Biological Effect Evaluation of Glutathione-Responsive Cyclodextrin-Based Nanosponges: 2D and 3D Studies
Abstract
1. Introduction
2. Results
2.1. Characterization of Glutathione Responsive β-cyclodextrin-Based Nanosponges
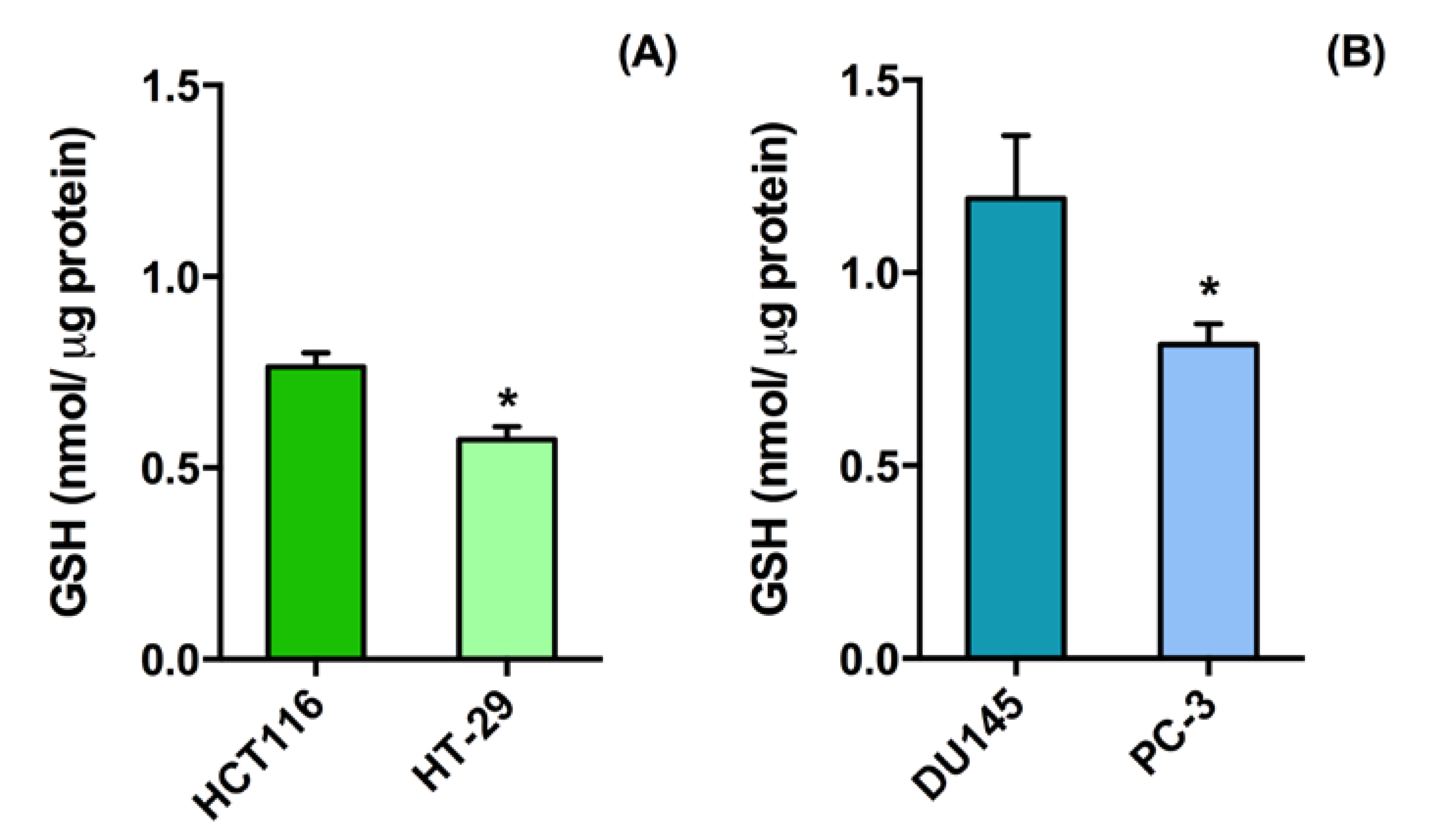
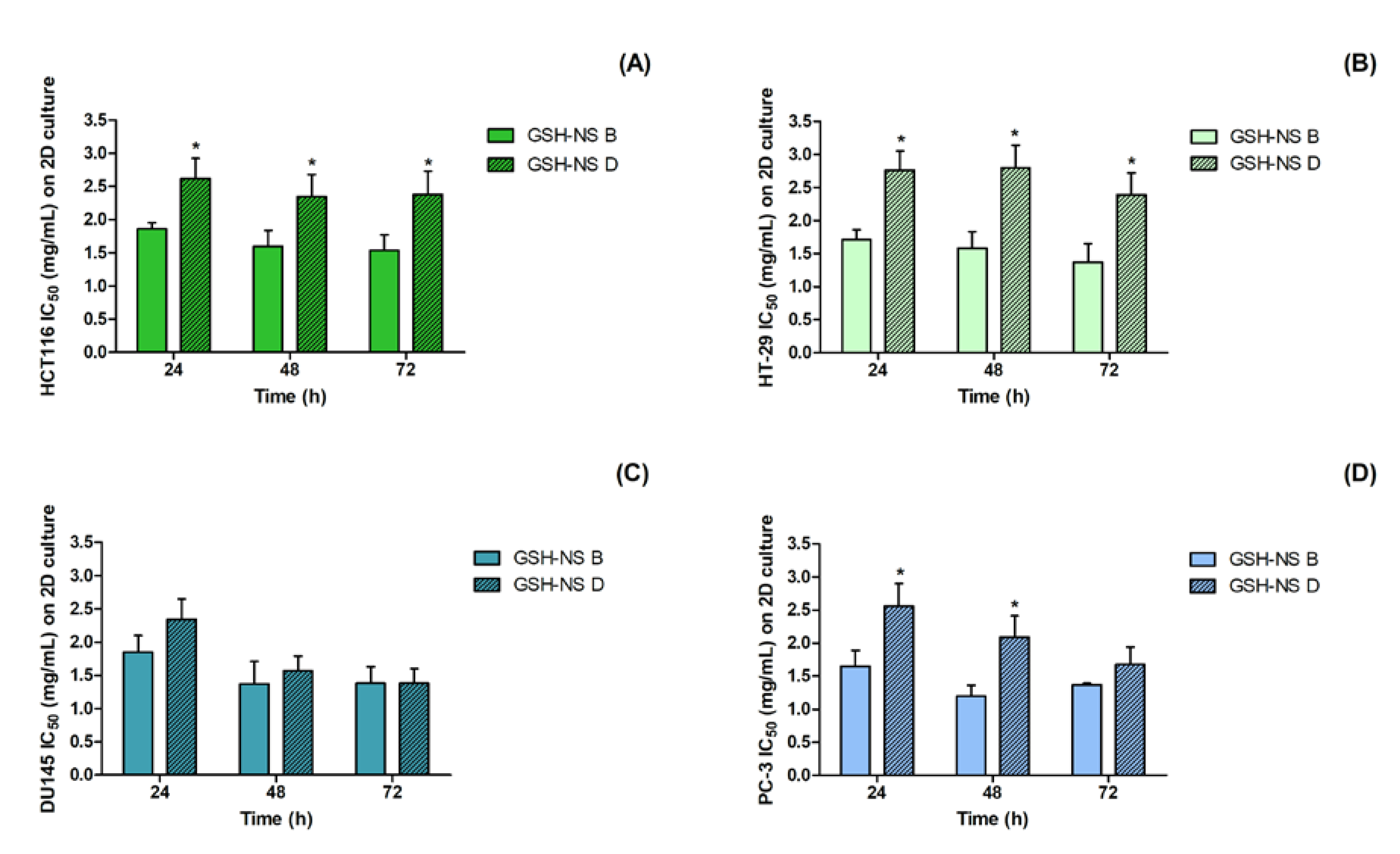
2.2. D Cell Culture Cytotoxicity of Glutathione Responsive β-cyclodextrin-Based Nanosponges
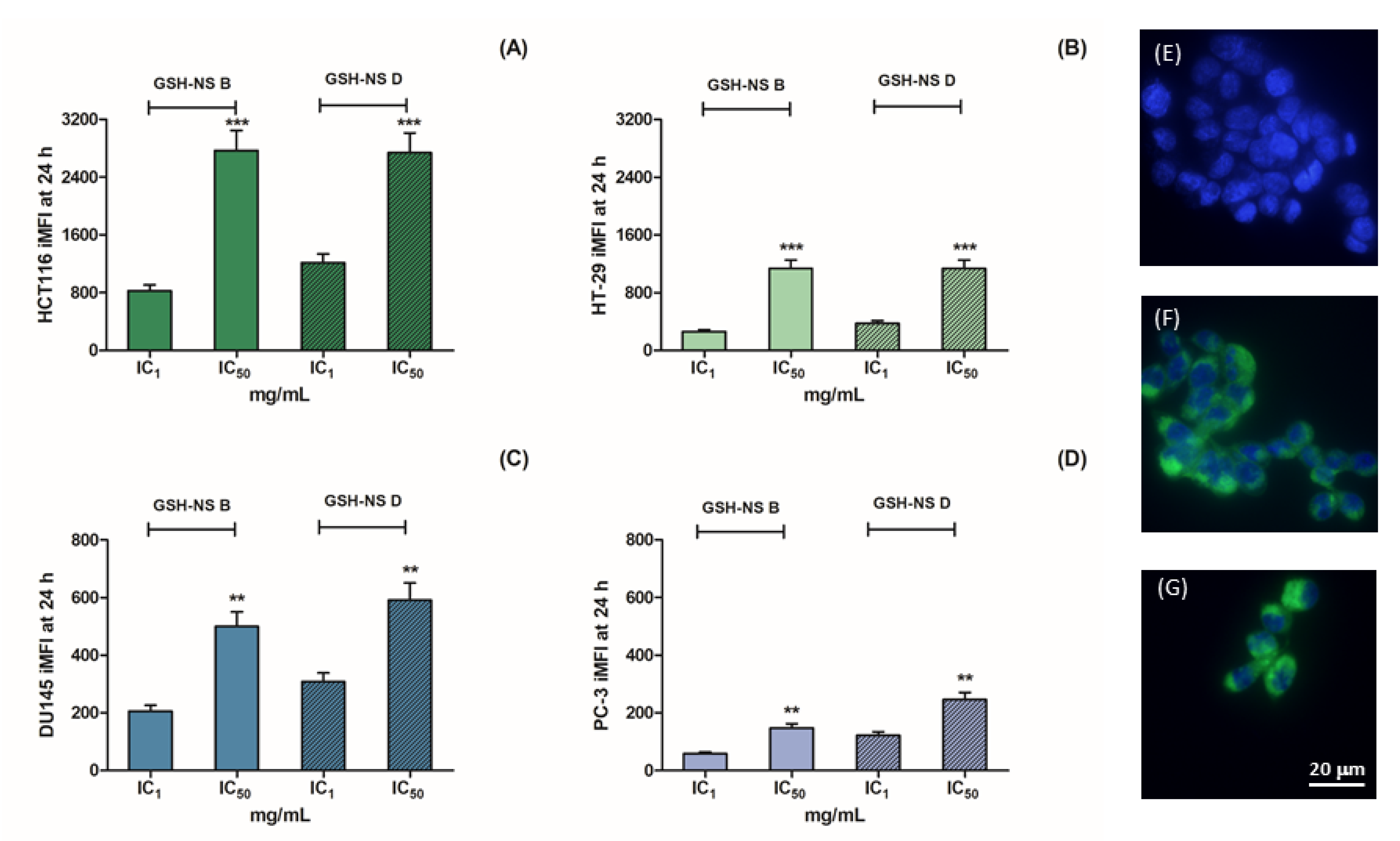
2.3. Glutathione Responsive β-cyclodextrin-Based Nanosponge Cellular Uptake on 2D Cell Cultures
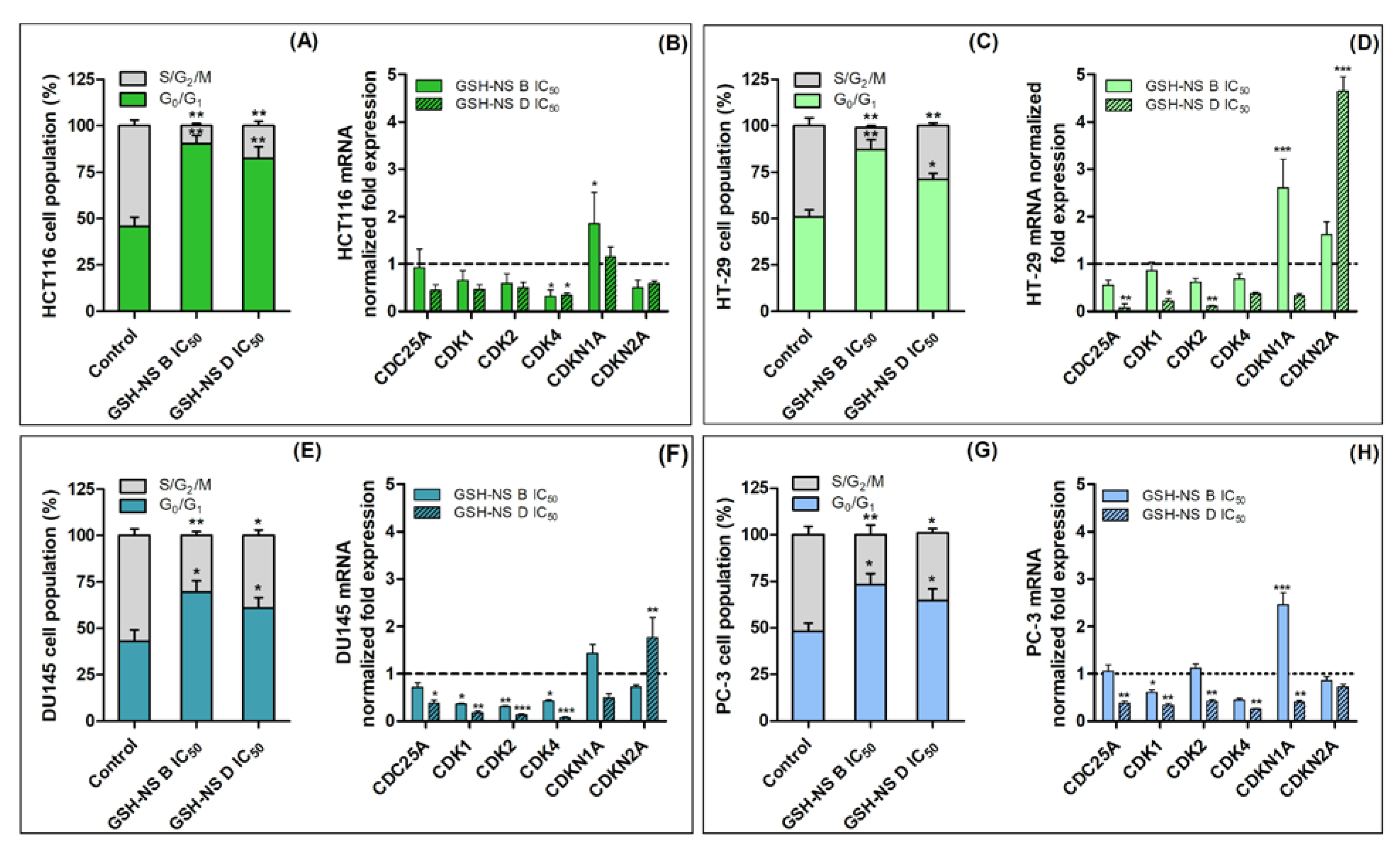
2.4. The Effect of Glutathione Responsive β-cyclodextrin-Based Nanosponges on Cell Death and Cell Cycle
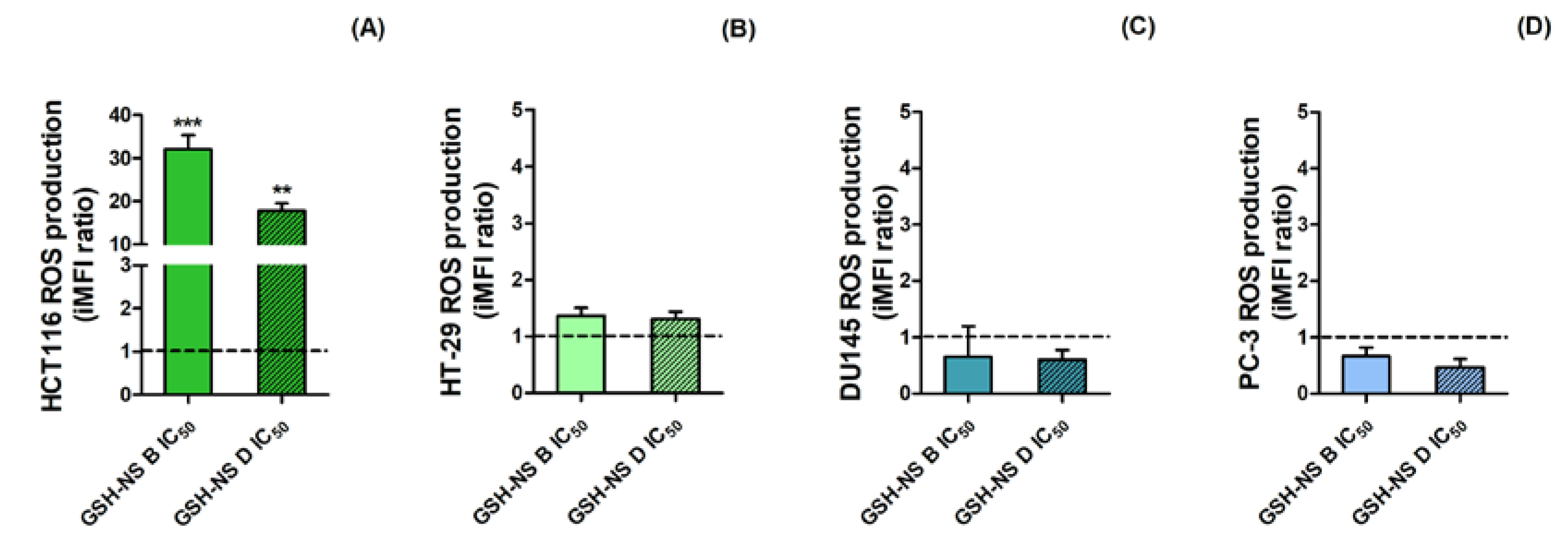
2.5. The Effect of Glutathione Responsive β-cyclodextrin-Based Nanosponges on Reactive Oxygen Species Production
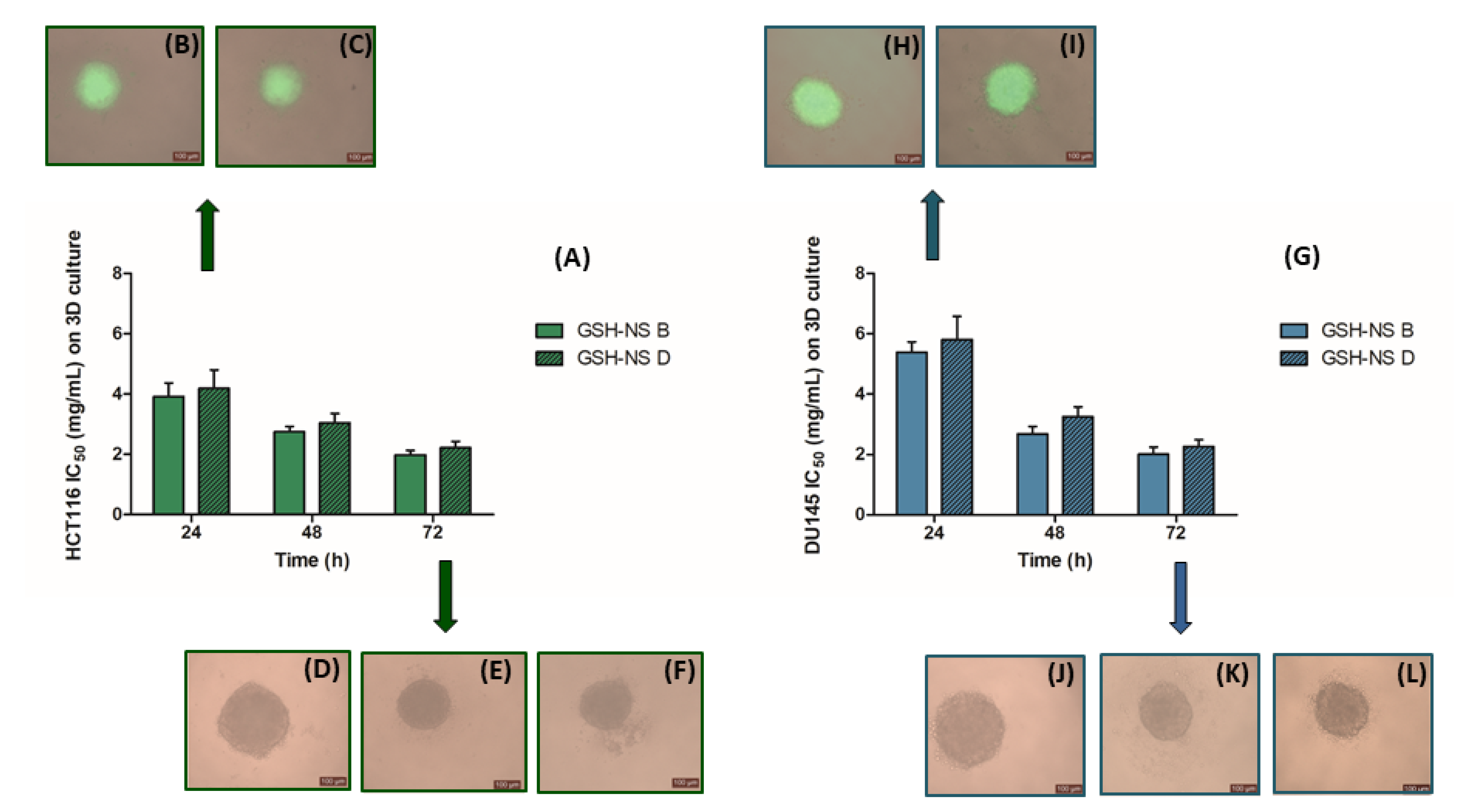
2.6. Glutathione Responsive β-cyclodextrin-Based Nanosponge Cytotoxicity in Three-Dimensional Cell Cultures
3. Discussion
4. Methods
4.1. Synthesis of Glutathione Responsive β-cyclodextrin-Based Nanosponges
4.2. Preparation of Glutathione Responsive β-cyclodextrin-Based Nanosponge Nanosuspension
4.3. Preparation of Fluorescent Glutathione Responsive β-cyclodextrin-Based Nanosponges
4.4. Characterization of Glutathione Responsive β-cyclodextrin-based Nanosponges
4.5. Cell Culture and Treatment with Glutathione Responsive β-cyclodextrin-Based Nanosponges
4.6. Measurement of Basal Intracellular Reduced Glutathione Levels
4.7. Cell Proliferation Assay
4.8. Nanosponge Cellular Uptake Assays
4.9. Lactate Dehydrogenase Leakage Assay
4.10. Cell Cycle Analysis
4.11. Real Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.12. Reactive Oxygen Species Production Assay
4.13. Cell Growth and Nanosponge Cellular Uptake Assays on Three-Dimensional Cell Culture
4.14. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Bawarski, W.E.; Chidlowsky, E.; Bharali, D.J.; Mousa, S.A. Emerging nanopharmaceuticals. Nanomedicine 2008, 4, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Huhn, D.; del Mercato, L.L.; Sasse, D.; Parak, W.J. Nanopharmacy: Inorganic nanoscale devices as vectors and active compounds. Pharm. Res. 2010, 62, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M. Nanotechnology-enabled medicine. Discov. Med. 2005, 5, 363–366. [Google Scholar] [PubMed]
- Malam, Y.; Loizidou, M.; Seifalian, A.M. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharm. Sci. 2009, 30, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Shin, D.M.; Simons, J.W.; Nie, S. Nanotechnology for targeted cancer therapy. Expert Rev. Anticancer Ther. 2007, 7, 833–837. [Google Scholar] [CrossRef]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Acharya, S.; Sahoo, S.K. Recent trends of nanomedicinal approaches in clinics. Int J. Pharm. 2018, 538, 263–278. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Curcio, M.; Diaz-Gomez, L.; Cirillo, G.; Concheiro, A.; Iemma, F.; Alvarez-Lorenzo, C. pH/redox dual-sensitive dextran nanogels for enhanced intracellular drug delivery. Eur. J. Pharm Biopharm. 2017, 117, 324–332. [Google Scholar] [CrossRef]
- Moya-Ortega, M.D.; Alvarez-Lorenzo, C.; Concheiro, A.; Loftsson, T. Cyclodextrin-based nanogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2012, 428, 152–163. [Google Scholar] [CrossRef]
- Ahmed, R.Z.; Patil, G.; Zaheer, Z. Nanosponges–A completely new nano-horizon: Pharmaceutical applications and recent advances. Drug Dev. Ind. Pharm. 2013, 39, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Selvamuthukumar, S.; Anandam, S.; Krishnamoorthy, K.; Rajappan, M. Nanosponges: A novel class of drug delivery system--review. J. Pharm. Pharm. Sci. 2012, 15, 103–111. [Google Scholar]
- Chilajwar, S.V.; Pednekar, P.P.; Jadhav, K.R.; Gupta, G.J.; Kadam, V.J. Cyclodextrin-based nanosponges: A propitious platform for enhancing drug delivery. Expert Opin. Drug Deliv. 2014, 11, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tejashri, G.; Amrita, B.; Darshana, J. Cyclodextrin based nanosponges for pharmaceutical use: A review. Acta. Pharm. 2013, 63, 335–358. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Cavalli, R. Cyclodextrin-based nanosponges as drug carriers. Beilstein J. Org. Chem. 2012, 8, 2091–2099. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-based nanosponges: A critical review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Allahyari, S.; Trotta, F.; Valizadeh, H.; Jelvehgari, M.; Zakeri-Milani, P. Cyclodextrin-based nanosponges as promising carriers for active agents. Expert Opin. Drug Deliv. 2019, 16, 467–479. [Google Scholar] [CrossRef]
- Pandey, P.; Purohit, D.; Dureja, H. Nanosponges -A Promising Novel Drug Delivery System. Recent Pat. Nanotechnol. 2018, 12, 180–191. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β-Cyclodextrin Nanosponge Hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef]
- Clemente, N.; Boggio, E.; Gigliotti, L.C.; Raineri, D.; Ferrara, B.; Miglio, G.; Argenziano, M.; Chiocchetti, A.; Cappellano, G.; Trotta, F.; et al. Immunotherapy of experimental melanoma with ICOS-Fc loaded in biocompatible and biodegradable nanoparticles. J. Control. Release 2020, 320, 112–124. [Google Scholar] [CrossRef]
- Clemente, N.; Argenziano, M.; Gigliotti, C.L.; Ferrara, B.; Boggio, E.; Chiocchetti, A.; Caldera, F.; Trotta, F.; Benetti, E.; Annaratone, A.; et al. Paclitaxel-Loaded Nanosponges Inhibit Growth and Angiogenesis in Melanoma Cell Models. Front. Pharm. 2019, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, M.; Gigliotti, C.L.; Clemente, N.; Boggio, E.; Ferrara, B.; Trotta, F.; Pizzimenti, S.; Barrera, G.; Boldorini, R.; Bessone, F.; et al. Improvement in the Anti-Tumor Efficacy of Doxorubicin Nanosponges in In Vitro and in Mice Bearing Breast Tumor Models. Cancers (Basel) 2020, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Pastero, L.; Serpe, L.; Trotta, F.; Vavia, P.; Aquilano, D.; Trotta, M.; Zara, G.P.; Cavalli, R. Cyclodextrin-based nanosponges encapsulating camptothecin: Physicochemical characterization; stability and cytotoxicity. Eur. J. Pharm. Biopharm. 2010, 74, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Trotta, F.; Dianzani, C.; Caldera, F.; Mognetti, B.; Cavalli, R. The application of nanosponges to cancer drug delivery. Expert Opin. Drug Deliv. 2014, 11, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef]
- Caldera, F.; Tannous, M.; Cavalli, R.; Zanetti, M.; Trotta, F. Evolution of Cyclodextrin Nanosponges. Int. J. Pharm. 2017, 531, 470–479. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Hu, X.; Ali, D.M.; Wang, M.H. Emerging Strategies in Stimuli-Responsive Nanocarriers as the Drug Delivery System for Enhanced Cancer Therapy. Curr. Pharm. Des. 2019, 25, 2609–2625. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Control. Release. 2011, 152, 2–12. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M. Iqbal HMN. Redox-responsive nano-carriers as tumor-targeted drug delivery systems. Eur. J. Med. Chem. 2018, 157, 705–715. [Google Scholar] [CrossRef]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2018, 24, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.S.; Seo, Y.R. Understanding of ROS-Inducing Strategy in Anticancer Therapy. Oxid. Med. Cell Longev. 2019, 2019, 5381692. [Google Scholar] [CrossRef]
- Traverso, N.; Ricciarelli, R.; Nitti, M.; Marengo, B.; Furfaro, A.L.; Pronzato, M.A.; Marinari, U.M.; Domenicotti, C. Role of glutathione in cancer progression and chemoresistance. Oxid. Med. Cell Longev. 2013, 2013, 972913. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease; that is the question. Cancer Biol. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Trotta, C.F.; Dianzani, C.; Argenziano, M.; Barrera, G.; Cavalli, R. Glutathione bioresponsive cyclodextrin nanosponges. Chem. Plus Chem. 2016, 81, 5. [Google Scholar]
- Yang, D.; Chen, W.; Hu, J. Design of controlled drug delivery system based on disulfide cleavage trigger. J. Phys. Chem. B 2014, 118, 12311–12317. [Google Scholar] [CrossRef]
- Daga, M.; Ullio, C.; Argenziano, M.; Dianzani, C.; Cavalli, R.; Trotta, F.; Ferretti, C.; Zara, G.P.; Gigliotti, C.L.; Ciamporcero, E.S.; et al. GSH-targeted nanosponges increase doxorubicin-induced toxicity “in vitro” and “in vivo” in cancer cells with high antioxidant defenses. Free Radic. Biol. Med. 2016, 97, 24–37. [Google Scholar] [CrossRef]
- Argenziano, M.; Lombardi, C.; Ferrara, B.; Trotta, F.; Caldera, F.; Blangetti, M.; Koltai, H.; Kapulnik, Y.; Yarden, R.; Gigliotti, L.; et al. Glutathione/pH-responsive nanosponges enhance strigolactone delivery to prostate cancer cells. Oncotarget 2018, 9, 35813–35829. [Google Scholar] [CrossRef]
- Kimlin, L.C.; Casagrande, G.; Virador, V.M. In vitro three-dimensional (3D) models in cancer research, an update. Mol. Carcinog. 2013, 52, 167–182. [Google Scholar] [CrossRef]
- Wang, X.; Zhen, X.; Wang, J.; Zhang, J.; Wu, W.; Jiang, X. Doxorubicin delivery to 3D multicellular spheroids and tumors based on boronic acid-rich chitosan nanoparticles. Biomaterials 2013, 34, 4667–4679. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Kreutz, M.; Knuechel, R. Multicellular spheroids: A three-dimensional in vitro culture system to study tumour biology. Int. J. Exp. Pathol. 1998, 79, 1–23. [Google Scholar] [CrossRef]
- Clemedson, C.; Ekwall, B. Overview of the Final MEIC Results: I. The In Vitro-In Vitro Evaluation. Toxicol. In Vitro 1999, 13, 657–663. [Google Scholar] [CrossRef]
- Scheers, E.M.; Ekwall, B.; Dierickx, P.J. In vitro long-term cytotoxicity testing of 27 MEIC chemicals on Hep G2 cells and comparison with acute human toxicity data. Toxicol. In Vitro 2001, 15, 153–161. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Grainger, D.W. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 2009, 61, 438–456. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef]
- Kramer, R.A.; Zakher, J.; Kim, G. Role of the glutathione redox cycle in acquired and de novo multidrug resistance. Science 1988, 241, 694–697. [Google Scholar] [CrossRef]
- Park, J.H.; Choi, K.H.; Kwak, H.S. Single- and 14-day repeat-dose toxicity of cross-linked beta-cyclodextrin in rats. Int. J. Toxicol. 2011, 30, 700–706. [Google Scholar] [CrossRef]
- Minelli, R.; Cavalli, R.; Ellis, L.; Pettazzoni, P.; Trotta, F.; Ciamporcero, E.; Barrera, G.; Fantozzi, R.; Dianzani, C.; Pili, R.; et al. Nanosponge-encapsulated camptothecin exerts anti-tumor activity in human prostate cancer cells. Eur. J. Pharm. Sci. 2012, 47, 686–694. [Google Scholar] [CrossRef]
- Torne, S.; Darandale, S.; Vavia, P.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges: Effective nanocarrier for tamoxifen delivery. Pharm. Dev. Technol. 2013, 18, 619–625. [Google Scholar] [CrossRef]
- Shende, P.; Kulkarni, Y.A.; Gaud, R.S.; Deshmukh, K.; Cavalli, R.; Trotta, F.; Caldera, F. Acute and repeated dose toxicity studies of different beta-cyclodextrin-based nanosponge formulations. J. Pharm. Sci. 2015, 104, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Daga, M.; de Graaf, I.A.M.; Argenziano, M.; Barranco, A.S.M.; Loeck, M.; Al-Adwi, Y.; Cucci, M.A.; Caldera, F.; Trotta, F.; Barrera, G.; et al. Glutathione-responsive cyclodextrin-nanosponges as drug delivery systems for doxorubicin: Evaluation of toxicity and transport mechanisms in the liver. Toxicol. In Vitro 2020, 65, 104800. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Kunwar, A.; Sandur, S.K.; Pandey, B.N.; Chaubey, R.C. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: Role of reactive oxygen species; GSH and Nrf2 in radiosensitivity. Biochim. Biophys. Acta 2014, 1840, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, N.; Slocum, S.L.; Skoko, J.J.; Shin, S.; Kensler, T.W. When NRF2 talks, who’s listening? Antioxid. Redox Signal. 2010, 13, 1649–1663. [Google Scholar] [CrossRef]
- Franco, R.; Cidlowski, J.A. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 2009, 16, 1303–1314. [Google Scholar] [CrossRef]
- Aubry, S.; Burlina, F.; Dupont, E.; Delaroche, D.; Joliot, A.; Lavielle, S.; Chassaing, G.; Sagan, S. Cell-surface thiols affect cell entry of disulfide-conjugated peptides. Faseb. J. 2009, 23, 2956–2967. [Google Scholar] [CrossRef]
- Feener, E.P.; Shen, W.C.; Ryser, H.J. Cleavage of disulfide bonds in endocytosed macromolecules. A processing not associated with lysosomes or endosomes. J. Biol. Chem. 1990, 265, 18780–18785. [Google Scholar]
- Choi, Y.A.; Chin, B.R.; Rhee, D.H.; Choi, H.G.; Chang, H.W.; Kim, J.H.; Baek, S.H. Methyl-beta-cyclodextrin inhibits cell growth and cell cycle arrest via a prostaglandin E(2) independent pathway. Exp. Mol. Med. 2004, 36, 78–84. [Google Scholar] [CrossRef]
- Lee, J.; Lilly, G.D.; Doty, R.C.; Podsiadlo, P.; Kotov, N.A. In vitro toxicity testing of nanoparticles in 3D cell culture. Small 2009, 5, 1213–1221. [Google Scholar] [CrossRef]
- Vandeputte, C.; Guizon, I.; Genestie-Denis, I.; Vannier, B.; Lorenzon, G. A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: Performance study of a new miniaturized protocol. Cell Biol. Toxicol. 1994, 10, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Haslam, G.; Wyatt, D.; Kitos, P.A. Estimating the number of viable animal cells in multi-well cultures based on their lactate dehydrogenase activities. Cytotechnology 2000, 32, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the GSH-NS are to be requested to Professor Francesco Trotta. |
| Average Diameter ± SD (nm) | Polydispersity Index (PDI) | Zeta Potential ± SD (mV) | |
|---|---|---|---|
| Blank GSH-NS B | 183.4 ± 15.3 | 0.23 ± 0.02 | −31.58 ± 3.82 |
| Blank GSH-NS D | 180.5 ± 6.7 | 0.21 ± 0.01 | −31.24 ± 3.05 |
| Fluorescent GSH-NS B | 188.3 ± 10.2 | 0.22 ± 0.01 | −29.98 ± 2.74 |
| Fluorescent GSH-NS D | 185.9 ± 12.5 | 0.22 ± 0.02 | −30.55 ± 2.66 |
| HCT116 IC Values (mg/mL ± St.Dev) on 2D Cultures | ||||||||
| 24 h | GSH-NSB | GSH-NSD | 48 h | GSH-NSB | GSH-NSD | 72 h | GSH-NS B | GSH-NSD |
| IC1 | 0.27 ± 0.03 | 0.51 ± 0.04 | IC1 | 0.47 ± 0.03 | 0.65 ± 0.03 | IC1 | 0.50 ± 0.04 | 0.66 ± 0.05 |
| IC50 | 1.86 ± 0.31 | 2.62 ± 0.39 * | IC50 | 1.60 ± 0.27 | 2.35 ± 0.40 * | IC50 | 1.54 ± 0.19 | 2.38 ± 0.41 * |
| HT-29 IC Values (mg/mL ± St.Dev) on 2D Cultures | ||||||||
| 24 h | GSH-NS B | GSH-NS D | 48 h | GSH-NSB | GSH-NS D | 72 h | GSH-NSB | GSH-NSD |
| IC1 | 0.05 ± 0.00 | 0.05 ± 0.00 | IC1 | 0.15 ± 0.01 | 0.65 ± 0.03 * | IC1 | 0.27 ± 0.02 | 0.65 ± 0.03 * |
| IC50 | 1.71 ± 0.25 | 2.76 ± 0.35 * | IC50 | 1.58 ± 0.21 | 2.80 ± 0.45 * | IC50 | 1.37 ± 0.30 | 2.39 ± 0.31 * |
| DU145 IC Values (mg/mL ± St.Dev) on 2D Cultures | ||||||||
| 24 h | GSH-NS B | GSH-NS D | 48 h | GSH-NSB | GSH-NS D | 72 h | GSH-NSB | GSH-NSD |
| IC1 | 0.01 ± 0.00 | 0.05 ± 0.00 | IC1 | 0.05 ± 0.00 | 0.03 ± 0.00 | IC1 | 0.17 ± 0.01 | 0.12 ± 0.01 |
| IC50 | 1.85 ± 0.40 | 2.34 ± 0.36 | IC50 | 1.37 ± 0.28 | 1.57 ± 0.32 | IC50 | 1.38 ± 0.25 | 1.56 ± 0.30 |
| PC-3 IC Values (mg/mL ± St.Dev) on 2D Cultures | ||||||||
| 24 h | GSH-NS B | GSH-NS D | 48 h | GSH-NSB | GSH-NSD | 72 h | GSH-NSB | GSH-NSD |
| IC1 | 0.04 ± 0.00 | 0.05 ± 0.00 | IC1 | 0.06 ± 0.01 | 0.43 ± 0.03 * | IC1 | 0.35 ± 0.02 | 0.43 ± 0.03 |
| IC50 | 1.65 ± 0.27 | 2.56 ± 0.38 * | IC50 | 1.20 ± 0.22 | 2.09 ± 0.25 * | IC50 | 1.37 ± 0.31 | 1.68 ± 0.27 |
| HCT116 IC Values (mg/mL ± St.Dev) on 3D Cultures | ||||||||
| 24 h | GSH-NS B | GSH-NS D | 48 h | GSH-NS B | GSH-NS D | 72 h | GSH-NS B | GSH-NS D |
| IC1 | 0.01 ± 0.00 | 0.02 ± 0.00 | IC1 | 0.01 ± 0.00 | 0.01 ± 0.00 | IC1 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| IC50 | 3.92 ± 0.95 | 4.19 ± 0.98 | IC50 | 2.75 ± 0.18 | 3.05 ± 0.31 | IC50 | 1.98 ± 0.15 | 2.22 ± 0.21 |
| DU145 IC Values (mg/mL ± St.Dev) on 3D Cultures | ||||||||
| 24 h | GSH-NS B | GSH-NS D | 48 h | GSH-NS B | GSH-NS D | 72 h | GSH-NS B | GSH-NS D |
| IC1 | 0.02 ± 0.00 | 0.04 ± 0.00 | IC1 | 0.03 ± 0.00 | 0.02 ± 0.00 | IC1 | 0.07 ± 0.01 | 0.02 ± 0.00 |
| IC50 | 5.38 ± 1.15 | 5.80 ± 1.78 | IC50 | 2.68 ± 0.25 | 3.24 ± 0.34 | IC50 | 2.01 ± 0.24 | 2.26 ± 0.23 |
| Gene | Primer Codes | Description |
|---|---|---|
| CDC25A | QT00001078 | cell division cycle 25 homolog A |
| CDK1 | QT00042672 | cyclin-dependent kinase 1 |
| CDK2 | QT00005586 | cyclin-dependent kinase 2 |
| CDK4 | QT00016107 | cyclin-dependent kinase 4 |
| CDKN1A | QT00031192 | cyclin-dependent kinase inhibitor 1A, p21 |
| CDKN2A | QT00089964 | cyclin-dependent kinase inhibitor 2A, p16 |
| RRN18S | QT00199367 | 18S ribosomal RNA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argenziano, M.; Foglietta, F.; Canaparo, R.; Spagnolo, R.; Della Pepa, C.; Caldera, F.; Trotta, F.; Serpe, L.; Cavalli, R. Biological Effect Evaluation of Glutathione-Responsive Cyclodextrin-Based Nanosponges: 2D and 3D Studies. Molecules 2020, 25, 2775. https://doi.org/10.3390/molecules25122775
Argenziano M, Foglietta F, Canaparo R, Spagnolo R, Della Pepa C, Caldera F, Trotta F, Serpe L, Cavalli R. Biological Effect Evaluation of Glutathione-Responsive Cyclodextrin-Based Nanosponges: 2D and 3D Studies. Molecules. 2020; 25(12):2775. https://doi.org/10.3390/molecules25122775
Chicago/Turabian StyleArgenziano, Monica, Federica Foglietta, Roberto Canaparo, Rita Spagnolo, Carlo Della Pepa, Fabrizio Caldera, Francesco Trotta, Loredana Serpe, and Roberta Cavalli. 2020. "Biological Effect Evaluation of Glutathione-Responsive Cyclodextrin-Based Nanosponges: 2D and 3D Studies" Molecules 25, no. 12: 2775. https://doi.org/10.3390/molecules25122775
APA StyleArgenziano, M., Foglietta, F., Canaparo, R., Spagnolo, R., Della Pepa, C., Caldera, F., Trotta, F., Serpe, L., & Cavalli, R. (2020). Biological Effect Evaluation of Glutathione-Responsive Cyclodextrin-Based Nanosponges: 2D and 3D Studies. Molecules, 25(12), 2775. https://doi.org/10.3390/molecules25122775










