Metabolomic Signature Discriminates Normal Human Cornea from Keratoconus—A Pilot GC/MS Study
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Tissue Collection
2.3. Sample Processing and Metabolite Extraction
2.4. GC/MS Analysis
2.5. Analysis of Spectral Data
2.6. Statistical and Chemometric Analyses
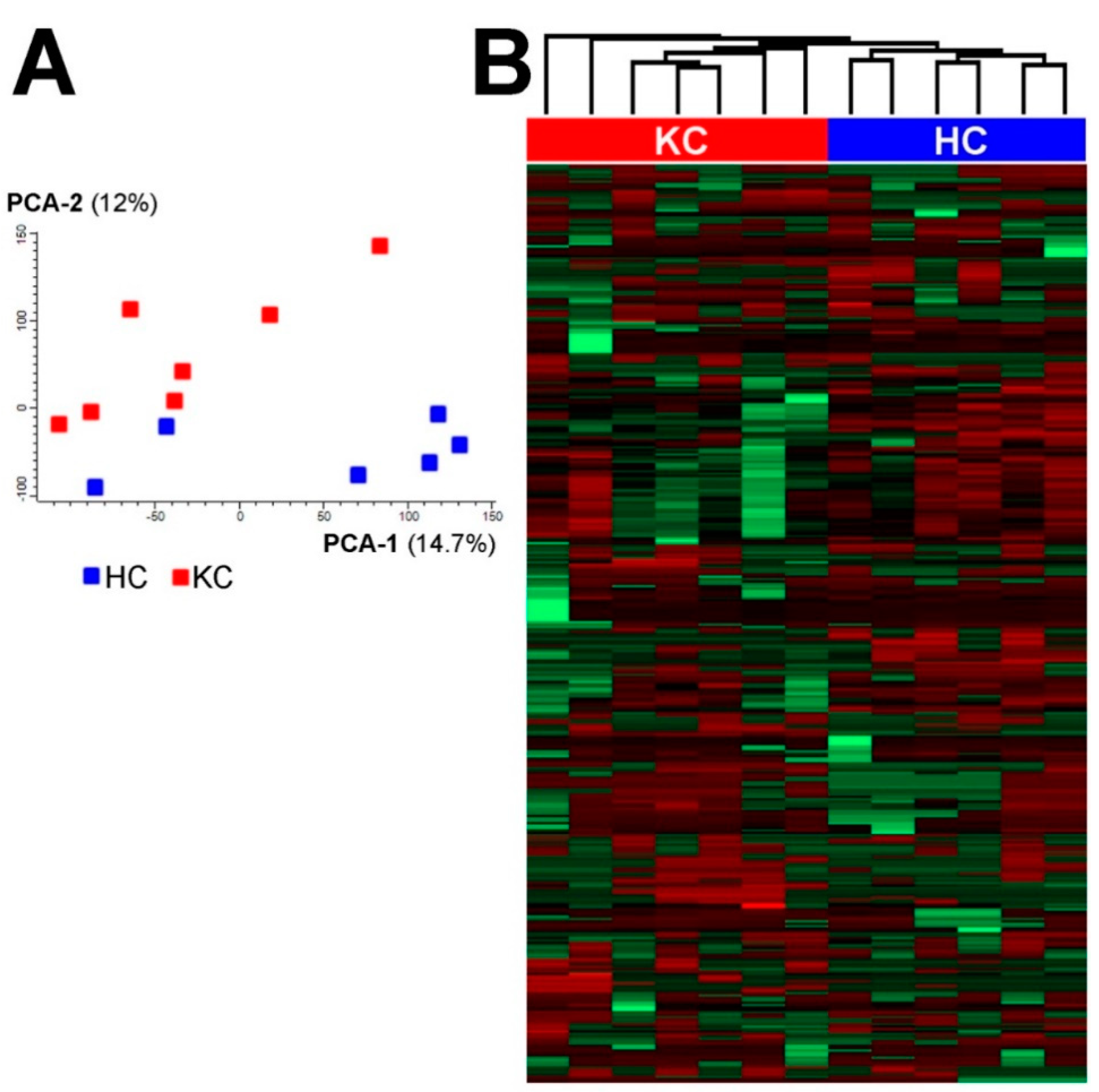
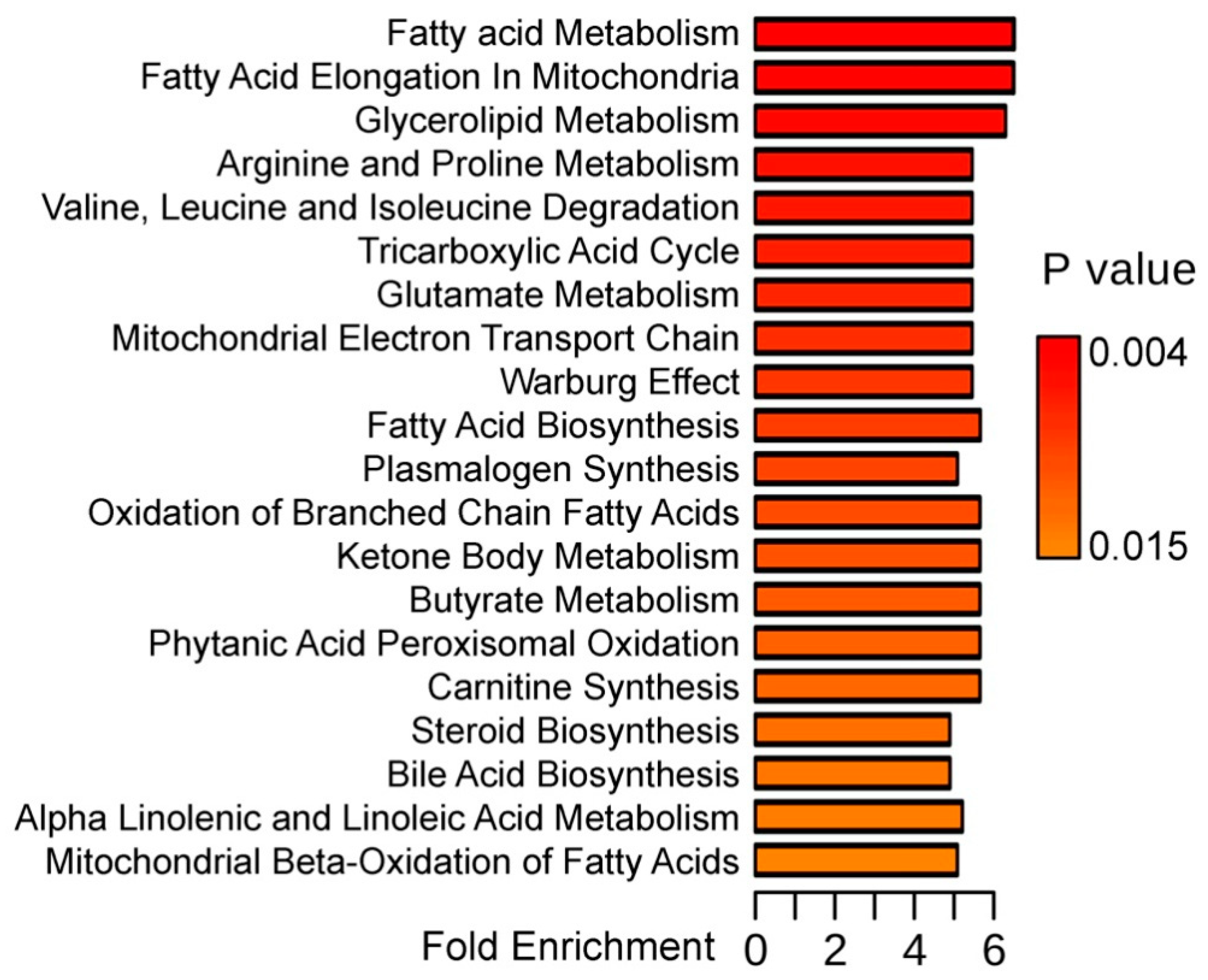
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mamalis, N.; Anderson, C.W.; Kreisler, K.R.; Lundergan, M.K.; Olson, R.J. Changing trends in the indications for penetrating keratoplasty. Arch. Ophthalmol. 1992, 110, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Lois, N.; Kowal, V.O.; Cohen, E.J.; Rapuano, C.J.; Gault, J.A.; Raber, I.M.; Laibson, P.R. Indications for penetrating keratoplasty and associated procedures, 1989–1995. Cornea 1997, 16, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Maeno, A.; Naor, J.; Lee, H.M.; Hunter, W.S.; Rootman, D.S. Three decades of corneal transplantation: Indications and patient characteristics. Cornea 2000, 19, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.H.; Bourne, W.M.; Dyer, J.A. A 48-year clinical and epidemiologic study of keratoconus. Am. J. Ophthalmol. 1986, 101, 267–273. [Google Scholar] [CrossRef]
- Godefrooij, D.A.; de Wit, G.A.; Uiterwaal, C.S.; Imhof, S.M.; Wisse, R.P.L. Age-specific Incidence and Prevalence of Keratoconus: A Nationwide Registration Study. Am. J. Ophthalmol. 2017, 175, 169–172. [Google Scholar] [CrossRef]
- Jonas, J.B.; Nangia, V.; Matin, A.; Kulkarni, M.; Bhojwani, K. Prevalence and Associations of Keratoconus in Rural Maharashtra in Central India: The Central India Eye and Medical Study. Am. J. Ophthalmol. 2009, 148, 760–765. [Google Scholar] [CrossRef]
- Georgiou, T.; Funnell, C.L.; Cassels-Brown, A.; O’Conor, R. Influence of ethnic origin on the incidence of keratoconus and associated atopic disease in Asians and white patients. Eye 2004, 18, 379–383. [Google Scholar] [CrossRef]
- Cursiefen, C.; Küchle, M.; Naumann, G.O. Changing indications for penetrating keratoplasty: Histopathology of 1250 corneal buttons. Cornea 1998, 17, 468–470. [Google Scholar] [CrossRef]
- Kenney, M.C.; Chwa, M.; Atilano, S.R.; Tran, A.; Carballo, M.; Saghizadeh, M.; Vasiliou, V.; Adachi, W.; Brown, D.J. Increased Levels of Catalase and Cathepsin V/L2 but Decreased TIMP-1 in Keratoconus Corneas: Evidence that Oxidative Stress Plays a Role in This Disorder. Investig. Opthalmol. Vis. Sci. 2005, 46, 823–832. [Google Scholar] [CrossRef]
- Arnal, E.; Peris-Martínez, C.; Menezo, J.L.; Johnsen-Soriano, S.; Romero, F.J. Oxidative Stress in Keratoconus? Investig. Opthalmol. Vis. Sci. 2011, 52, 8592. [Google Scholar] [CrossRef]
- Dudakova, L.; Liskova, P.; Trojek, T.; Palos, M.; Kalasova, S.; Jirsova, K. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp. Eye Res. 2012, 104, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Behndig, A.; Karlsson, K.; Johansson, B.O.; Brännström, T.; Marklund, S.L. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2293–2296. [Google Scholar]
- Buddi, R.; Lin, B.; Atilano, S.R.; Zorapapel, N.C.; Kenney, M.C.; Brown, D.J. Evidence of Oxidative Stress in Human Corneal Diseases. J. Histochem. Cytochem. 2002, 50, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Podskochy, A.; Gan, L.; Fagerholm, P. Apoptosis in UV-exposed rabbit corneas. Cornea 2000, 19, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Chwa, M.; Atilano, S.R.; Reddy, V.; Jordan, N.; Kim, D.W.; Kenney, M.C. Increased Stress-Induced Generation of Reactive Oxygen Species and Apoptosis in Human Keratoconus Fibroblasts. Investig. Opthalmol. Vis. Sci. 2006, 47, 1902. [Google Scholar] [CrossRef] [PubMed]
- Čejková, J.; Čejka, Č. The role of oxidative stress in corneal diseases and injuries. Histol. Histopathol. 2015, 30, 893–900. [Google Scholar] [CrossRef]
- Tan, S.Z.; Begley, P.; Mullard, G.; Hollywood, K.A.; Bishop, P.N. Introduction to metabolomics and its applications in ophthalmology. Eye 2016, 30, 773–783. [Google Scholar] [CrossRef]
- Chen, L.; Gao, Y.; Wang, L.Z.; Cheung, N.; Tan, G.S.W.; Cheung, G.C.M.; Beuerman, R.W.; Wong, T.Y.; Chan, E.C.Y.; Zhou, L. Recent advances in the applications of metabolomics in eye research. Anal. Chim. Acta 2018, 1037, 28–40. [Google Scholar] [CrossRef]
- Laíns, I.; Gantner, M.; Murinello, S.; Lasky-Su, J.A.; Miller, J.W.; Friedlander, M.; Husain, D. Metabolomics in the study of retinal health and disease. Prog. Retin. Eye Res. 2019, 69, 57–79. [Google Scholar] [CrossRef]
- Snytnikova, O.A.; Yanshole, L.V.; Iskakov, I.A.; Yanshole, V.V.; Chernykh, V.V.; Stepakov, D.A.; Novoselov, V.P.; Tsentalovich, Y.P. Quantitative metabolomic analysis of the human cornea and aqueous humor. Metabolomics 2017, 13, 152. [Google Scholar] [CrossRef]
- Karamichos, D.; Zieske, J.D.; Sejersen, H.; Sarker-Nag, A.; Asara, J.M.; Hjortdal, J. Tear metabolite changes in keratoconus. Exp. Eye Res. 2015, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Priyadarsini, S.; Nicholas, S.E.; Sarker-Nag, A.; Allegood, J.; Chalfant, C.E.; Mandal, N.A.; Karamichos, D. Analysis of sphingolipids in human corneal fibroblasts from normal and keratoconus patients. J. Lipid Res. 2017, 58, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Galvis, V.; Sherwin, T.; Tello, A.; Merayo, J.; Barrera, R.; Acera, A. Keratoconus: An inflammatory disorder? Eye 2015, 29, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Kuffová, L.; Holán, V.; Lumsden, L.; Forrester, J.V.; Filipec, M. Cell subpopulations in failed human corneal grafts. Br. J. Ophthalmol. 1999, 83, 1364–1369. [Google Scholar] [CrossRef][Green Version]
- Wisse, R.P.; Kuiper, J.J.; Gans, R.; Imhof, S.; Radstake, T.R.; Van der Lelij, A. Cytokine Expression in Keratoconus and its Corneal Microenvironment: A Systematic Review. Ocul. Surf. 2015, 13, 272–283. [Google Scholar] [CrossRef]
- Kenney, C.M.; Brown, D.J. The cascade hypothesis of keratoconus. Cont. Lens Anterior Eye 2003, 26, 139–146. [Google Scholar] [CrossRef]
- Kryczka, T.; Ehlers, N.; Nielsen, K.; Wylegala, E.; Dobrowolski, D.; Midelfart, A. Metabolic Profile of Keratoconic Cornea. Curr. Eye Res. 2013, 38, 305–309. [Google Scholar] [CrossRef]
- Saijyothi, A.V.; Fowjana, J.; Madhumathi, S.; Rajeshwari, M.; Thennarasu, M.; Prema, P.; Angayarkanni, N. Tear fluid small molecular antioxidants profiling shows lowered glutathione in keratoconus. Exp. Eye Res. 2012, 103, 41–46. [Google Scholar] [CrossRef]
- Greiner, J.V.; Lass, J.H.; Reinhart, W.J.; Medcalf, S.K.; Glonek, T. Phosphatic metabolites in keratoconus. Exp. Eye Res. 1989, 49, 799–806. [Google Scholar] [CrossRef]
- Tsentalovich, Y.P.; Verkhovod, T.D.; Yanshole, V.V.; Kiryutin, A.S.; Yanshole, L.V.; Fursova, A.Z.; Stepakov, D.A.; Novoselov, V.P.; Sagdeev, R.Z. Metabolomic composition of normal aged and cataractous human lenses. Exp. Eye Res. 2015, 134, 15–23. [Google Scholar] [CrossRef]
- Priyadarsini, S.; McKay, T.B.; Sarker-Nag, A.; Allegood, J.; Chalfant, C.; Ma, J.-X.; Karamichos, D. Complete metabolome and lipidome analysis reveals novel biomarkers in the human diabetic corneal stroma. Exp. Eye Res. 2016, 153, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wojakowska, A.; Marczak, Ł.; Jelonek, K.; Polanski, K.; Widlak, P.; Pietrowska, M. An optimized method of metabolite extraction from formalin-fixed paraffin-embedded tissue for GC/MS analysis. PLoS ONE 2015, 10, e0136902. [Google Scholar] [CrossRef] [PubMed]
- Lass, J.H.; Greiner, J.V.; Merchant, T.E.; Glonek, T. The effects of age on phosphatic metabolites of the human cornea. Cornea 1995, 14, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Kryczka, T.; Szaflik, J.P.; Szaflik, J.; Midelfart, A. Influence of donor age, post-mortem time and cold storage on metabolic profile of human cornea. Acta Ophthalmol. 2013, 91, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Kryczka, T.; Wylęgała, E.; Dobrowolski, D.; Midelfart, A. NMR spectroscopy of human eye tissues: A new insight into ocular biochemistry. Sci. World J. 2014, 2014, 546192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Muehlbauer, M.; O’Neal, S.; Newgard, C.; Hauser, E.; Bain, J.; Shah, S. Recommendations for Improving Identification and Quantification in Non-Targeted, GC-MS-Based Metabolomic Profiling of Human Plasma. Metabolites 2017, 7, 45. [Google Scholar] [CrossRef]
- Lema, I.; Sobrino, T.; Durán, J.A.; Brea, D.; Díez-Feijoo, E. Subclinical keratoconus and inflammatory molecules from tears. Br. J. Ophthalmol. 2009, 93, 820–824. [Google Scholar] [CrossRef]
- McKay, T.B.; Hjortdal, J.; Sejersen, H.; Asara, J.M.; Wu, J.; Karamichos, D. Endocrine and Metabolic Pathways Linked to Keratoconus: Implications for the Role of Hormones in the Stromal Microenvironment. Sci. Rep. 2016, 6, 25534. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Tschetter, R.T. Lipid analysis of the human cornea with and without arcus senilis. Arch. Ophthalmol. 1966, 76, 403–405. [Google Scholar] [CrossRef]
- Kenchegowda, S.; Bazan, H.E.P. Significance of lipid mediators in corneal injury and repair. J. Lipid Res. 2010, 51, 879–891. [Google Scholar] [CrossRef]
- Bazan, H.E.; Birkle, D.L.; Beuerman, R.W.; Bazan, N.G. Inflammation-induced stimulation of the synthesis of prostaglandins and lipoxygenase-reaction products in rabbit cornea. Curr. Eye Res. 1985, 4, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Erdinest, N.; Shmueli, O.; Grossman, Y.; Ovadia, H.; Solomon, A. Anti-Inflammatory Effects of Alpha Linolenic Acid on Human Corneal Epithelial Cells. Investig. Opthalmol. Vis. Sci. 2012, 53, 4396–4406. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Thies, R.S.; Mandel, L.J. Role of glucose in corneal metabolism. Am. J. Physiol. Physiol. 1985, 249, C409–C416. [Google Scholar] [CrossRef]
- Karamichos, D.; Hutcheon, A.E.K.; Rich, C.B.; Trinkaus-Randall, V.; Asara, J.M.; Zieske, J.D. In vitro model suggests oxidative stress involved in keratoconus disease. Sci. Rep. 2015, 4, 4608. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Metabolite Name | Class | HC Mean Abundance | KC Mean Abundance | HC/KC | |
|---|---|---|---|---|---|
| FC | p-Value | ||||
| Benzoic acid | Carboxylic acids | 180 | 119 | 1.51 | 0.0338 |
| Glycolic acid | Carboxylic acids | 6.18 | 2.65 | 2.33 | 0.0258 |
| Succinic acid | Carboxylic acids | 771 | 536 | 1.44 | 0.0096 |
| Gluconic acid | Carboxylic acids | N.D. | 0.56 | - | - |
| Linoleic acid | Fatty acids | 103 | 38.8 | 2.65 | 0.0144 |
| Myristic acid | Fatty acids | 175 | 112 | 1.56 | 0.0197 |
| Palmitic acid | Fatty acids | 7240 | 4250 | 1.70 | 0.0042 |
| Pentadecanoic acid | Fatty acids | 122 | 74.3 | 1.64 | 0.0163 |
| Stearic acid | Fatty acids | 7440 | 4810 | 1.55 | 0.0159 |
| trans-13-Octadecenoic acid | Fatty acids | 149 | 83.3 | 1.79 | <0.0001 |
| Petroselinic acid | Fatty acids | 256 | N.D. | - | - |
| Cholesta-3,5-diene—isomer 1 | Sterols | 83.6 | 17.6 | 4.75 | 0.0032 |
| Cholesta-3,5-diene—isomer 2 | Sterols | 421 | 85.5 | 4.92 | 0.0016 |
| Cholesterol | Sterols | 5680 | 1960 | 2.90 | 0.0218 |
| Cholesterol propionate | Sterol esters | N.D. | 0.12 | - | - |
| Hexadecanol | Alcohols | 109 | 76.3 | 1.43 | 0.0147 |
| Phosphoric acid | Others | 56,900 | 67,700 | 0.84 | 0.0228 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojakowska, A.; Pietrowska, M.; Widlak, P.; Dobrowolski, D.; Wylęgała, E.; Tarnawska, D. Metabolomic Signature Discriminates Normal Human Cornea from Keratoconus—A Pilot GC/MS Study. Molecules 2020, 25, 2933. https://doi.org/10.3390/molecules25122933
Wojakowska A, Pietrowska M, Widlak P, Dobrowolski D, Wylęgała E, Tarnawska D. Metabolomic Signature Discriminates Normal Human Cornea from Keratoconus—A Pilot GC/MS Study. Molecules. 2020; 25(12):2933. https://doi.org/10.3390/molecules25122933
Chicago/Turabian StyleWojakowska, Anna, Monika Pietrowska, Piotr Widlak, Dariusz Dobrowolski, Edward Wylęgała, and Dorota Tarnawska. 2020. "Metabolomic Signature Discriminates Normal Human Cornea from Keratoconus—A Pilot GC/MS Study" Molecules 25, no. 12: 2933. https://doi.org/10.3390/molecules25122933
APA StyleWojakowska, A., Pietrowska, M., Widlak, P., Dobrowolski, D., Wylęgała, E., & Tarnawska, D. (2020). Metabolomic Signature Discriminates Normal Human Cornea from Keratoconus—A Pilot GC/MS Study. Molecules, 25(12), 2933. https://doi.org/10.3390/molecules25122933






