Possible Effect of Climate Change on Surface-Water Photochemistry: A Model Assessment of the Impact of Browning on the Photodegradation of Pollutants in Lakes during Summer Stratification. Epilimnion vs. Whole-Lake Phototransformation
Abstract
1. Introduction
2. Theoretical Background
3. Results and Discussion
3.1. Photodegradation in the Epilimnion
- Compounds that are mainly degraded by direct photolysis would be poorly affected by browning, as far as photodegradation in the epilimnion is concerned. Indeed, the DOC increase would be offset by the dth decrease, and vice versa. Compounds belonging to this class are many UV filters, some pharmaceuticals, and pesticides [42,43]. It is unfortunately very difficult to carry out a structure–activity relationship, with which to predict the importance of the direct photolysis from molecular structure information only, without experimental data [44,45].
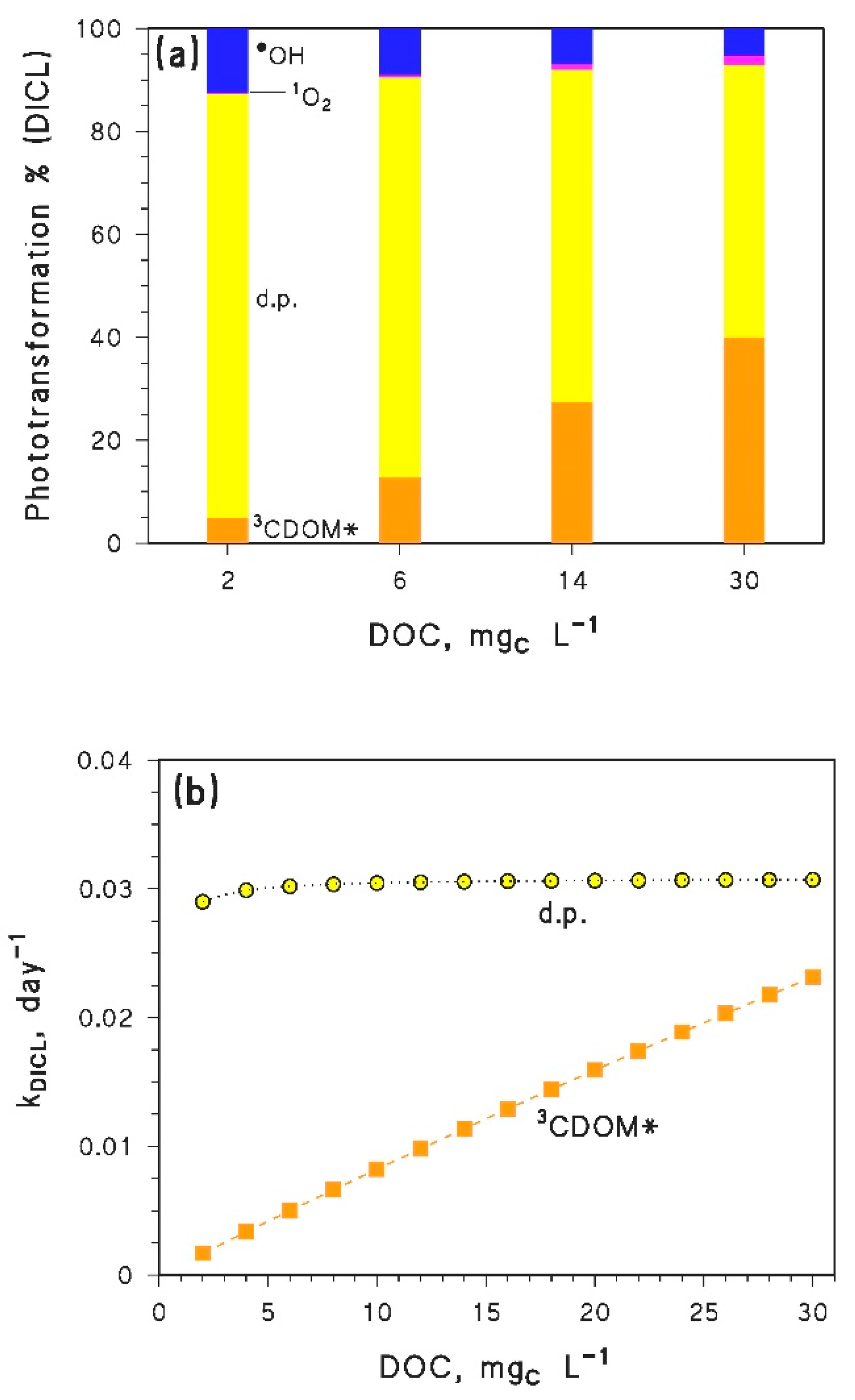
- Compounds that mainly/only react with •OH should show a slight decrease in photodegradation kinetics because of the browning phenomenon. However, these compounds would be too photostable to be significantly degraded in the epilimnion during the summer season. More photolabile compounds, reacting through other pathways in addition to •OH, could be highly affected by the DOC trend of the additional photoreactions, because [•OH] does not vary much with varying DOC (see Figure 2).
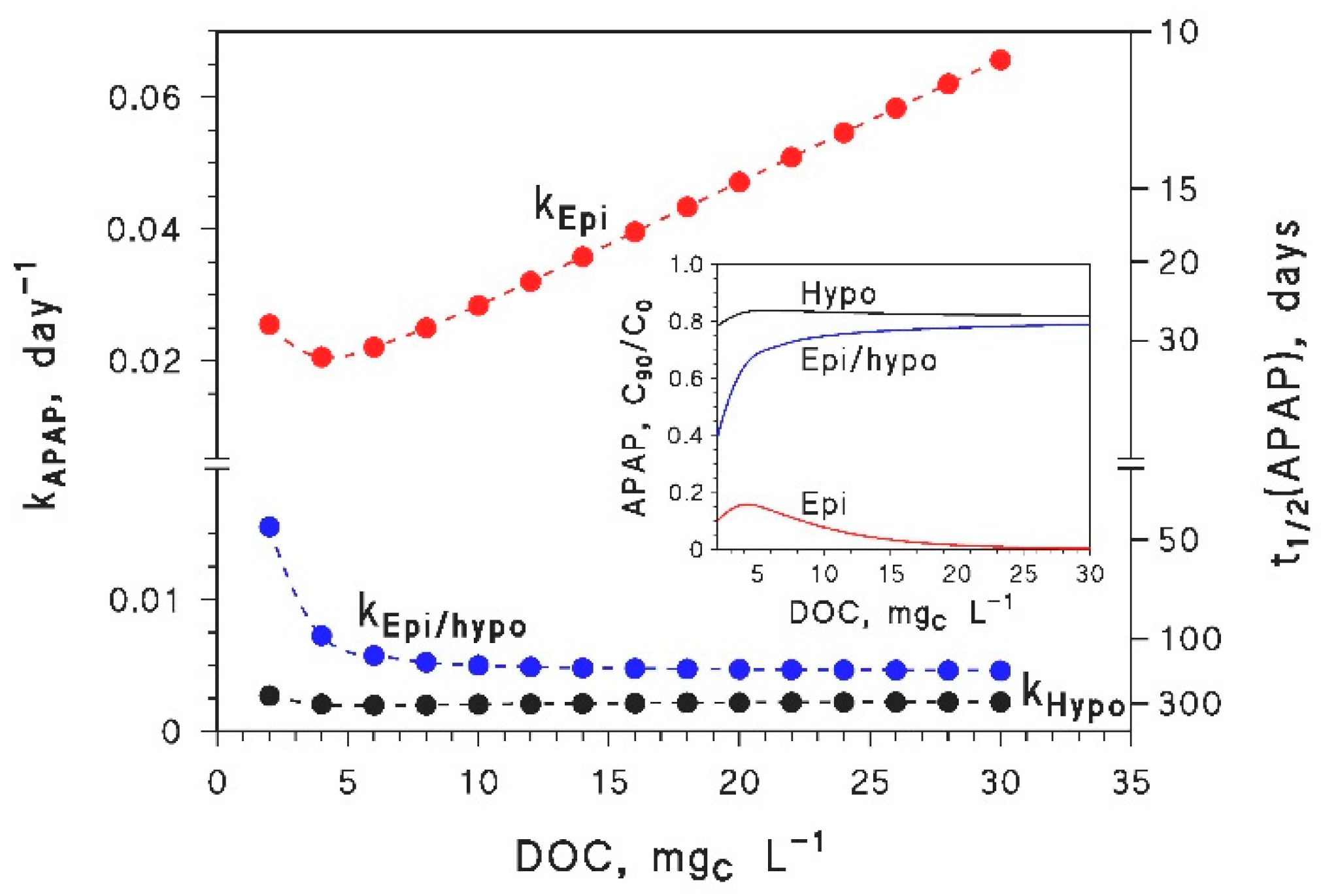
- Inhibition of epilimnion photodegradation due to browning is predicted for compounds that mainly react with CO3−. However, because the reduction potential of CO3•− is lower than the reduction potentials of many 3CDOM* [4], it is very difficult to find compounds that react with CO3•− at low DOC and do not react with 3CDOM* (or 1O2) at high DOC. Therefore, most pollutants that react fast with CO3•− (e.g., phenols, anilines, sulphur-containing compounds [8,46,47]) are expected to show a minimum in their epilimnion photodegradation rate constants, in a similar way as APAP. The position of the minimum would depend on the respective values of the second-order rate constants and , and on the irradiance and spectrum of sunlight.
- Because of the above considerations, in many cases the browning of medium- to high-DOC waters would accelerate the epilimnion photodegradation kinetics of pollutants, especially when photodegradation is quite fast. Indeed, despite the fact that browning makes water more colored, and thus less conducive to sunlight penetration [21], the combination of a shallower thermocline with the photoreaction pathways occurring at high (C)DOM (3CDOM* and, where applicable, 1O2) would speed-up photodegradation in the epilimnion.
3.2. Whole-Lake Photodegradation (Epilimnion + Hypolimnion)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Barreca, S. Environmental organic photochemistry: Advances and perspectives. Curr. Org. Chem. 2014, 17, 3032–3041. [Google Scholar] [CrossRef]
- Mc Neill, K.; Canonica, S. Triplet state dissolved organic matter in aquatic photochemistry: Reaction mechanisms, substrate scope, and photophysical properties. Environ. Sci.-Process Impacts 2016, 18, 1381–1399. [Google Scholar] [CrossRef] [PubMed]
- Vione, D.; Minella, M.; Maurino, V.; Minero, C. Indirect photochemistry in sunlit surface waters: Photoinduced production of reactive transient species. Chem.-Eur. J. 2014, 20, 10590–10606. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Ortiz, F.L.; Canonica, S. Probe compounds to assess the photochemical activity of dissolved organic matter. Environ. Sci. Technol. 2016, 50, 12532–12547. [Google Scholar] [CrossRef]
- Gligorovski, S.; Strekowski, R.; Barbati, S.; Vione, D. Environmental implications of hydroxyl radicals (•OH). Chem. Rev. 2015, 115, 13051–13092. [Google Scholar] [CrossRef]
- Canonica, S.; Kohn, T.; Mac, M.; Real, F.J.; Wirz, J.; Von Gunten, U. Photosensitizer method to determine rate constants for the reaction of carbonate radical with organic compounds. Environ. Sci. Technol. 2005, 39, 9182–9188. [Google Scholar] [CrossRef]
- Vione, D. A critical view of the application of the APEX software (Aqueous Photochemistry of Environmentally-occurring Xenobiotics) to predict photoreaction kinetics in surface freshwaters. Molecules 2020, 25, 9. [Google Scholar] [CrossRef]
- Adrian, R.; O’Reilly, C.M.; Zagarese, H.; Baines, S.B.; Hessen, D.O.; Keller, W.; Livingstone, D.M.; Sommaruga, R.; Straile, D.; Van Donk, E.; et al. Lakes as sentinels of climate change. Limnol. Oceanogr. 2009, 54, 2283–2297. [Google Scholar] [CrossRef]
- Vione, D.; Scozzaro, A. Photochemistry of surface fresh waters in the framework of climate change. Environ. Sci. Technol. 2019, 53, 7945–7963. [Google Scholar] [CrossRef] [PubMed]
- Koehler, B.; Landelius, T.; Weyhenmeyer, G.A.; Machida, N.; Tranvik, L.J. Sunlight-induced carbon dioxide emissions from inland waters. Glob. Biogeochem. Cycle 2014, 28, 696–711. [Google Scholar] [CrossRef]
- Koehler, B.; Barsotti, F.; Minella, M.; Landelius, T.; Minero, C.; Tranvik, L.J.; Vione, D. Simulation of photoreactive transients and of photochemical transformation of organic pollutants in sunlit boreal lakes across 14 degrees of latitude: A photochemical mapping of Sweden. Water Res. 2018, 129, 94–104. [Google Scholar] [CrossRef]
- Weyhenmeyer, G.A.; Müller, R.A.; Norman, M.; Tranvik, L.J. Sensitivity of freshwaters to browning in response to future climate change. Clim. Chang. 2016, 134, 225–239. [Google Scholar] [CrossRef]
- Sobek, S.; Tranvik, L.J.; Prairie, Y.T.; Kortelainen, P.; Cole, J.J. Patterns and regulation of dissolved organic carbon: An analysis of 7,500 widely distributed lakes. Limnol. Oceanogr. 2007, 58, 1208–1219. [Google Scholar] [CrossRef]
- Williamson, C.E.; Overholt, E.P.; Pilla, R.M.; Leach, T.H.; Brentrup, J.A.; Knoll, L.B.; Mette, E.M.; Moeller, R.E. Ecological consequences of long-term browning in lakes. Sci. Rep. 2015, 5, 18666. [Google Scholar] [CrossRef] [PubMed]
- Futter, M.N.; Starr, M.; Forsius, M.; Holmberg, M. Modeling the effects of climate on long-term patterns of dissolved organic carbon concentrations in the surface waters of a boreal catchment. Hydrol. Earth Syst. Sci. 2008, 12, 437–447. [Google Scholar] [CrossRef]
- Jane, S.F.; Winslow, L.A.; Remucal, C.K.; Rose, K.C. Long-term trends and synchrony in dissolved organic matter characteristics in Wisconsin, USA, lakes: Quality, not quantity, is highly sensitive to climate. J. Geophys. Res. Biogeosci. 2017, 122, 546–561. [Google Scholar] [CrossRef]
- Solomon, C.T.; Jones, S.E.; Weidel, B.C.; Buffam, I.; Fork, M.L.; Karlsson, J.; Larsen, S.; Lennon, J.T.; Read, J.S.; Sadro, S.; et al. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: Current knowledge and future challenges. Ecosystems 2015, 18, 376–389. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Bracchini, L.; Loiselle, S.; Dattilo, A.M.; Mazzuoli, S.; Cozar, A.; Rossi, C. The spatial distribution of optical properties in the ultraviolet and visible in an aquatic ecosystem. Photochem. Photobiol. 2004, 80, 139–149. [Google Scholar] [CrossRef]
- Vione, D.; Caringella, R.; De Laurentiis, E.; Pazzi, M.; Minero, C. Phototransformation of the sunlight filter benzophenone-3 (2-hydroxy-4-methoxybenzophenone) under conditions relevant to surface waters. Sci. Total Environ. 2013, 463, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Avetta, P.; Fabbri, D.; Minella, M.; Brigante, M.; Maurino, V.; Minero, C.; Pazzi, M.; Vione, D. Assessing the phototransformation of diclofenac, clofibric acid and naproxen in surface waters: Model predictions and comparison with field data. Water Res. 2016, 105, 383–394. [Google Scholar] [CrossRef] [PubMed]
- De Laurentiis, E.; Prasse, C.; Ternes, T.A.; Minella, M.; Maurino, V.; Minero, C.; Sarakha, M.; Brigante, M.; Vione, D. Assessing the photochemical transformation pathways of acetaminophen relevant to surface waters: Transformation kinetics, intermediates, and modeling. Water Res. 2014, 53, 235–248. [Google Scholar] [CrossRef]
- Minella, M.; Giannakis, S.; Mazzavillani, A.; Maurino, V.; Minero, C.; Vione, D. Phototransformation of Acesulfame K in surface waters: Comparison of two techniques for the measurement of the second-order rate constants of indirect photodegradation, and modeling of photoreaction kinetics. Chemosphere 2017, 186, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.S.; Gold, A.J.; Bernal, S.; Tank, J.L. Diverse water quality responses to extreme climate events: An introduction. Biogeochemistry 2018, 141, 273–279. [Google Scholar] [CrossRef]
- Bodrato, M.; Vione, D. APEX (Aqueous Photochemistry of Environmentally occurring Xenobiotics): A free software tool to predict the kinetics of photochemical processes in surface waters. Environ. Sci.-Process Impacts 2014, 16, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Page, S.E.; Arnold, W.A.; McNeill, K. Assessing the contribution of free hydroxyl radical in organic matter-sensitized photohydroxylation reactions. Environ. Sci. Technol. 2011, 45, 2818–2825. [Google Scholar] [CrossRef]
- Sun, L.N.; Qian, J.G.; Blough, N.V.; Mopper, K. Insights into the photoproduction sites of hydroxyl radicals by dissolved organic matter in natural waters. Environ. Sci. Technol. Lett. 2015, 2, 352–356. [Google Scholar] [CrossRef]
- Vione, D.; Das, R.; Rubertelli, F.; Maurino, V.; Minero, C.; Barbati, S.; Chiron, S. Modeling the occurrence and reactivity of hydroxyl radicals in surface waters: Implications for the fate of selected pesticides. Intern. J. Environ. Anal. Chem. 2010, 90, 260–275. [Google Scholar] [CrossRef]
- Zepp, R.G.; Hoigné, J.; Bader, H. Nitrate-induced photooxidation of trace organic chemicals in water. Environ. Sci. Technol. 1987, 21, 443–450. [Google Scholar] [CrossRef]
- Braslavsky, S.E. Glossary of terms used in photochemistry. third edition. Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Minella, M.; De Laurentiis, E.; Maurino, V.; Minero, C.; Vione, D. Dark production of hydroxyl radicals by aeration of anoxic lake water. Sci Total Environ. 2015, 527–528, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Galgani, L.; Tognazzi, A.; Rossi, C.; Ricci, M.; Angel Galvez, J.; Dattilo, A.M.; Cozar, A.; Bracchini, L.; Loiselle, S.A. Assessing the optical changes in dissolved organic matter in humic lakes by spectral slope distributions. J. Photochem. Photobiol. B Biol. 2011, 102, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Tixier, C.; Singer, H.P.; Oellers, S.; Mueller, S.R. Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ. Sci. Technol. 2003, 37, 1061–1068. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nakamura, Y.; Moriguchi, S.; Nakamura, Y.; Honda, Y.; Tamura, I.; Hirata, Y.; Hayashi, A.; Sekizawa, J. Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: Laboratory photolysis, biodegradation, and sorption experiments. Water Res. 2009, 43, 351–362. [Google Scholar] [CrossRef]
- Zou, H.; Radke, M.; Kierkegaard, A.; MacLeod, M.; McLachlan, M.S. Using chemical benchmarking to determine the persistence of chemicals in a Swedish lake. Environ. Sci. Technol. 2015, 49, 1646–1653. [Google Scholar] [CrossRef]
- Zou, H.; Radke, M.; Kierkegaard, A.; McLachlan, M.S. Temporal variation of chemical persistence in a Swedish lake assessed by benchmarking. Environ. Sci. Technol. 2015, 49, 9881–9888. [Google Scholar] [CrossRef]
- Frank, R.; Klöpffer, W. Spectral solar photo irradiance in Central Europe and the adjacent North Sea. Chemosphere 1988, 17, 985–994. [Google Scholar] [CrossRef]
- Wenk, J.; Eustis, S.N.; McNeill, K.; Canonica, S. Quenching of excited triplet states by dissolved natural organic matter. Environ. Sci. Technol. 2013, 47, 12802–12810. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O− in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photodegradation of pharmaceuticals in the aquatic environment: A review. Aquat. Sci. 2003, 65, 320–341. [Google Scholar] [CrossRef]
- Calza, P.; Vione, D.; Galli, F.; Fabbri, D.; Dal Bello, F.; Medana, C. Study of the photochemical transformation of 2-ethylhexyl 4-(dimethylamino)benzoate (OD-PABA) under conditions relevant to surface waters. Water Res. 2016, 88, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peijnenburg, W.J.G.M.; Quan, X.; Yang, F. Quantitative structure–property relationships for direct photolysis quantum yields of selected polycyclic aromatic hydrocarbons. Sci. Total Environ. 2000, 246, 11–20. [Google Scholar] [CrossRef]
- Chen, J.; Quan, X.; Peijnenburg, W.J.G.M.; Yang, F. Quantitative structure–property relationships (QSPRs) on direct photolysis quantum yields of PCDDs. Chemosphere 2001, 43, 235–241. [Google Scholar] [CrossRef]
- Canonica, S. Oxidation of aquatic organic contaminants induced by excited triplet states. Chimia 2007, 61, 641–644. [Google Scholar] [CrossRef]
- Vione, D. A model assessment of the role played by the carbonate (CO3•−) and dibromide (Br2•−) radicals in the photodegradation of glutathione in sunlit fresh- and salt-waters. Chemosphere 2018, 209, 401–410. [Google Scholar] [CrossRef]
Sample Availability: No samples were used in this work. |
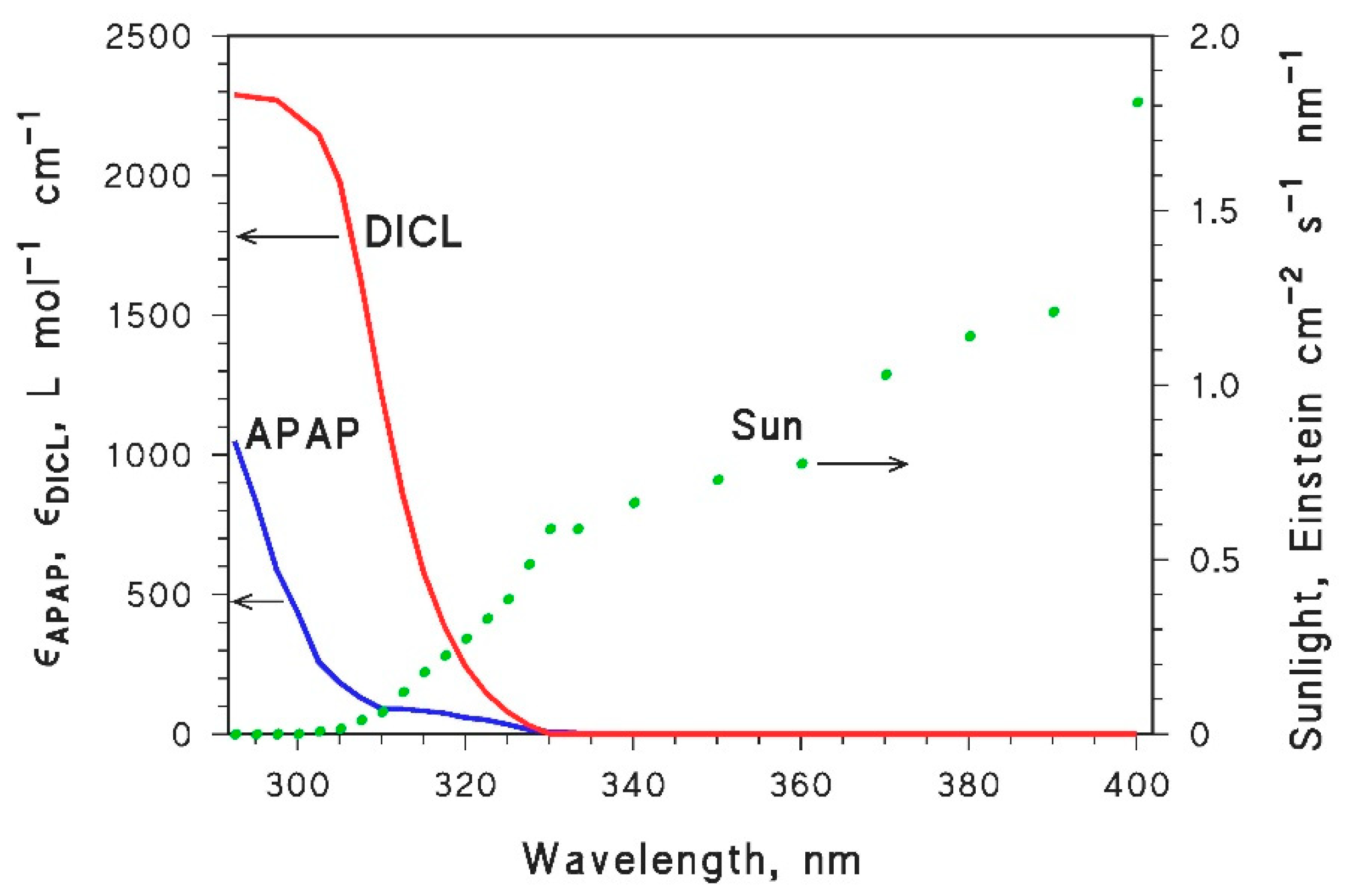
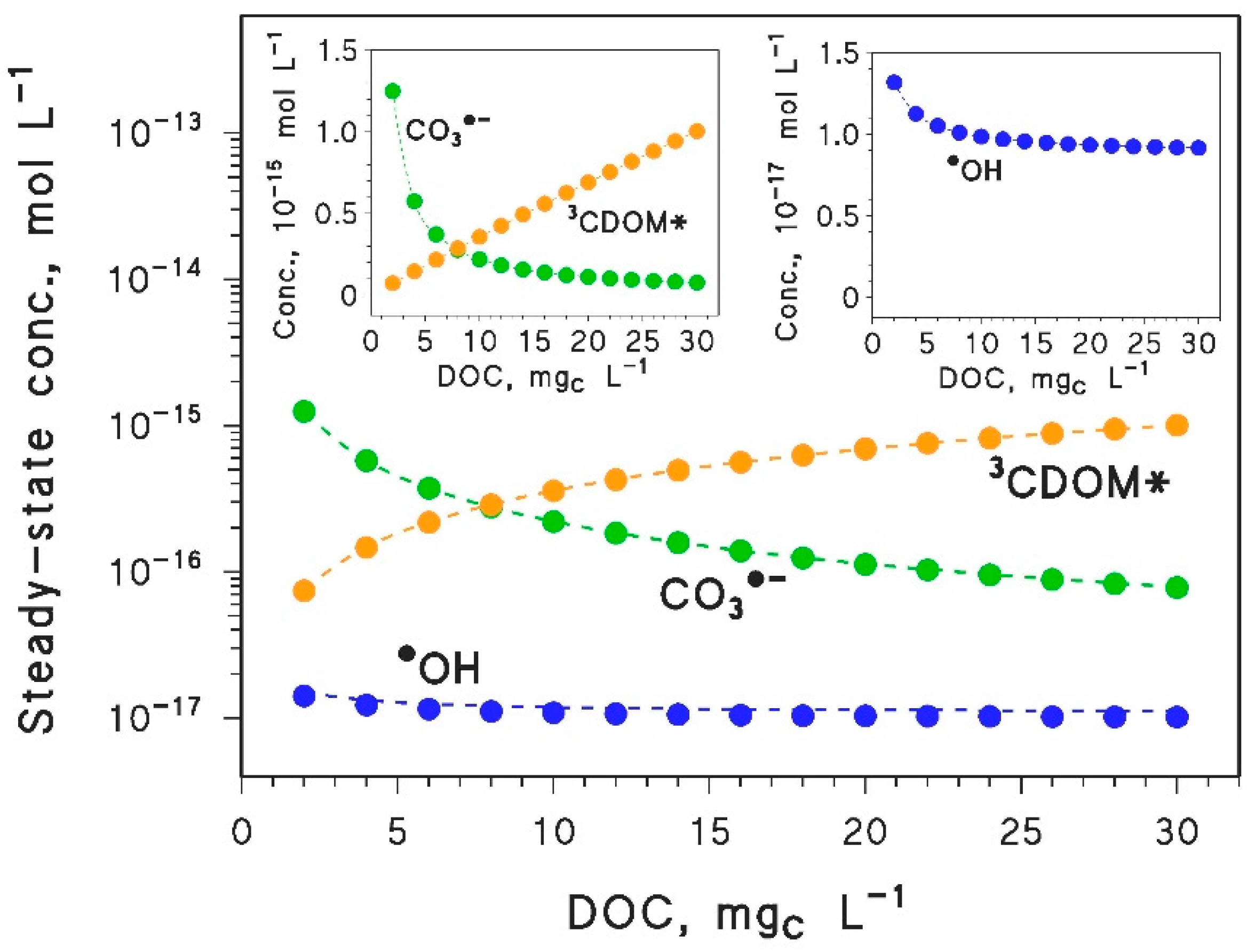
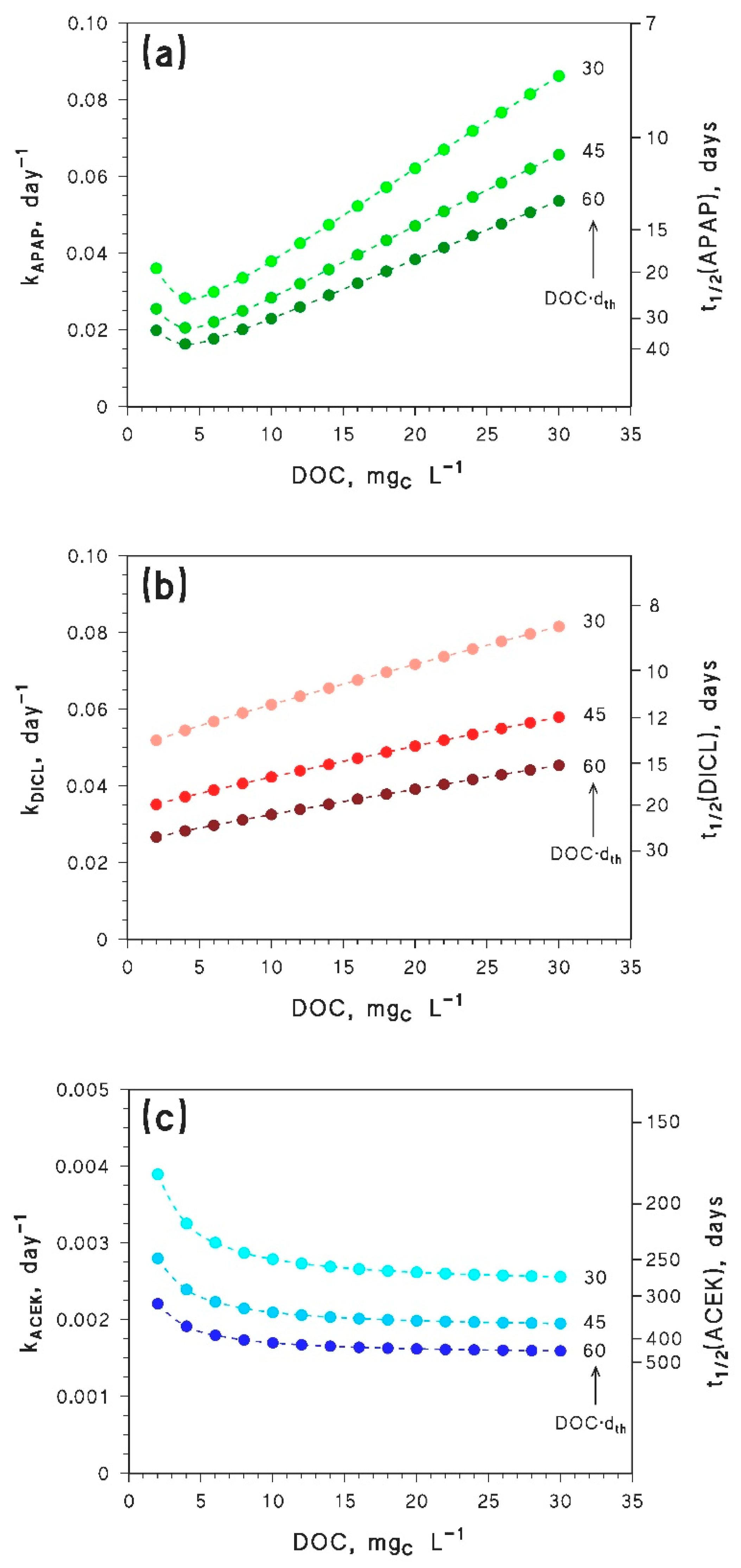
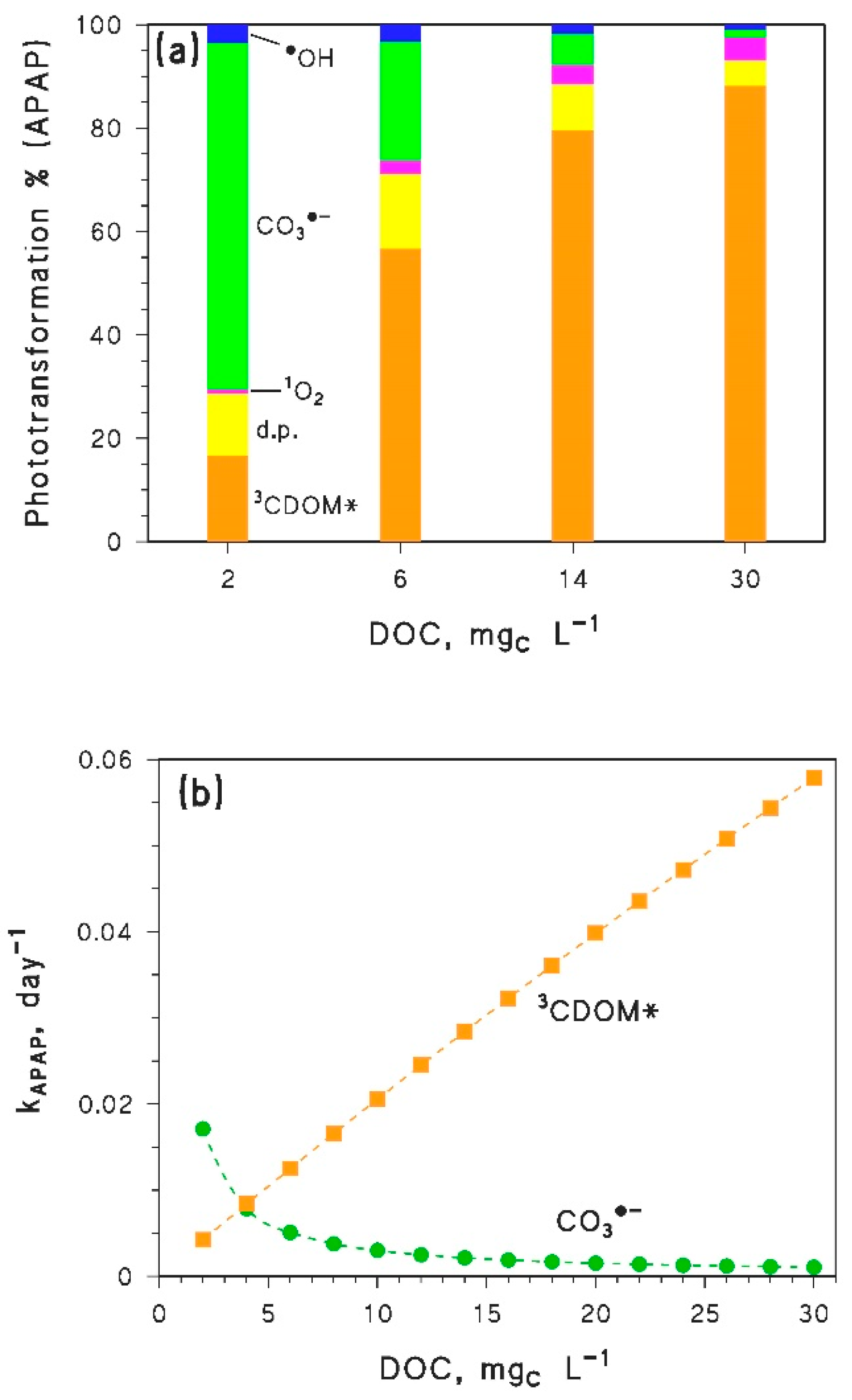
| Parameter | Paracetamol (APAP) | Diclofenac (DICL) | Acesulfame K (ACEK) |
|---|---|---|---|
| ΦS, unitless | 4.6 × 10−2 | 9.4 × 10−2 | 5.5 × 10−3 |
| , L mol−1 s−1 | 1.9 × 109 | 9.3 × 109 | 5.9 × 109 |
| , L mol−1 s−1 | 3.8 × 108 | Negligible | Negligible |
| , L mol−1 s−1 | 3.7 × 107 | 1.3 × 107 | 2.8 × 104 |
| , L mol−1 s−1 | 1.6 × 109 | 6.4 × 108 | Negligible |
| Parameter | Field Lifetime, Days | Location | Modeled Lifetime, Days |
|---|---|---|---|
| APAP | 1.5–2.5 [36] | Tokushima (Japan) | 2.5 |
| DICL | 8.3 [35] | Greinfensee (Switzerland) | 7–8 |
| ACEK | >1200 [37] | Norra Bergundasjön (Sweden) | >700 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderaro, F.; Vione, D. Possible Effect of Climate Change on Surface-Water Photochemistry: A Model Assessment of the Impact of Browning on the Photodegradation of Pollutants in Lakes during Summer Stratification. Epilimnion vs. Whole-Lake Phototransformation. Molecules 2020, 25, 2795. https://doi.org/10.3390/molecules25122795
Calderaro F, Vione D. Possible Effect of Climate Change on Surface-Water Photochemistry: A Model Assessment of the Impact of Browning on the Photodegradation of Pollutants in Lakes during Summer Stratification. Epilimnion vs. Whole-Lake Phototransformation. Molecules. 2020; 25(12):2795. https://doi.org/10.3390/molecules25122795
Chicago/Turabian StyleCalderaro, Federico, and Davide Vione. 2020. "Possible Effect of Climate Change on Surface-Water Photochemistry: A Model Assessment of the Impact of Browning on the Photodegradation of Pollutants in Lakes during Summer Stratification. Epilimnion vs. Whole-Lake Phototransformation" Molecules 25, no. 12: 2795. https://doi.org/10.3390/molecules25122795
APA StyleCalderaro, F., & Vione, D. (2020). Possible Effect of Climate Change on Surface-Water Photochemistry: A Model Assessment of the Impact of Browning on the Photodegradation of Pollutants in Lakes during Summer Stratification. Epilimnion vs. Whole-Lake Phototransformation. Molecules, 25(12), 2795. https://doi.org/10.3390/molecules25122795







