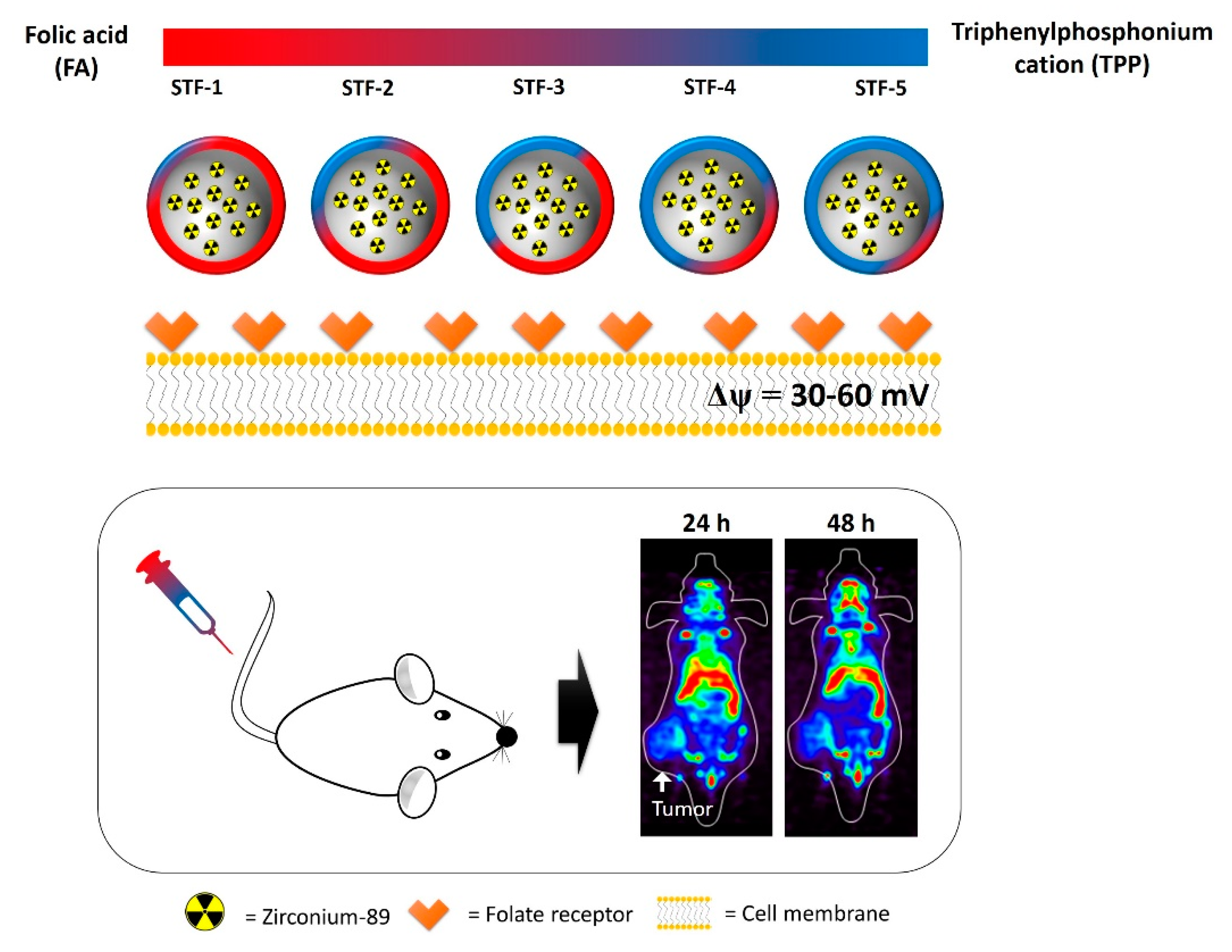
Tumor Targeting Effect of Triphenylphosphonium Cations and Folic Acid Coated with Zr-89-Labeled Silica Nanoparticles
Abstract
1. Introduction
2. Results
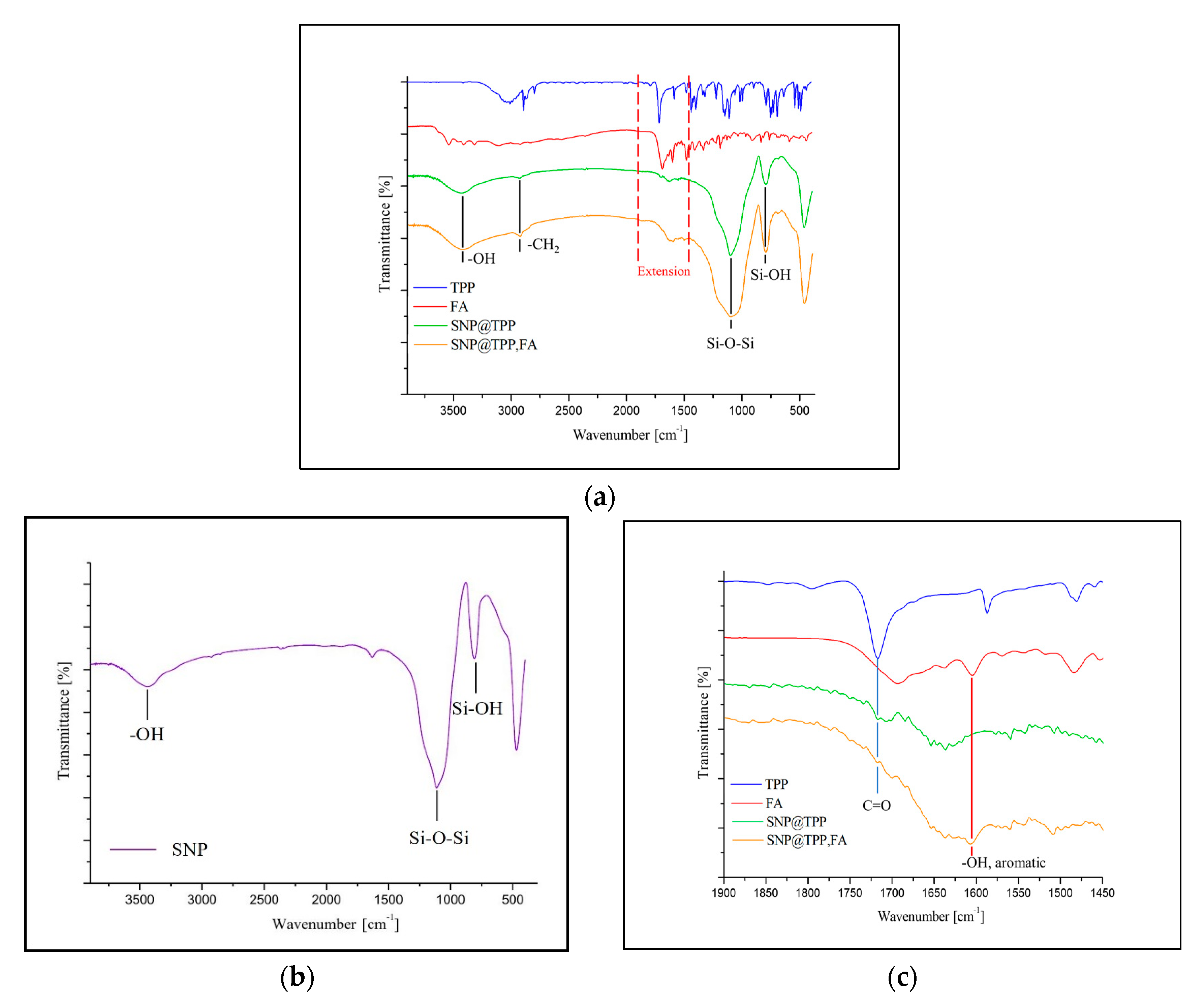
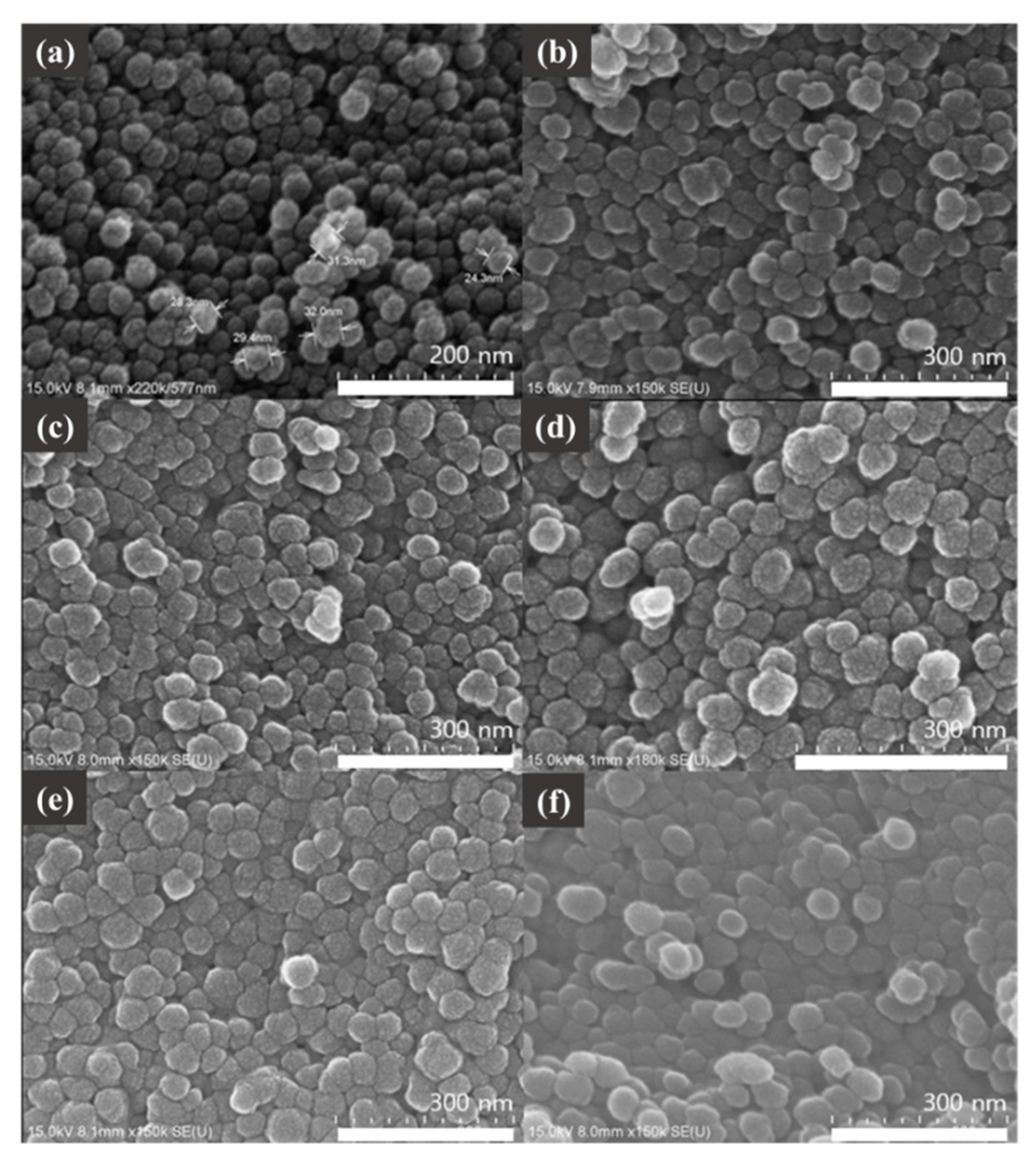
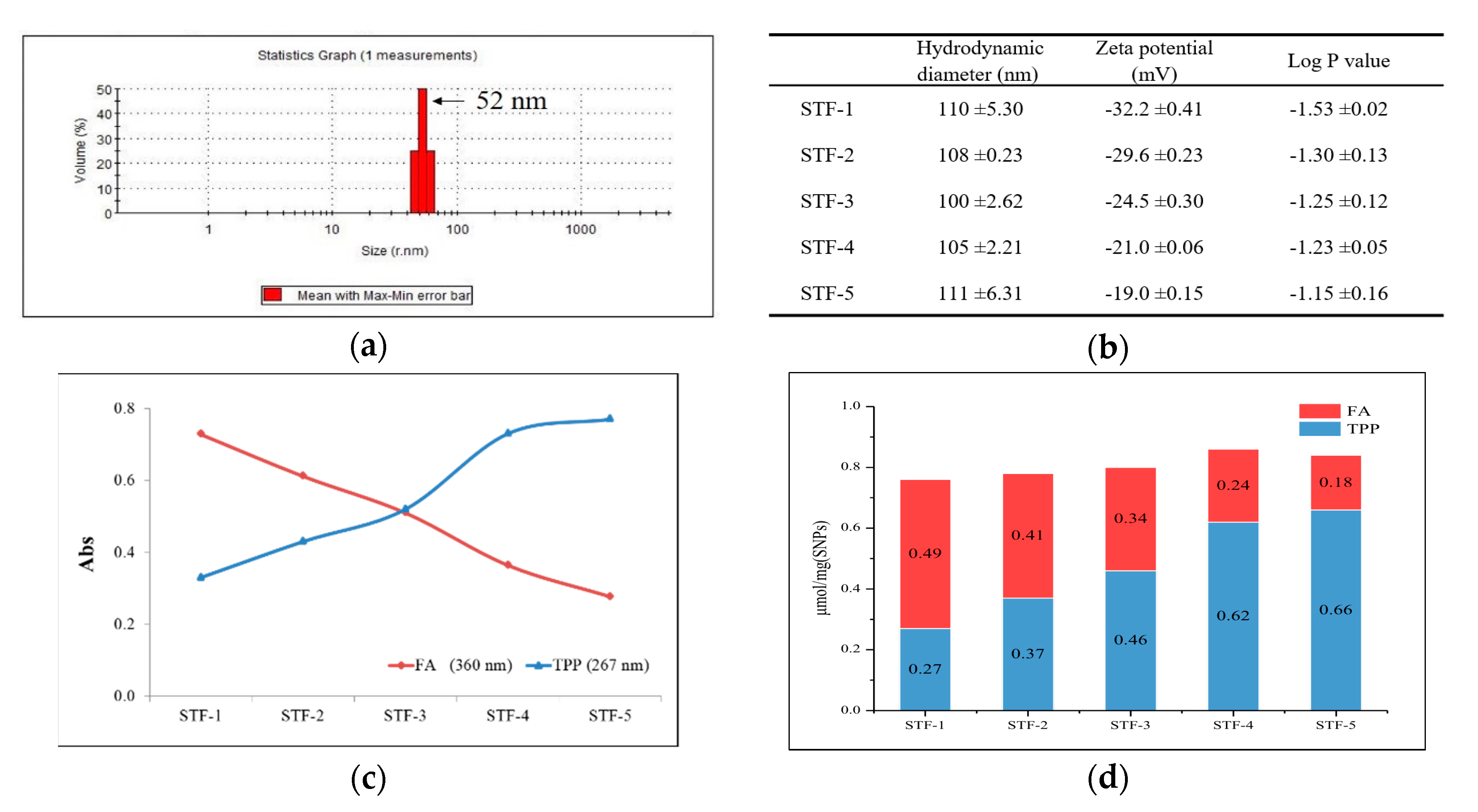
2.1. Synthesis of TPP and FA Conjugated SNPs
2.2. Zr-89 Radiolabeling
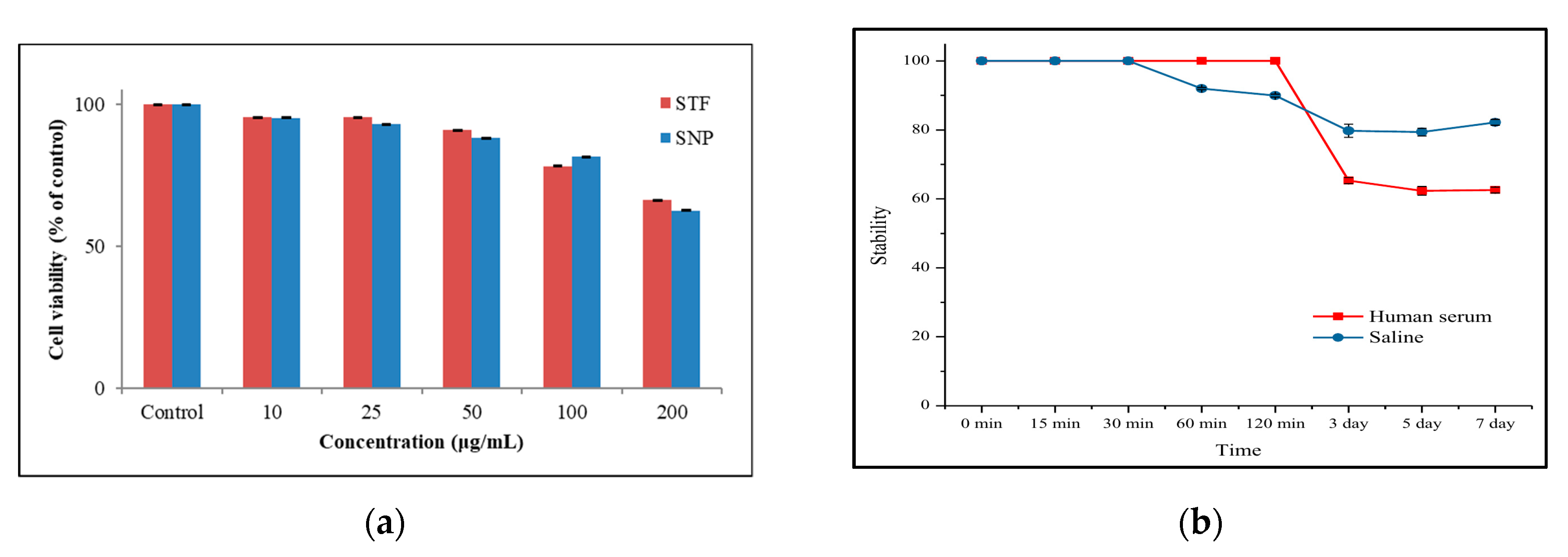
2.3. In-Vitro Studies: Cytotoxicity and Stability Test
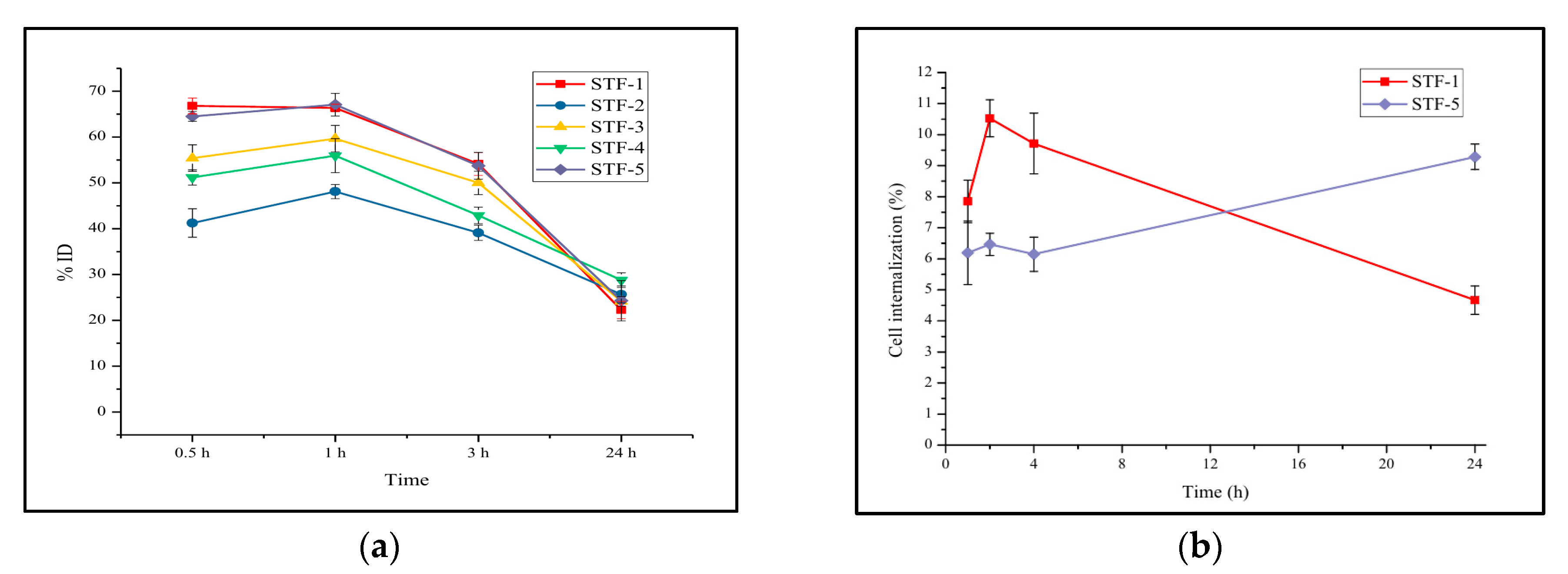
2.4. Cellular Uptake and Cell Internalization Assay
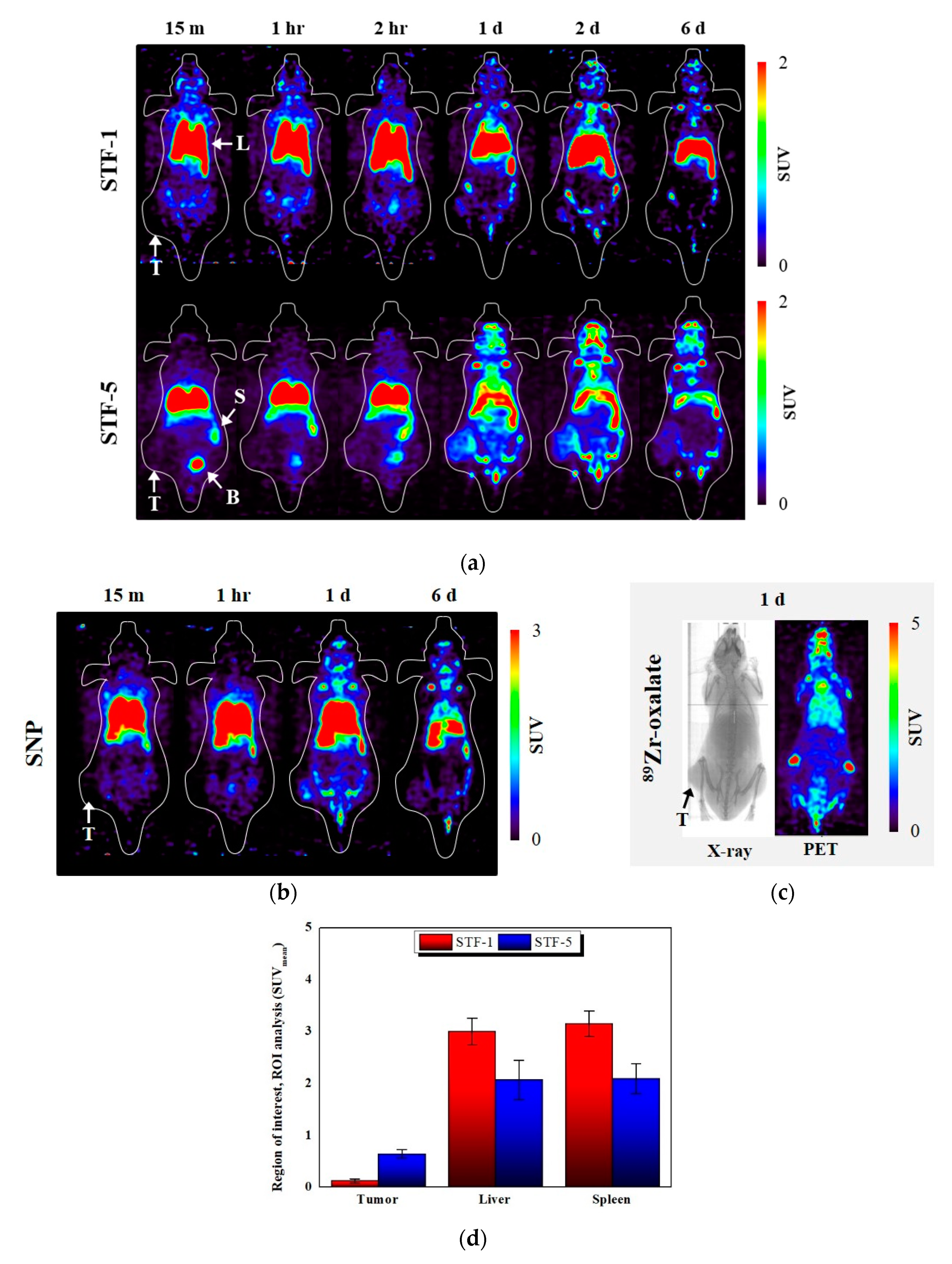
2.5. In-Vivo Study: PET Imaging
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of TPP Conjugated SNPs (ST)
4.3. Synthesis of FA Conjugated ST (STF)
4.4. Zr-89 Radiolabeling
4.5. Partition Coefficient (Log P Value)
4.6. In-Vitro Stability Studies
4.7. Cell Viability Study (MTT Assay)
4.8. Cellular Uptake
4.9. Cell Internalization Assay
4.10. PET Imaging
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, R.A.J.; Porteous, C.M.; Gane, A.M. Murphy, M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 5407–5412. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Cha, M.-Y.; Kim, D.; Kim, D.K.; Soh, M.; Shin, K.; Hyeon, T.; Mook-Jung, I. Mitochondria-Targeting Ceria Nanoparticles as Antioxidants for Alzheimer’s Disease. ACS Nano 2016, 10, 2860–2870. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.J.; Hartley, R.C.; Cochemé, H.M.; Murphy, M.P. Mitochondrial pharmacology. Trends Pharmacol. Sci. 2012, 33, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Frantz, M.-C.; Wipf, P. Mitochondria as a target in treatment. Environ. Mol. Mutagen. 2010, 51, 462–475. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhang, P.-Y. Mitochondria targeting nano agents in cancer therapeutics. Oncol. Lett. 2016, 12, 4887–4890. [Google Scholar] [CrossRef]
- Davis, S.; Weiss, M.J.; Wong, J.R.; Lampidis, T.J.; Chen, L.B. Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J. Biol. Chem. 1985, 260, 13844–13850. [Google Scholar]
- Modica-Napolitano, J.S.; Aprille, J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv. Drug Deliv. Rev. 2001, 49, 63–70. [Google Scholar] [CrossRef]
- Dairkee, S.; Hackett, A.J. Differential retention of rhodamine 123 by breast carcinoma and normal human mammary tissue. Breast Cancer Res. Treat. 1991, 18, 57–61. [Google Scholar] [CrossRef]
- Low, P.S.; Kularatne, S.A. Folate-targeted therapeutic and imaging agents for cancer. Curr. Opin. Chem. Biol. 2009, 13, 256–262. [Google Scholar] [CrossRef]
- Elnakat, H. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef]
- Salazar, M.D.; Ratnam, M. The folate receptor: What does it promise in tissue-targeted therapeutics? Cancer Metastasis Rev. 2007, 26, 141–152. [Google Scholar] [CrossRef]
- Parker, N.; Turk, M.J.; Westrick, E.; Lewis, J.D.; Low, P.S.; Leamon, C.P. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal. Biochem. 2005, 338, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Weitman, S.D.; Lark, R.H.; Coney, L.R.; Fort, D.W.; Frasca, V.; Zurawski, V.R.; Kamen, B.A. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992, 52, 3396–3401. [Google Scholar] [PubMed]
- Low, P.S.; Henne, W.A.; Doorneweerd, D.D. ChemInform Abstract: Discovery and Development of Folic-Acid-Based Receptor Targeting for Imaging and Therapy of Cancer and Inflammatory Diseases. ChemInform 2008, 39, 120–129. [Google Scholar] [CrossRef]
- Hilgenbrink, A.R.; Low, P.S. Folate Receptor-Mediated Drug Targeting: From Therapeutics to Diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Barbe, C.; Bartlett, J.; Kong, L.; Finnie, K.; Lin, H.Q.; Larkin, M.; Calleja, S.; Bush, A.; Calleja, G. Silica Particles: A Novel Drug-Delivery System. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Choi, P.S.; Lee, J.Y.; Vyas, C.K.; Yang, S.D.; Kim, S.W.; Park, J.H. One-pot synthesis of chelator-free 89Zr-incorporated hierarchical hematite nanoclusters for in vitro evaluation. J. Nanoparticle Res. 2019, 21, 240. [Google Scholar] [CrossRef]
- Benezra, M.; Medina, O.P.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef]
- Bradbury, M.S.; Phillips, E.; Montero, P.H.; Cheal, S.M.; Stambuk, H.; Durack, J.; Sofocleous, C.T.; Meester, R.J.C.; Wiesner, U.; Patel, S. Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr. Biol. 2013, 5, 74–86. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A. Historical perspective on advanced drug delivery: How engineering design and mathematical modeling helped the field mature. Adv. Drug Deliv. Rev. 2013, 65, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Slowingab, I.I.; Vivero-Escoto, J.L.; Wu, C.W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Cheng, J. Nonporous Silica Nanoparticles for Nanomedicine Application. Nano Today 2013, 8, 290–312. [Google Scholar] [CrossRef]
- Lee, J.Y.; Vyas, C.K.; Kim, G.G.; Choi, P.S.; Hur, M.G.; Yang, S.D.; Kong, Y.B.; Lee, E.J.; Park, J.H. Red Blood Cell Membrane Bioengineered Zr-89 Labelled Hollow Mesoporous Silica Nanosphere for Overcoming Phagocytosis. Sci. Rep. 2019, 9, 7419. [Google Scholar] [CrossRef]
- Vilaça, N.; Machado, A.F.B.; Morais-Santos, F.; Amorim, R.; Neto, A.P.; Logodin, E.; Pereira, M.F.R.; Sardo, M.; Rocha, J.; Parpot, P.; et al. Comparison of different silica microporous structures as drug delivery systems for in vitro models of solid tumors. RSC Adv. 2017, 7, 13104–13111. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2012, 65, 104–120. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Min, J.-J. Radiolabeled Phosphonium Salts as Mitochondrial Voltage Sensors for Positron Emission Tomography Myocardial Imaging Agents. Nucl. Med. Mol. Imaging 2016, 50, 185–195. [Google Scholar] [CrossRef]
- Goel, S.; Chen, F.; Luan, S.; Valdovinos, H.; Shi, S.; Graves, S.; Ai, F.; Barnhart, T.; Theuer, C.P.; Cai, W. Engineering Intrinsically Zirconium-89 Radiolabeled Self-Destructing Mesoporous Silica Nanostructures for In Vivo Biodistribution and Tumor Targeting Studies. Adv. Sci. 2016, 3, 1600122. [Google Scholar] [CrossRef]
- Chen, F.; Goel, S.; Valdovinos, H.F.; Luo, H.; Hernandez, R.; Barnhart, T.E.; Cai, W. In Vivo Integrity and Biological Fate of Chelator-Free Zirconium-89-Labeled Mesoporous Silica Nanoparticles. ACS Nano 2015, 9, 7950–7959. [Google Scholar] [CrossRef]
- Chen, F.; Ma, K.; Zhang, L.; Madajewski, B.; Zanzonico, P.; Sequeira, S.; Gonen, M.; Wiesner, U.; Bradbury, M.S. Target-or-Clear Zirconium-89 Labeled Silica Nanoparticles for Enhanced Cancer-Directed Uptake in Melanoma: A Comparison of Radiolabeling Strategies. Chem. Mater. 2017, 29, 8269–8281. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.G.; Lee, J.Y.; Choi, P.S.; Vyas, C.K.; Yang, S.D.; Hur, M.G.; Park, J.H. Synthesis and evaluation of triphenylphosphonium conjugated 18F-labeled silica nanoparticles for PET imaging. J. Radioanal. Nucl. Chem. 2018, 316, 1099–1106. [Google Scholar] [CrossRef]
Sample Availability: Samples of the silica nanoparticles are available on request to the authors. |
| Samples | Labeling Yield (%) | Radiochemical Purity (%) |
|---|---|---|
| STF-1 | 67.5 ± 28.6 | 98.7 ± 0.95 |
| STF-2 | 68.7 ± 3.70 | 99.6 ± 0.26 |
| STF-3 | 70.3 ± 2.36 | 99.8 ± 0.17 |
| STF-4 | 69.3 ± 1.59 | 99.6 ± 0.26 |
| STF-5 | 71.2 ± 2.99 | 99.8 ± 0.10 |
| Mean | 69.4 ± 1.43 | 99.5 ± 0.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.G.; Lee, J.Y.; Choi, P.S.; Kim, S.W.; Park, J.H. Tumor Targeting Effect of Triphenylphosphonium Cations and Folic Acid Coated with Zr-89-Labeled Silica Nanoparticles. Molecules 2020, 25, 2922. https://doi.org/10.3390/molecules25122922
Kim GG, Lee JY, Choi PS, Kim SW, Park JH. Tumor Targeting Effect of Triphenylphosphonium Cations and Folic Acid Coated with Zr-89-Labeled Silica Nanoparticles. Molecules. 2020; 25(12):2922. https://doi.org/10.3390/molecules25122922
Chicago/Turabian StyleKim, Gun Gyun, Jun Young Lee, Pyeong Seok Choi, Sang Wook Kim, and Jeong Hoon Park. 2020. "Tumor Targeting Effect of Triphenylphosphonium Cations and Folic Acid Coated with Zr-89-Labeled Silica Nanoparticles" Molecules 25, no. 12: 2922. https://doi.org/10.3390/molecules25122922
APA StyleKim, G. G., Lee, J. Y., Choi, P. S., Kim, S. W., & Park, J. H. (2020). Tumor Targeting Effect of Triphenylphosphonium Cations and Folic Acid Coated with Zr-89-Labeled Silica Nanoparticles. Molecules, 25(12), 2922. https://doi.org/10.3390/molecules25122922







