(Poly)phenolic Content and Profile and Antioxidant Capacity of Whole-Grain Cookies are Better Estimated by Simulated Digestion than Chemical Extraction
Abstract
1. Introduction
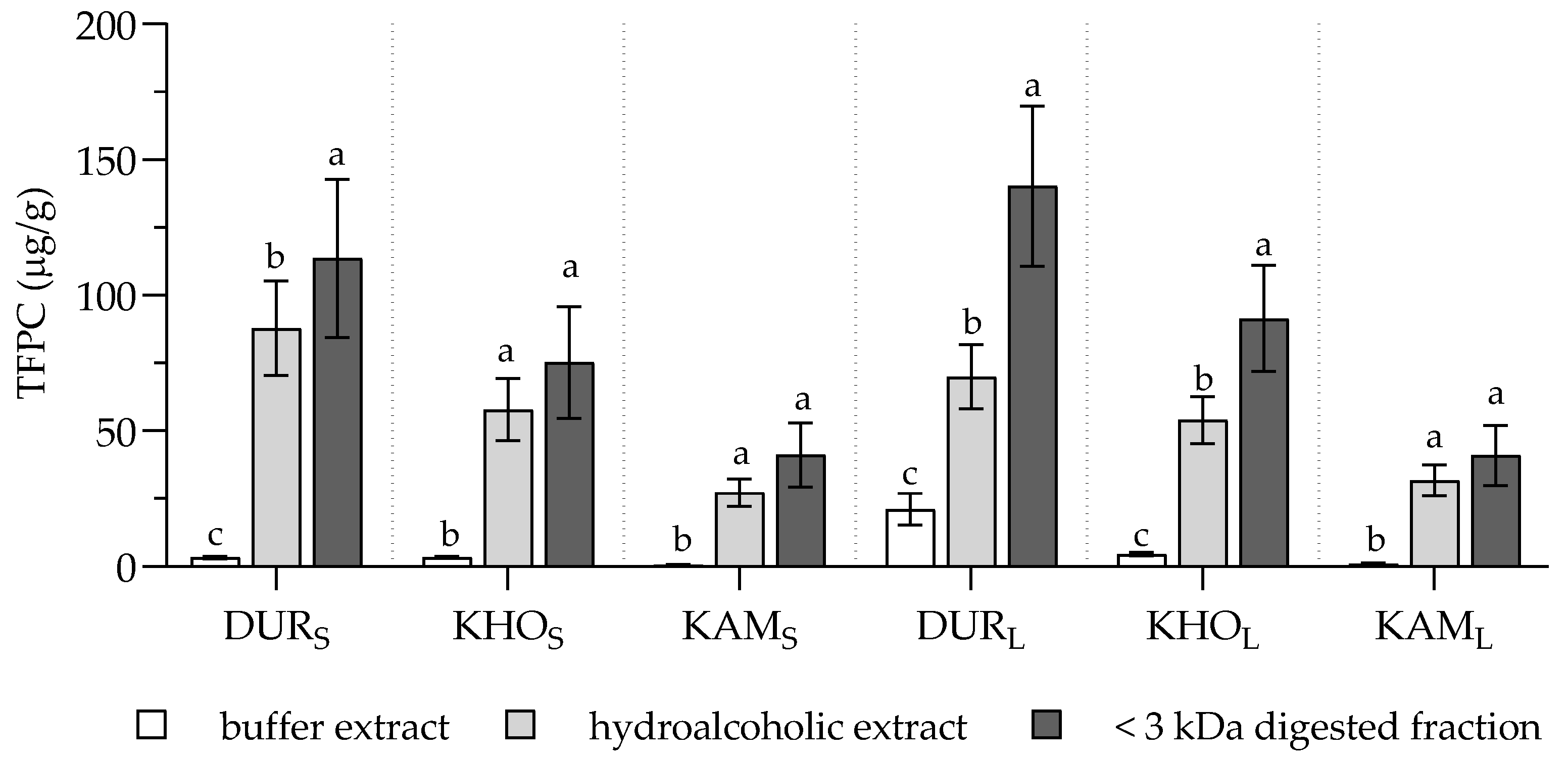
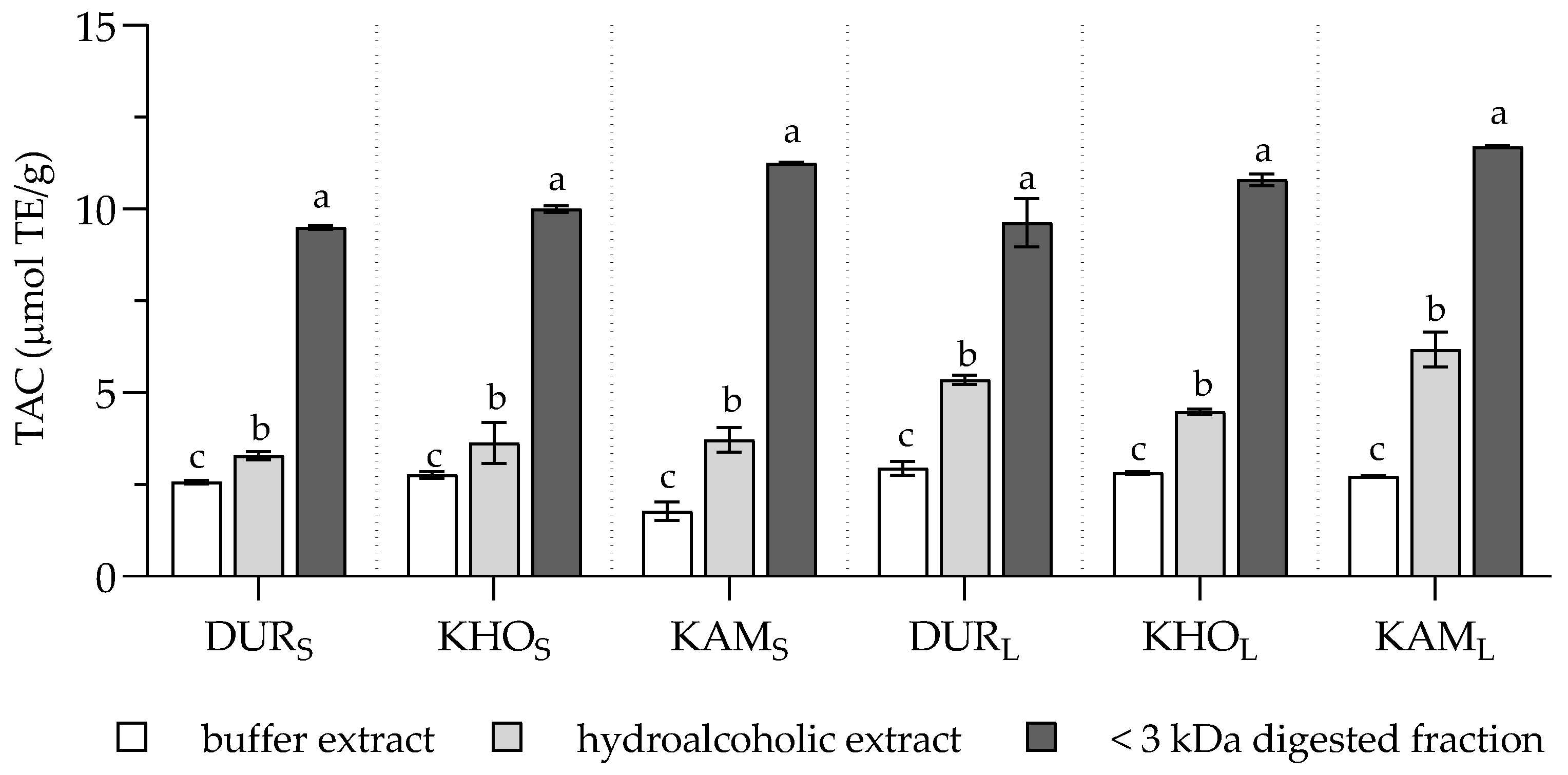
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Cookie Preparation
3.3. Cookie Chemical Extraction
3.4. Cookie Simulated Digestion
3.5. Total Antioxidant Capacity (TAC)
3.6. (Poly)phenolic Profile and Content
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegria, A.; Alminger, M.; Arrigoni, E.; Aura, A.M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap-deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- Lampe, J.W.; Chang, J.L. Interindividual differences in phytochemical metabolism and disposition. Semin. Cancer Biol. 2007, 17, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ross, K. Concepts important in understanding the health benefits of phenolics in fruits and vegetables: Extractable & non-extractable phenolics and the influence of cell wall polysaccharides on bioaccessibility & bioavailability. Res. Health Nutr. 2014, 2, 29–43. [Google Scholar]
- Rubió, L.; Macià, A.; Castell-Auví, A.; Pinent, M.; Blay, M.T.; Ardévol, A.; Romero, M.P.; Motilva, M.J. Effect of the co-occurring olive oil and thyme extracts on the phenolic bioaccesibility and bioavailability assessed by in vitro digestion and cell models. Food Chem. 2014, 149, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Danesi, F.; Govoni, M.; D’Antuono, L.F.; Bordoni, A. The molecular mechanism of the cholesterol-lowering effect of dill and kale: The influence of the food matrix components. Electrophoresis 2016, 37, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; Da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Guerin-Dubiard, C.; Jardin, J.; Lechevalier, V.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, E.; Hingyi, H.; et al. The food matrix affects the anthocyanin profile of fortified egg and dairy matrices during processing and in vitro digestion. Food Chem. 2017, 214, 486–496. [Google Scholar] [CrossRef]
- Nagah, A.M.; Seal, C.J. In vitro procedure to predict apparent antioxidant release from wholegrain foods measured using three different analytical methods. J. Sci. Food Agric. 2005, 85, 1177–1185. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Concept and health-related properties of nonextractable polyphenols: The missing dietary polyphenols. J. Agric. Food Chem. 2012, 60, 11195–11200. [Google Scholar] [CrossRef]
- Serrano, J.; Goñi, I.; Saura-Calixto, F. Food antioxidant capacity determined by chemical methods may underestimate the physiological antioxidant capacity. Food Res. Int. 2007, 40, 15–21. [Google Scholar] [CrossRef]
- Valli, V.; Danesi, F.; Gianotti, A.; Di Nunzio, M.; Taneyo Saa, D.L.; Bordoni, A. Antioxidative and anti-inflammatory effect of in vitro digested cookies baked using different types of flours and fermentation methods. Food Res. Int. 2016, 88, 256–262. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E.; Rémésy, C. Is the in vitro antioxidant potential of whole-grain cereals and cereal products well reflected in vivo? J. Cereal Sci. 2008, 48, 258–276. [Google Scholar] [CrossRef]
- Vitali, D.; Dragojević, I.V.; Šebečić, B. Effects of incorporation of integral raw materials and dietary fibre on the selected nutritional and functional properties of biscuits. Food Chem. 2009, 114, 1462–1469. [Google Scholar] [CrossRef]
- Quinn, R.M. Kamut®: Ancient grain, new cereal. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, Egypt, 1999; pp. 182–183. [Google Scholar]
- Bordoni, A.; Danesi, F.; Di Nunzio, M.; Taccari, A.; Valli, V. Ancient wheat and health: A legend or the reality? A review on KAMUT khorasan wheat. Int. J. Food Sci. Nutr. 2017, 68, 278–286. [Google Scholar] [CrossRef]
- Poutanen, K.; Flander, L.; Katina, K. Sourdough and cereal fermentation in a nutritional perspective. Food Microbiol. 2009, 26, 693–699. [Google Scholar] [CrossRef]
- Khosravi, A.; Razavi, S.H.; Fadda, A.M. Advanced assessments on innovative methods to improve the bioaccessibility of polyphenols in wheat. Process Biochem. 2020, 88, 1–14. [Google Scholar] [CrossRef]
- Brandolini, A.; Castoldi, P.; Plizzari, L.; Hidalgo, A. Phenolic acids composition, total polyphenols content and antioxidant activity of Triticum monococcum, Triticum turgidum and Triticum aestivum: A two-years evaluation. J. Cereal Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Dall’Asta, M.; Bresciani, L.; Calani, L.; Cossu, M.; Martini, D.; Melegari, C.; Del Rio, D.; Pellegrini, N.; Brighenti, F.; Scazzina, F. In vitro bioaccessibility of phenolic acids from a commercial aleurone-enriched bread compared to a whole grain bread. Nutrients 2016, 8, 42. [Google Scholar] [CrossRef]
- Brazier-Hicks, M.; Evans, K.M.; Gershater, M.C.; Puschmann, H.; Steel, P.G.; Edwards, R. The C-glycosylation of flavonoids in cereals. J. Biol. Chem. 2009, 284, 17926–17934. [Google Scholar] [CrossRef] [PubMed]
- Courts, F.L.; Williamson, G. The occurrence, fate and biological activities of C-glycosyl flavonoids in the human diet. Crit. Rev. Food Sci. Nutr. 2015, 55, 1352–1367. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, G.Y.; Mares, D.J. Apigenin di-C-glycosides (ACG) content and composition in grains of bread wheat (Triticum aestivum) and related species. J. Cereal Sci. 2012, 56, 260–267. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Isolani, L.; Arfaioli, P.; Ghiselli, L.; Romani, A. Polyphenol content of modern and old varieties of Triticum aestivum L. and T. durum Desf. grains in two years of production. J. Agric. Food Chem. 2010, 58, 7329–7334. [Google Scholar] [CrossRef]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMSn and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef]
- Yu, L.; Beta, T. Identification and antioxidant properties of phenolic compounds during production of bread from purple wheat grains. Molecules 2015, 20, 15525–15549. [Google Scholar] [CrossRef]
- Hole, A.S.; Rud, I.; Grimmer, S.; Sigl, S.; Narvhus, J.; Sahlstrøm, S. Improved bioavailability of dietary phenolic acids in whole grain barley and oat groat following fermentation with probiotic Lactobacillus acidophilus, Lactobacillus johnsonii, and Lactobacillus reuteri. J. Agric. Food Chem. 2012, 60, 6369–6375. [Google Scholar] [CrossRef]
- Ripari, V.; Bai, Y.; Ganzle, M.G. Metabolism of phenolic acids in whole wheat and rye malt sourdoughs. Food Microbiol. 2019, 77, 43–51. [Google Scholar] [CrossRef]
- Dynkowska, W.M.; Cyran, M.R.; Ceglińska, A. Soluble and cell wall-bound phenolic acids and ferulic acid dehydrodimers in rye flour and five bread model systems: Insight into mechanisms of improved availability. J. Sci. Food Agric. 2015, 95, 1103–1115. [Google Scholar] [CrossRef]
- Skrajda-Brdak, M.; Konopka, I.; Tańska, M.; Czaplicki, S. Changes in the content of free phenolic acids and antioxidative capacity of wholemeal bread in relation to cereal species and fermentation type. Eur. Food Res. Technol. 2019, 245, 2247–2256. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Literature data may underestimate the actual antioxidant capacity of cereals. J. Agric. Food Chem. 2005, 53, 5036–5040. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B.D. Simulated gastrointestinal digestion and in vitro colonic fermentation of spent coffee (Coffea arabica L.): Bioaccessibility and intestinal permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, K.; Martinez-Saez, N.; Rebollo-Hernanz, M.; Del Castillo, M.D.; Gaytán-Martínez, M.; Campos-Vega, R. In vitro health promoting properties of antioxidant dietary fiber extracted from spent coffee (Coffee arabica L.) grounds. Food Chem. 2018, 261, 253–259. [Google Scholar] [CrossRef]
- Bulmer, A.C.; Ried, K.; Blanchfield, J.T.; Wagner, K.H. The anti-mutagenic properties of bile pigments. Mutat. Res. 2008, 658, 28–41. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Danesi, F.; Pasini, F.; Caboni, M.F.; D’Antuono, L.F.; Bordoni, A.; on behalf of the BaSeFood Consortium. Traditional foods for health: Screening of the antioxidant capacity and phenolic content of selected Black Sea area local foods. J. Sci. Food Agric. 2013, 93, 3595–3603. [Google Scholar] [CrossRef]
- Bordoni, A.; Picone, G.; Babini, E.; Vignali, M.; Danesi, F.; Valli, V.; Di Nunzio, M.; Laghi, L.; Capozzi, F. NMR comparison of in vitro digestion of Parmigiano Reggiano cheese aged 15 and 30 months. Magn. Reson. Chem. 2011, 49, S61–S70. [Google Scholar] [CrossRef]
- Valli, V.; Taccari, A.; Di Nunzio, M.; Danesi, F.; Bordoni, A. Health benefits of ancient grains. Comparison among bread made with ancient, heritage and modern grain flours in human cultured cells. Food Res. Int. 2018, 107, 206–215. [Google Scholar] [CrossRef]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compound | RT | [M − H]− (m/z) | MS2 ions (m/z) |
|---|---|---|---|
| Phenolic Acids | |||
| p-Hydroxybenzoic acid | 5.8 | 137 | 93 |
| p-Coumaric acid | 9.5 | 163 | 119 |
| Caffeic acid | 7.7 | 179 | 135 |
| Ferulic acid | 10.3 | 193 | 149, 178, 134 |
| Sinapic acid | 10.6 | 223 | 208, 179, 164 |
| Sinapic acid isomer | 10.8 | 223 | 208, 164, 179 |
| Flavones | |||
| Apigenin-C-hexoside-C-pentoside | 9.9 | 563 | 473, 443, 503, 545, 383, 353 |
| Apigenin-C-hexoside-C-pentoside | 10.4 | 563 | 473, 443, 503, 545, 383, 353 |
| Apigenin-C-hexoside-C-pentoside (1st) | 10.6 | 563 | 473, 443, 503, 545, 383, 353 |
| Apigenin-C-hexoside-C-pentoside (2nd) | 11.1 | 563 | 473, 443, 503, 545, 383, 353 |
| Apigenin-C-hexoside-C-pentoside | 12.1 | 563 | 473, 443, 503, 545, 383, 353 |
| Apigenin-C-hexoside-C-pentoside | 12.4 | 563 | 473, 443, 503, 545, 383, 353 |
| Apigenin-C-hexoside-C-hexoside (1st) | 9.7 | 593 | 473, 503, 353, 575, 383 |
| Apigenin-C-hexoside-C-hexoside (2nd) | 10.1 | 593 | 473, 503, 353, 575, 383, 533 |
| Apigenin-C-hexoside-O-hexoside | 10.8 | 593 | 431, 473, 311, 503, 341, 413, 383 |
| Apigenin-C-hexoside-C-hexoside (3rd) | 11.6 | 593 | 473, 503, 383, 575, 413, 533 |
| Luteolin-O-rutinoside | 12.6 | 593 | 285; MS3(285): 241, 175, 199, 217, 243, 197 |
| Compound | DURS (µg/g of Cookie) | KHOS (µg/g of Cookie) | KAMS (µg/g of Cookie) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Buffer Extract | Hydroalcoholic Extract | <3 kDa Digested Fraction | Buffer Extract | Hydroalcoholic Extract | <3 kDa Digested Fraction | Buffer Extract | Hydroalcoholic Extract | <3 kDa Digested Fraction | |
| Apigenin-C-hex-C-pen (1st) | 0.28 ± 0.07c | 23.14 ± 1.67b | 30.43 ± 2.29a | 0.17 ± 0.02c | 13.68 ± 0.22b | 17.73 ± 0.66a | 0.05 ± 0.06c | 6.71 ± 0.19b | 10.70 ± 0.36a |
| Apigenin-C-hex-C-pen (2nd) | 0.80 ± 0.09c | 54.19 ± 3.02b | 73.87 ± 0.50a | 0.61 ± 0.09c | 36.09 ± 0.68b | 49.84 ± 0.84a | 0.19 ± 0.06c | 15.78 ± 0.24b | 26.65 ± 0.15a |
| Apigenin-C-dihex (1st) | nd | 0.55 ± 0.05b | 2.02 ± 0.15a | nd | 0.56 ± 0.02b | 1.25 ± 0.02a | nd | 0.20 ± 0.01 | nd |
| Apigenin-C-dihex (2nd) | nd | 1.84 ± 0.10b | 2.38 ± 0.02a | nd | 0.79 ± 0.04 | nd | nd | 0.32 ± 0.00 | nd |
| Apigenin-C-dihex (3rd) | nd | 1.91 ± 0.02 | nd | nd | 0.37 ± 0.02 | nd | nd | 0.16 ± 0.01 | nd |
| p-Hydroxybenzoic acid | 0.40 ± 0.08a | 0.25 ± 0.02b | nd | 0.59 ± 0.09a | 0.32 ± 0.03b | nd | 0.27 ± 0.05a | 0.18 ± 0.03b | nd |
| p-Coumaric acid | 0.52 ± 0.03b | 0.45 ± 0.02b | 0.64 ± 0.06a | 0.96 ± 0.10a,b | 0.78 ± 0.03b | 1.07 ± 0.15a | 0.17 ± 0.03c | 0.22 ± 0.00b | 0.41 ± 0.02a |
| Caffeic acid | nd | 0.13 ± 0.01 | nd | nd | 0.11 ± 0.00 | nd | 0.05 ± 0.02a | 0.05 ± 0.00a | nd |
| Ferulic acid | 1.38 ± 0.11b | 4.72 ± 0.17a,b | 4.24 ± 0.79a | 1.14 ± 0.19b | 4.80 ± 0.08a,b | 5.30 ± 1.21a | nd | 3.11 ± 0.05a | 3.40 ± 0.35a |
| Sinapic acid isomers | nd | 0.65 ± 0.04 | nd | nd | 0.38 ± 0.04 | nd | nd | 0.52 ± 0.07 | nd |
| Compound | DURL (µg/g of Cookie) | KHOL (µg/g of Cookie) | KAML (µg/g of Cookie) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Buffer Extract | Hydroalcoholic Extract | <3 kDa Digested Fraction | Buffer Extract | Hydroalcoholic Extract | <3 kDa Digested Fraction | Buffer Extract | Hydroalcoholic Extract | <3 Kda Digested Fraction | |
| Apigenin-C-hex-C-pen (1st) | 1.31 ± 1.18c | 15.71 ± 0.32b | 31.47 ± 1.32a | 0.17 ± 0.04c | 9.54 ± 0.45b | 17.21 ± 0.23a | nd | 7.73 ± 0.46b | 10.77 ± 0.29a |
| Apigenin-C-hex-C-pen (2nd) | 3.73 ± 3.49c | 36.26 ± 1.02b | 81.38 ± 0.20a | 0.56 ± 0.09c | 26.57 ± 0.33b | 49.37 ± 1.15a | 0.20 ± 0.02c | 18.22 ± 1.00b | 25.86 ± 0.14a |
| Apigenin-C-dihex (1st) | nd | 0.34 ± 0.02b | 2.06 ± 0.17a | nd | 0.38 ± 0.03b | 1.05 ± 0.07a | nd | 0.23 ± 0.01 | nd |
| Apigenin-C-dihex (2nd) | nd | 1.09 ± 0.03 | nd | nd | 0.55 ± 0.01 | nd | nd | 0.38 ± 0.03 | nd |
| Apigenin-C-dihex (3rd) | nd | 1.23 ± 0.02b | 2.35 ± 0.24a | nd | 0.27 ± 0.03 | nd | nd | 0.18 ± 0.02 | nd |
| p-Hydroxybenzoic acid | 0.76 ± 0.12a | 0.41 ± 0.01b | nd | 1.36 ± 0.14a | 0.66 ± 0.01b | nd | 0.79 ± 0.07a | 0.64 ± 0.04b | nd |
| p-Coumaric acid | 0.76 ± 0.6a | 0.60 ± 0.03a | 1.07 ± 0.02a | 1.91 ± 0.19b | 1.44 ± 0.03c | 2.49 ± 0.08a | 0.06 ± 0.00b | 0.08 ± 0.01a | nd |
| Caffeic acid | nd | 0.24 ± 0.01 | nd | nd | 0.34 ± 0.01 | nd | nd | 0.08 ± 0.00 | nd |
| Ferulic acid | 14.55 ± 1.85b | 12.34 ± 0.26b | 20.21 ± 1.44a | 0.59 ± 0.16c | 13.03 ± 0.45b | 21.31 ± 0.26a | nd | 3.34 ± 0.16b | 4.31 ± 0.17a |
| Sinapic acid isomers | nd | 1.76 ± 0.05a | 1.69 ± 0.12a | nd | 1.17 ± 0.07 | nd | nd | 0.88 ± 0.06 | nd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danesi, F.; Calani, L.; Valli, V.; Bresciani, L.; Del Rio, D.; Bordoni, A. (Poly)phenolic Content and Profile and Antioxidant Capacity of Whole-Grain Cookies are Better Estimated by Simulated Digestion than Chemical Extraction. Molecules 2020, 25, 2792. https://doi.org/10.3390/molecules25122792
Danesi F, Calani L, Valli V, Bresciani L, Del Rio D, Bordoni A. (Poly)phenolic Content and Profile and Antioxidant Capacity of Whole-Grain Cookies are Better Estimated by Simulated Digestion than Chemical Extraction. Molecules. 2020; 25(12):2792. https://doi.org/10.3390/molecules25122792
Chicago/Turabian StyleDanesi, Francesca, Luca Calani, Veronica Valli, Letizia Bresciani, Daniele Del Rio, and Alessandra Bordoni. 2020. "(Poly)phenolic Content and Profile and Antioxidant Capacity of Whole-Grain Cookies are Better Estimated by Simulated Digestion than Chemical Extraction" Molecules 25, no. 12: 2792. https://doi.org/10.3390/molecules25122792
APA StyleDanesi, F., Calani, L., Valli, V., Bresciani, L., Del Rio, D., & Bordoni, A. (2020). (Poly)phenolic Content and Profile and Antioxidant Capacity of Whole-Grain Cookies are Better Estimated by Simulated Digestion than Chemical Extraction. Molecules, 25(12), 2792. https://doi.org/10.3390/molecules25122792