Magnetic Solid-Phase Extraction Based on Poly 4-Vinyl Pyridine for HPLC-FLD Analysis of Naproxen in Urine Samples
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Characterization of Magnetics Solids
2.2. Adsorption Capacity of Magnetic Solids
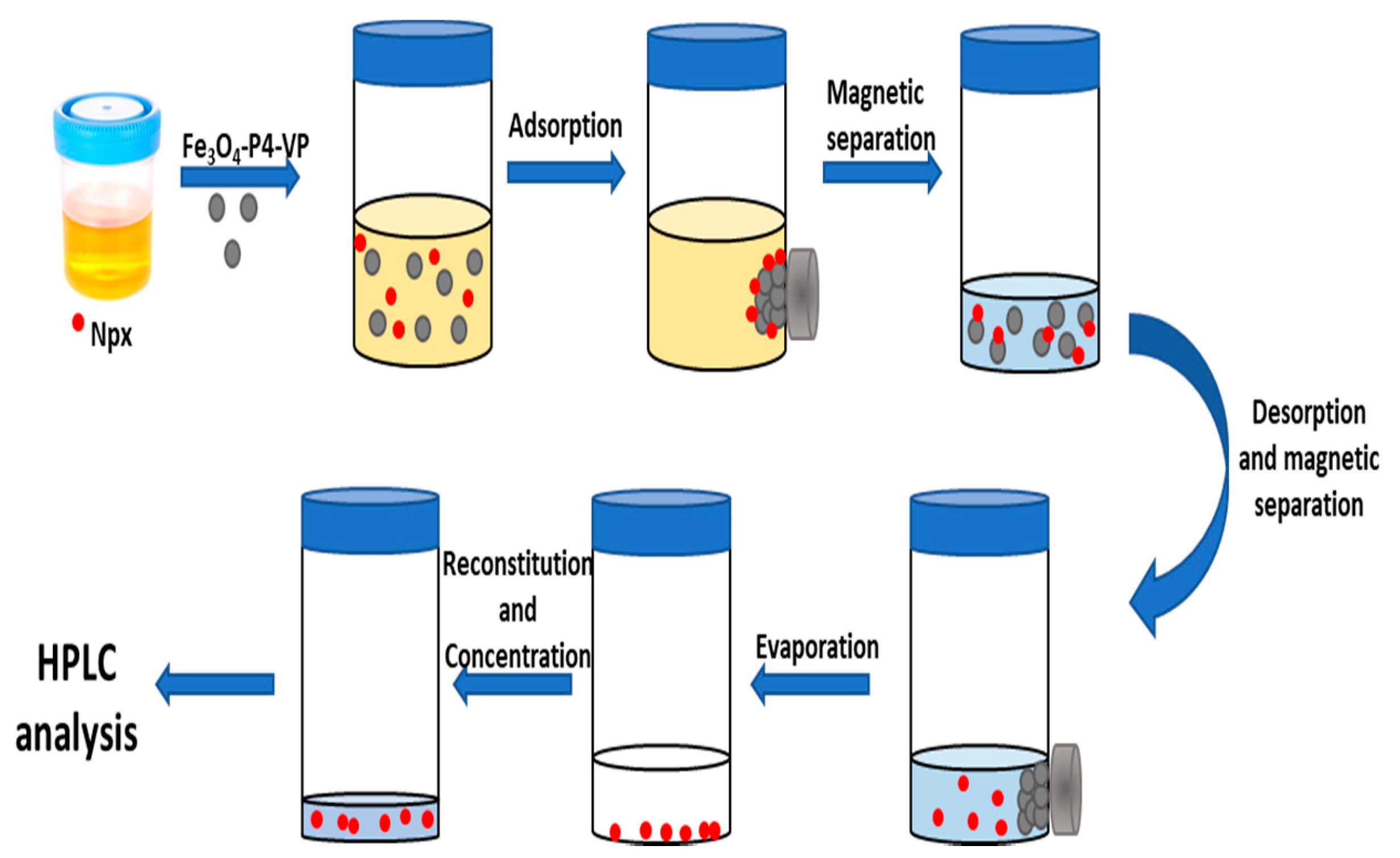
2.3. Optimization of the MSPE Conditions
2.4. Method Validation and Urine Samples Analysis
3. Materials and Methods
3.1. Chemicals
3.2. Equipment
3.3. Synthesis of Magnetic Solids
3.4. Characterization of the Solid
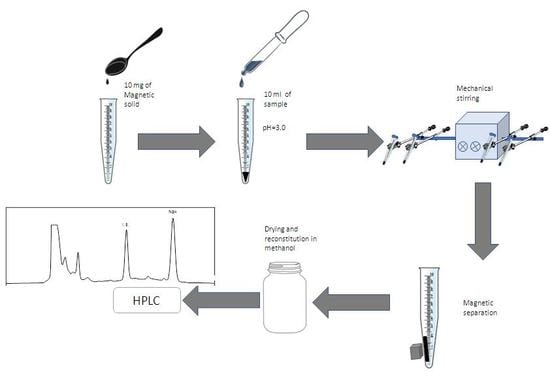
3.5. Sample Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santini, A.O.; Oliveira, J.E.D.; Pezza, H.R.; Pezza, L. A novel potentiometric naproxenate ion sensor immobilized in a graphite matrix for determination of naproxen in pharmaceuticals. J. Braz. Chem. Soc. 2006, 17, 785–791. [Google Scholar] [CrossRef]
- Adhoum, N.; Monser, L.; Toumi, M.; Boujlel, K. Determination of naproxen in pharmaceuticals by differential pulse voltammetry at a platinum electrode. Anal. Chim. Acta 2003, 495, 69–75. [Google Scholar] [CrossRef]
- Madrakian, T.; Ahmadi, M.; Afkhami, A.; Soleimani, M. Selective solid-phase extraction of naproxen drug from human urine samples using molecularly imprinted polymer-coated magnetic multi-walled carbon nanotubes prior to its spectrofluorometric determination. Analyst 2013, 138, 4542–4549. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.; Talukder, M.M.U.; Laila, S.; Fatema, K.; Kabir, A.K.L. Simultaneous estimation of naproxen and ranitidine HCl by using UV spectrophotometer. Stamford J. Pharm. Sci. 2008, 1, 18–24. [Google Scholar] [CrossRef][Green Version]
- Sebők, Á.; Vasanits-Zsigrai, A.; Palkó, G.; Záray, G.; Molnár-Perl, I. Identification and quantification of ibuprofen, naproxen, ketoprofen and diclofenac present in waste-waters, as their trimethylsilyl derivatives, by gas chromatography mass spectrometry. Talanta 2008, 76, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Song, C.; Liao, J.; Huang, Z.; Li, G. Water stable metal-organic framework packed microcolumn for online sorptive extraction and direct analysis of naproxen and its metabolite from urine sample. J. Chromatogr. A 2013, 1294, 17–24. [Google Scholar] [CrossRef]
- Phillips, T.M.; Wellner, E.F. Measurement of naproxen in human plasma by chip-based immunoaffinity capillary electrophoresis. Biomed. Chromatogr. 2006, 20, 662–667. [Google Scholar] [CrossRef]
- Seidi, S.; Sanàti, S.E. Nickel-iron layered double hydroxide nanostructures for micro solid phase extraction of nonsteroidal anti-inflammatory drugs, followed by quantitation by HPLC-UV. Microchim. Acta 2019, 186, 297. [Google Scholar] [CrossRef]
- Wang, J.Q.; Zheng, B.G.; Zhang, J.B.; Zheng, Z. Degradation of the emerging contaminant naproxen in aqueous solutions by dielectric barrier discharge. Asian J. Chem. 2013, 25, 3595–3600. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, I.; Alonso, E.; Callejón, M. Simultaneous determination of pharmaceutically active compounds in wastewater samples by solid phase extraction and high-performance liquid chromatography with diode array and fluorescence detectors. Anal. Chim. Acta 2005, 550, 116–122. [Google Scholar] [CrossRef]
- Jim, T.Y.; Bisceglia, K.J.; Bouwer, E.J.; Roberts, A.L.; Coelhan, M. Determination of pharmaceuticals and antiseptics in water by solid-phase extraction and gas chromatography/mass spectrometry: Analysis via pentafluorobenzylation and stable isotope dilution. Anal. Bioanal. Chem. 2012, 403, 583–591. [Google Scholar] [CrossRef]
- Duan, C.; Shen, Z.; Wu, D.; Guan, Y. Recent developments in solid-phase microextraction for on-site sampling and sample preparation. TrAC, Trends Anal. Chem. 2011, 30, 1568–1574. [Google Scholar] [CrossRef]
- Aqda, T.G.; Behkami, S.; Bagheri, H. Porous eco–friendly fibers for on–line micro solid–phase extraction of nonsteroidal anti–inflammatory drugs from urine and plasma samples. J. Chromatogr. A 2018, 1574, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Chamsaz, M.; Rounaghi, G.H. Ultrasound-assisted magnetic dispersive solid-phase microextraction: A novel approach for the rapid and efficient microextraction of naproxen and ibuprofen employing experimental design with high-performance liquid chromatography. J. Sep. Sci. 2016, 39, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Aghamohammadhasan, M.; Shams, A.; Tajfirooz, F.; Pourhassan, R.; Khosravi, S.R.B.; Jampour, A. Ultrasonic assisted magnetic dispersive solid phase microextraction for preconcentration of two nonsteroidal anti-inflammatory drugs in real water, biological and milk samples employing an experimental design. Microchem. J. 2019, 145, 1026–1035. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). TrAC, Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Khezeli, T.; Daneshfar, A. Development of dispersive micro-solid phase extraction based on micro and nano sorbents. TrAC Trends Anal. Chem. 2017, 89, 99–118. [Google Scholar] [CrossRef]
- Asgharinezhad, A.A.; Ebrahimzadeh, H. Poly (2-aminobenzothiazole)-coated graphene oxide/magnetite nanoparticles composite as an efficient sorbent for determination of non-steroidal anti-inflammatory drugs in urine sample. J. Chromatogr. A 2016, 1435, 18–29. [Google Scholar] [CrossRef]
- Li, G.; Deng, R.; Peng, G.; Yang, C.; He, Q.; Lu, Y.; Shi, H. Magnetic solid-phase extraction for the analysis of bisphenol A, naproxen and triclosan in wastewater samples. Water Sci. Technol. 2018, 77, 2220–2227. [Google Scholar] [CrossRef]
- Abujaber, F.; Zougagh, M.; Jodeh, S.; Ríos, Á.; Bernardo, F.J.G.; Martín-Doimeadios, R.C.R. Magnetic cellulose nanoparticles coated with ionic liquid as a new material for the simple and fast monitoring of emerging pollutants in waters by magnetic solid phase extraction. Microchem. J. 2018, 137, 490–495. [Google Scholar] [CrossRef]
- Mohammadi Nodeh, M.K.; Radfard, M.; Zardari, L.A.; Rashidi Nodeh, H. Enhanced removal of naproxen from wastewater using silica magnetic nanoparticles decorated onto graphene oxide; parametric and equilibrium study. Sep. Sci. Technol. 2018, 53, 2476–2485. [Google Scholar] [CrossRef]
- Aguilar-Arteaga, K.; Rodriguez, J.A.; Miranda, J.M.; Medina, J.; Barrado, E. Determination of non-steroidal anti-inflammatory drugs in wastewaters by magnetic matrix solid phase dispersion–HPLC. Talanta 2010, 80, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Rafighi, P.; Yaftian, M.R.; Haghighi, B. Magnetic nanofibrous polyaniline nanocomposite for solid-phase extraction of naproxen from biological samples prior to its spectrofluorimetric determination. J. Iran. Chem. Soc. 2018, 15, 1209–1221. [Google Scholar] [CrossRef]
- Barrado, E.; Rodríguez, J.A. Extraction. Magnetic Materials in Separation Science. Encycl Anal. Sci. 2019, 3, 63–66. [Google Scholar] [CrossRef]
- Kempe, M.; Mosbach, K. Direct resolution of naproxen on a non-covalently molecularly imprinted chiral stationary phase. J. Chromatogr. A 1994, 664, 276–279. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; He, L.; Meng, L.; Lu, H.; Li, X. Preparation of poly(4-vinylpyridine)-functionalized magnetic Al-MOF for the removal of naproxen from aqueous solution. J. Hazard. Mater. 2020, 383, 121144. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Kim, J.K. Solvothermal synthesis and magnetic properties of magnetite nanoplatelets. Mater. Lett. 2009, 63, 428–430. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Bazrafshan, A.A.; Jannesar, R. Comparative study on ultrasonic assisted adsorption of dyes from single system onto Fe3O4 magnetite nanoparticles loaded on activated carbon: Experimental design methodology. Ultrason. Sonochem. 2017, 34, 294–304. [Google Scholar] [CrossRef]
- Sahiner, N.; Ilgin, P. Multiresponsive polymeric particles with tunable morphology and properties based on acrylonitrile (AN) and 4-vinylpyridine (4-VP). Polymer 2010, 51, 3156–3163. [Google Scholar] [CrossRef]
- Ibarra, I.S.; Rodriguez, J.A.; Miranda, J.M.; Vega, M.; Barrado, E. Magnetic solid phase extraction based on phenyl silica adsorbent for the determination of tetracyclines in milk samples by capillary electrophoresis. J. Chromatogr. A 2011, 1218, 2196–2202. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Rutnakornpituk, B.; Wichai, U.; Vilaivan, T.; Rutnakornpituk, M. Surface-initiated atom transfer radical polymerization of poly (4-vinylpyridine) from magnetite nanoparticle. J. Nanopart. Res. 2011, 13, 6847–6857. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Espinosa, J.; Aguilar-Arteaga, K.; Ibarra, I.S.; Miranda, J.M. Determination of tetracyclines in milk samples by magnetic solid phase extraction flow injection analysis. Microchim. Acta 2010, 171, 407–413. [Google Scholar] [CrossRef]
- Aresta, A.; Palmisano, F.; Zambonin, C.G. Determination of naproxen in human urine by solid-phase microextraction coupled to liquid chromatography. J. Pharm. Biomed. Anal. 2005, 39, 643–647. [Google Scholar] [CrossRef]
- Sarafraz-Yazdi, A.; Amiri, A.; Rounaghi, G.; Eshtiagh-Hosseini, H. Determination of non-steroidal anti-inflammatory drugs in water samples by solid-phase microextraction based sol–gel technique using poly (ethylene glycol) grafted multi-walled carbon nanotubes coated fiber. Anal. Chim. Acta 2012, 720, 134–141. [Google Scholar] [CrossRef]
- Ayazi, Z.; Matin, A.A. Development of carbon nanotube–polyamide nanocomposite-based stir bar sorptive extraction coupled to HPLC-UV applying response surface methodology for the analysis of bisphenol a in aqueous samples. J. Chromatogr. Sci. 2016, 54, 1841–1850. [Google Scholar] [CrossRef]
- Nagai, K.; Shibata, T.; Shinkura, S.; Ohnishi, A. Poly (4-vinylpyridine) based novel stationary phase investigated under supercritical fluid chromatography conditions. J. Chromatogr. A 2018, 1572, 119–127. [Google Scholar] [CrossRef]
- Mafra, G.; Spudeit, D.; Brognoli, R.; Merib, J.; Carasek, E. Expanding the applicability of cork as extraction phase for disposable pipette extraction in multiresidue analysis of pharmaceuticals in urine samples. J. Chromatogr. B 2018, 1102, 159–166. [Google Scholar] [CrossRef]
- Food and Drug Administration. Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2-r1-validation-analytical-procedures-text-and-methodology (accessed on 15 June 2020).
- Caro, E.; Marcé, R.M.; Cormack, P.A.; Sherrington, D.C.; Borrull, F. A new molecularly imprinted polymer for the selective extraction of naproxen from urine samples by solid-phase extraction. J. Chromatogr. B 2004, 813, 137–143. [Google Scholar] [CrossRef]
- Bagheri, H.; Khanipour, P.; Asgari, S. Magnetic field assisted μ-solid phase extraction of anti-inflammatory and loop diuretic drugs by modified polybutylene terephthalate nanofibers. Anal. Chim. Acta 2016, 934, 88–97. [Google Scholar] [CrossRef]
- Ibarra, I.S.; Miranda, J.M.; Rodriguez, J.A.; Nebot, C.; Cepeda, A. Magnetic solid phase extraction followed by high-performance liquid chromatography for the determination of sulphonamides in milk samples. Food Chem. 2014, 157, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Hamulić, D.; Rodič, P.; Poberžnik, M.; Jereb, M.; Kovač, J.; Milošev, I. The Effect of the Methyl and Ethyl Group of the Acrylate Precursor in Hybrid Silane Coatings Used for Corrosion Protection of Aluminium Alloy 7075-T6. Coatings 2020, 10, 172. [Google Scholar] [CrossRef]
Sample Availability: Samples of the solids compounds A, B and C are available from the authors. |





| Solid | Kd (×10−6 M) | Active Sites (mmoL H+ kg−1) | Qmax (mmoL kg−1) |
|---|---|---|---|
| A | 24.5 ± 0.1 | 700 ± 3 | 79.8 ± 1.0 |
| B | 20.4 ± 0.1 | 500 ± 2 | 78.8 ± 5.7 |
| C | 3.5 ± 0.1 | 1200 ± 4 | 82.5 ± 1.4 |
| Experiment | SC | NaCl (M) | MV (mL) | pH | [Area Npx]/[Area IS] |
|---|---|---|---|---|---|
| 1 | A | 0.5 | 1 | 1 | 0.97 |
| 2 | A | 1.0 | 2 | 2 | 0.56 |
| 3 | A | 1.5 | 3 | 3 | 1.58 |
| 4 | B | 0.5 | 2 | 3 | 0.98 |
| 5 | B | 1.0 | 3 | 1 | 0.82 |
| 6 | B | 1.5 | 1 | 2 | 4.90 |
| 7 | C | 0.5 | 3 | 2 | 0.94 |
| 8 | C | 1 | 1 | 3 | 2.15 |
| 9 | C | 1.5 | 2 | 1 | 0.60 |
| [Npx]added (µg L−1) | [Npx]calculated (µg L−1) | Repeatability, Within-Day (%RSD, n = 3) | Repeatability, Between-Days (%RSD, n = 3) | % Recovery |
|---|---|---|---|---|
| 5.0 | 4.9 | 2.2 | 3.7 | 98.0 |
| 30.0 | 30.2 | 1.6 | 1.9 | 100.7 |
| 50.0 | 49.7 | 1.5 | 2.1 | 99.4 |
| Sample | [Npx]added (µg L−1) | [Npx]calculated (µg L−1, %RSD, n = 3) | [Npx]total (µg L−1) a | % Recovery b |
|---|---|---|---|---|
| M1 | 8.2 (1.9) | |||
| 15.0 | 22.1 (1.7) | 23.2 | 95.3 | |
| 35.0 | 42.7 (1.3) | 43.2 | 98.8 | |
| M2 | 7.8 (2.0) | |||
| 15.0 | 23.1 (2.0) | 22.8 | 101.3 | |
| 35.0 | 42.0 (0.7) | 42.8 | 98.1 | |
| W1 | 6.0 (2.5) | |||
| 15.0 | 20.3 (2.3) | 21.0 | 96.7 | |
| 35.0 | 40.7 (2.7) | 41.0 | 99.3 | |
| W2 | 1.3 (4.6) | |||
| 15.0 | 16.4 (1.9) | 16.3 | 100.6 | |
| 35.0 | 35.7 (1.3) | 36.3 | 98.3 |
| Sample Preparation Method | Detection System | LOD (µg L−1) | RSD (%) | Sample Volume (mL) | Reconstitution Volume (mL) | Reference |
|---|---|---|---|---|---|---|
| MMOF-SPME a | HPLC-UV | 0.03 | <4.7 | 5.0 | 2.0 | [40] |
| LDH-PS-μSPE b | HPLC-UV | 5.0 | <7.4 | 0.25 | 0.2 | [8] |
| MFA-SPE c | HPLC-UV | 2.0 | <6.7 | 50.0 | 5.0 | [41] |
| MIP-SPE d | FLD | 2.0 | <1.0 | 40.0–100.0 | 2.0 | [3] |
| MSPE | HPLC-FLD | 0.02 | <5.2 | 1.0 | 0.5 | This work |
| Solid | Magnetite (mmoL) | 4-VP (mmoL) | MA (mmoL) | EGDMA (mmoL) |
|---|---|---|---|---|
| A | 3.0 | 0.3 | 0.3 | 5.0 |
| B | 6.0 | 0.3 | 0.3 | 5.0 |
| C | 12.0 | 0.3 | 0.3 | 5.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla-Lara, K.A.; Heredia, A.C.; Peña-Alvarez, A.; Ibarra, I.S.; Barrado, E.; Rodriguez, J.A. Magnetic Solid-Phase Extraction Based on Poly 4-Vinyl Pyridine for HPLC-FLD Analysis of Naproxen in Urine Samples. Molecules 2020, 25, 2924. https://doi.org/10.3390/molecules25122924
Escamilla-Lara KA, Heredia AC, Peña-Alvarez A, Ibarra IS, Barrado E, Rodriguez JA. Magnetic Solid-Phase Extraction Based on Poly 4-Vinyl Pyridine for HPLC-FLD Analysis of Naproxen in Urine Samples. Molecules. 2020; 25(12):2924. https://doi.org/10.3390/molecules25122924
Chicago/Turabian StyleEscamilla-Lara, Karen A., Ana C. Heredia, Araceli Peña-Alvarez, Israel S. Ibarra, Enrique Barrado, and Jose A. Rodriguez. 2020. "Magnetic Solid-Phase Extraction Based on Poly 4-Vinyl Pyridine for HPLC-FLD Analysis of Naproxen in Urine Samples" Molecules 25, no. 12: 2924. https://doi.org/10.3390/molecules25122924
APA StyleEscamilla-Lara, K. A., Heredia, A. C., Peña-Alvarez, A., Ibarra, I. S., Barrado, E., & Rodriguez, J. A. (2020). Magnetic Solid-Phase Extraction Based on Poly 4-Vinyl Pyridine for HPLC-FLD Analysis of Naproxen in Urine Samples. Molecules, 25(12), 2924. https://doi.org/10.3390/molecules25122924