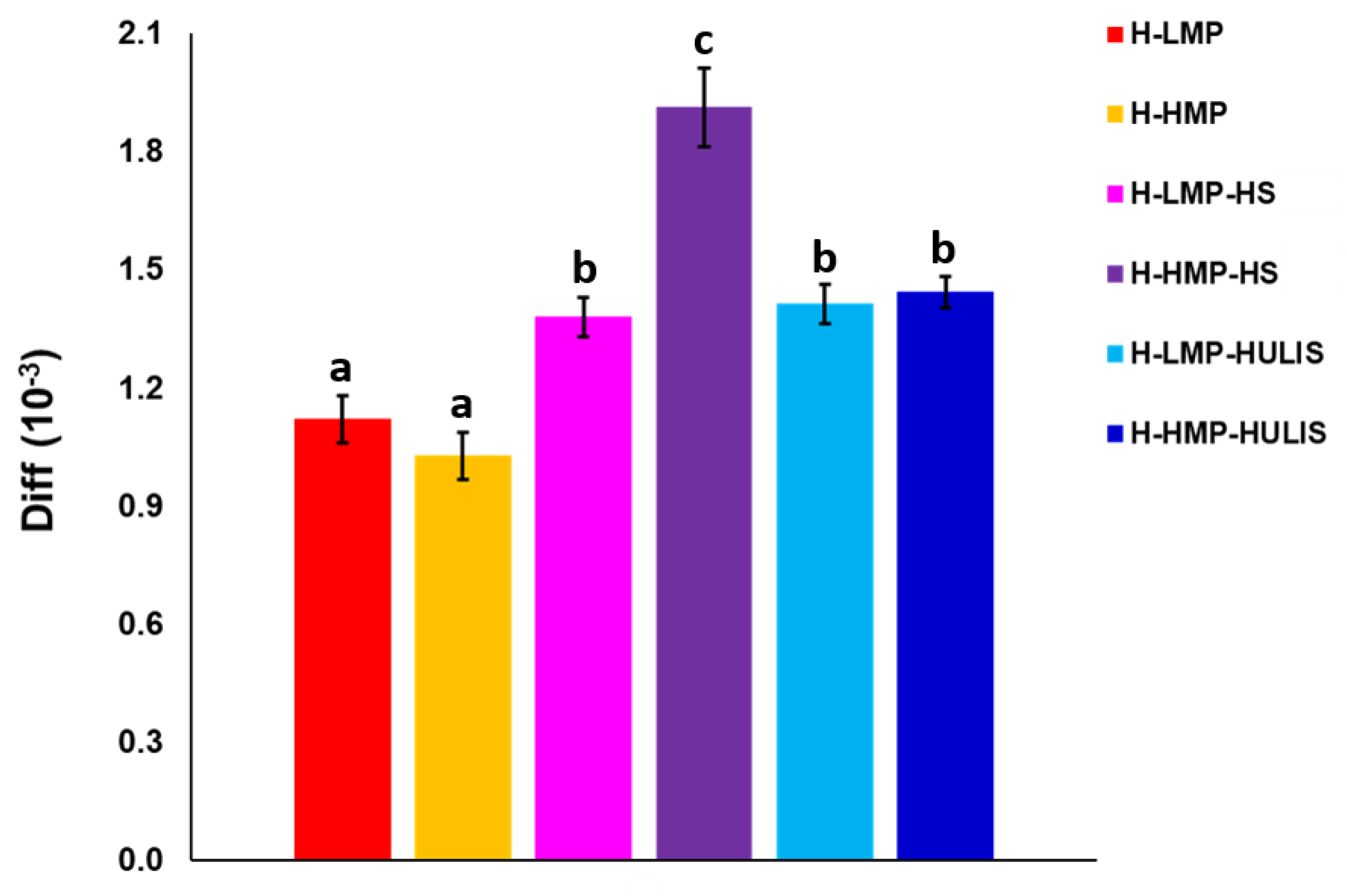
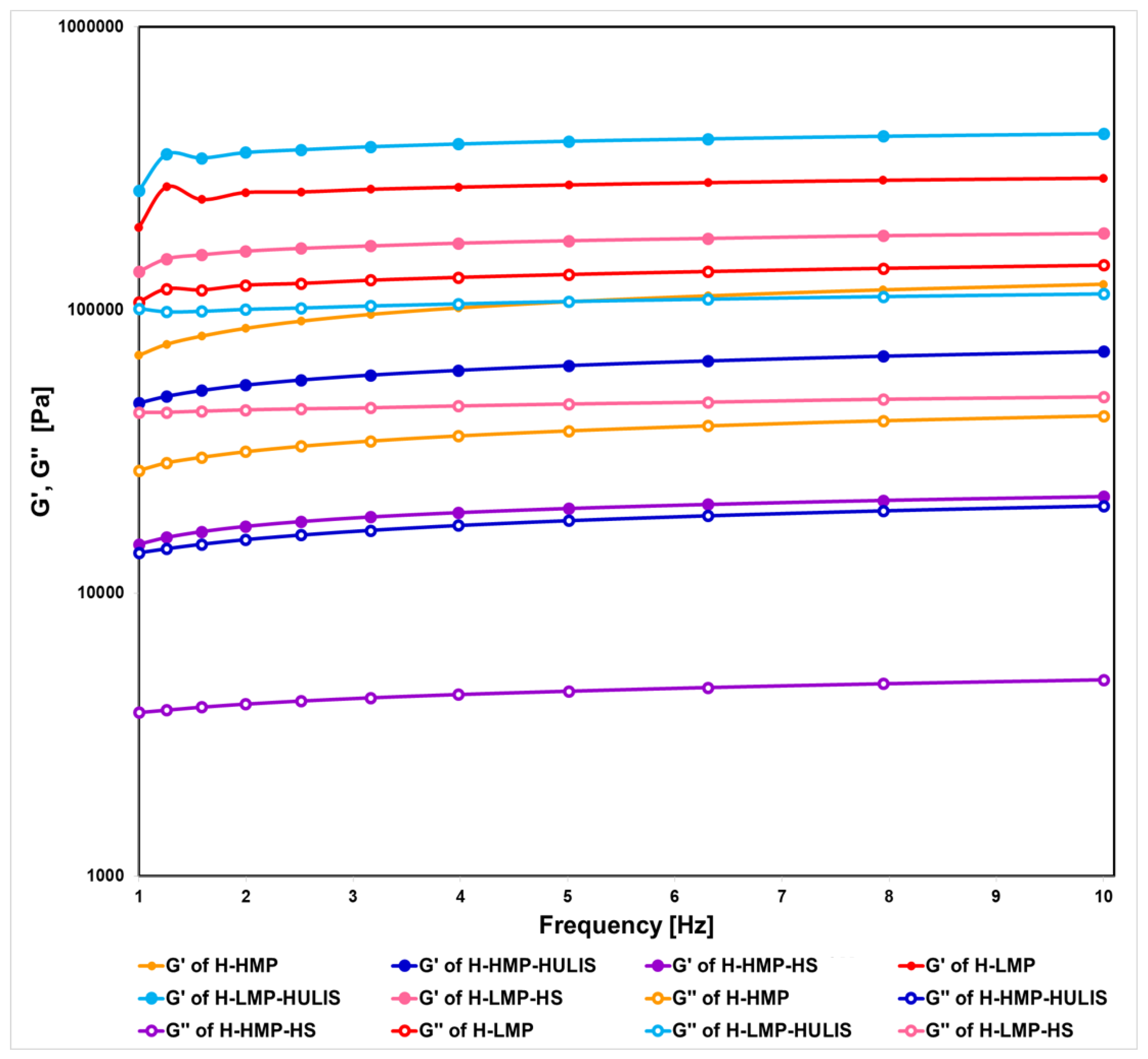
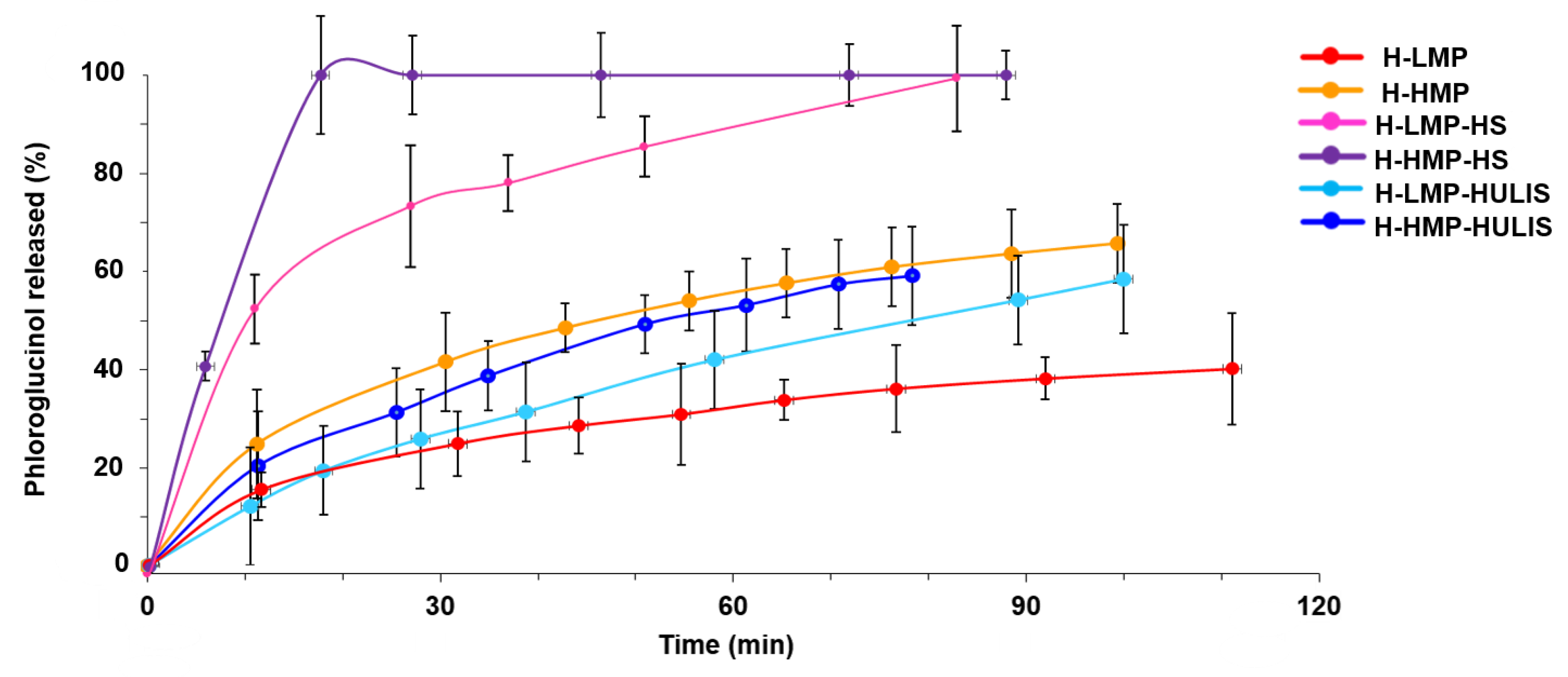
| Sample | Carboxyl-C | Phenol-C | Aryl-C | O-alkyl-C | C-O/C-N | Alkyl-C | HB/HI a |
|---|---|---|---|---|---|---|---|
| 190–160 | 160–145 | 145–110 | 110–60 | 60–45 | 45–0 | ||
| H-LMP | 10.70 | 0.00 | 0.00 | 81.16 | 6.99 | 1.15 | 0.01 |
| H-HMP | 7.30 | 0.00 | 0.00 | 80.14 | 11.72 | 0.84 | 0.01 |
| H-LMP-HS | 4.57 | 0.00 | 0.78 | 81.74 | 10.81 | 2.11 | 0.03 |
| H-HMP-HS | 2.88 | 1.09 | 7.68 | 66.60 | 12.11 | 9.64 | 0.23 |
| H-LMP-HULIS | 4.01 | 0.00 | 0.00 | 82.43 | 10.95 | 2.61 | 0.03 |
| H-HMP-HULIS | 0.00 | 0.00 | 0.97 | 85.51 | 13.52 | 0.00 | 0.01 |
| Hydrogels | K a | n b | R2 |
|---|---|---|---|
| H-LMP | 5.7382 | 0.4072 | 0.9938 |
| H-HMP | 9.3832 | 0.4225 | 0.9908 |
| H-LMP-HS | 44.4927 | 0.2851 | 0.5809 |
| H-HMP-HS | 28.3910 | 0.1990 | 0.9920 |
| H-LMP-HULIS | 2.7121 | 0.6576 | 0.9987 |
| H-HMP-HULIS | 5.2196 | 0.5545 | 0.9953 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, A.; Mazzei, P.; Savy, D.; Di Meo, V.; Piccolo, A. Bio-Based Hydrogels Composed of Humic Matter and Pectins of Different Degree of Methyl-Esterification. Molecules 2020, 25, 2936. https://doi.org/10.3390/molecules25122936
Nuzzo A, Mazzei P, Savy D, Di Meo V, Piccolo A. Bio-Based Hydrogels Composed of Humic Matter and Pectins of Different Degree of Methyl-Esterification. Molecules. 2020; 25(12):2936. https://doi.org/10.3390/molecules25122936
Chicago/Turabian StyleNuzzo, Assunta, Pierluigi Mazzei, Davide Savy, Vincenzo Di Meo, and Alessandro Piccolo. 2020. "Bio-Based Hydrogels Composed of Humic Matter and Pectins of Different Degree of Methyl-Esterification" Molecules 25, no. 12: 2936. https://doi.org/10.3390/molecules25122936
APA StyleNuzzo, A., Mazzei, P., Savy, D., Di Meo, V., & Piccolo, A. (2020). Bio-Based Hydrogels Composed of Humic Matter and Pectins of Different Degree of Methyl-Esterification. Molecules, 25(12), 2936. https://doi.org/10.3390/molecules25122936







