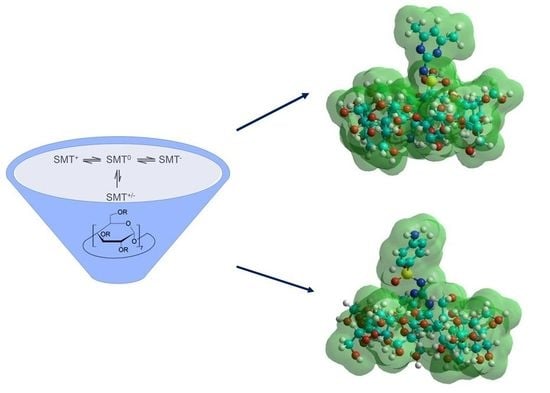
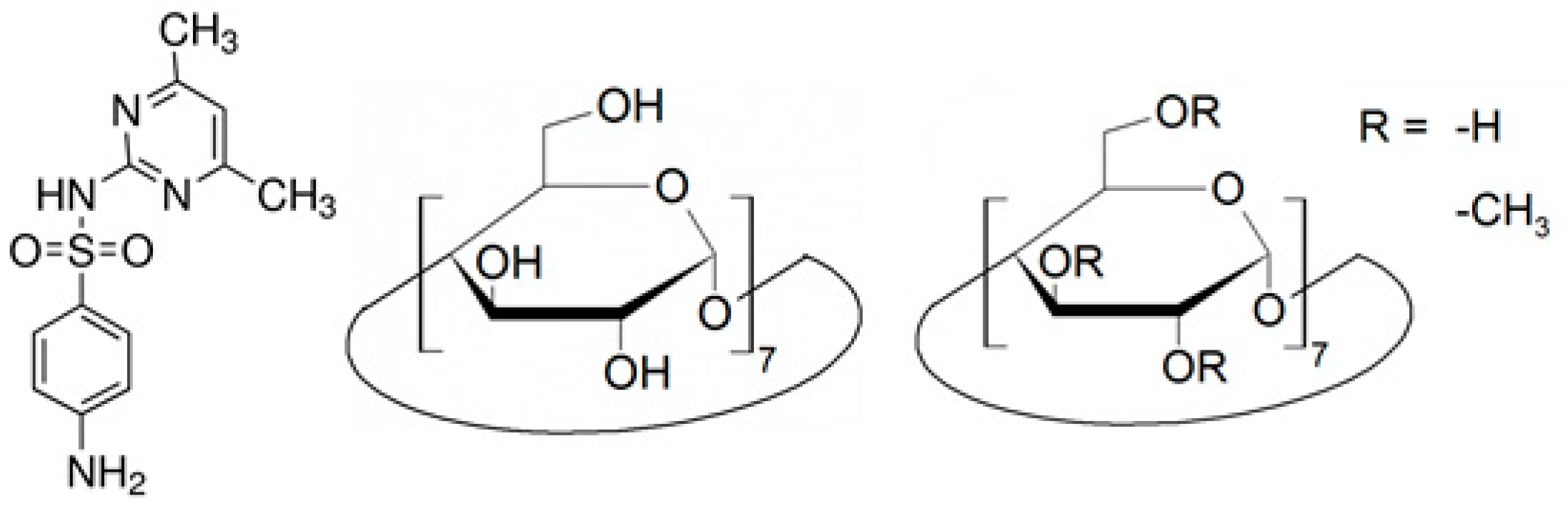
Thermodynamic Characterization of the Interaction between the Antimicrobial Drug Sulfamethazine and Two Selected Cyclodextrins
Abstract
1. Introduction
2. Results and Discussion
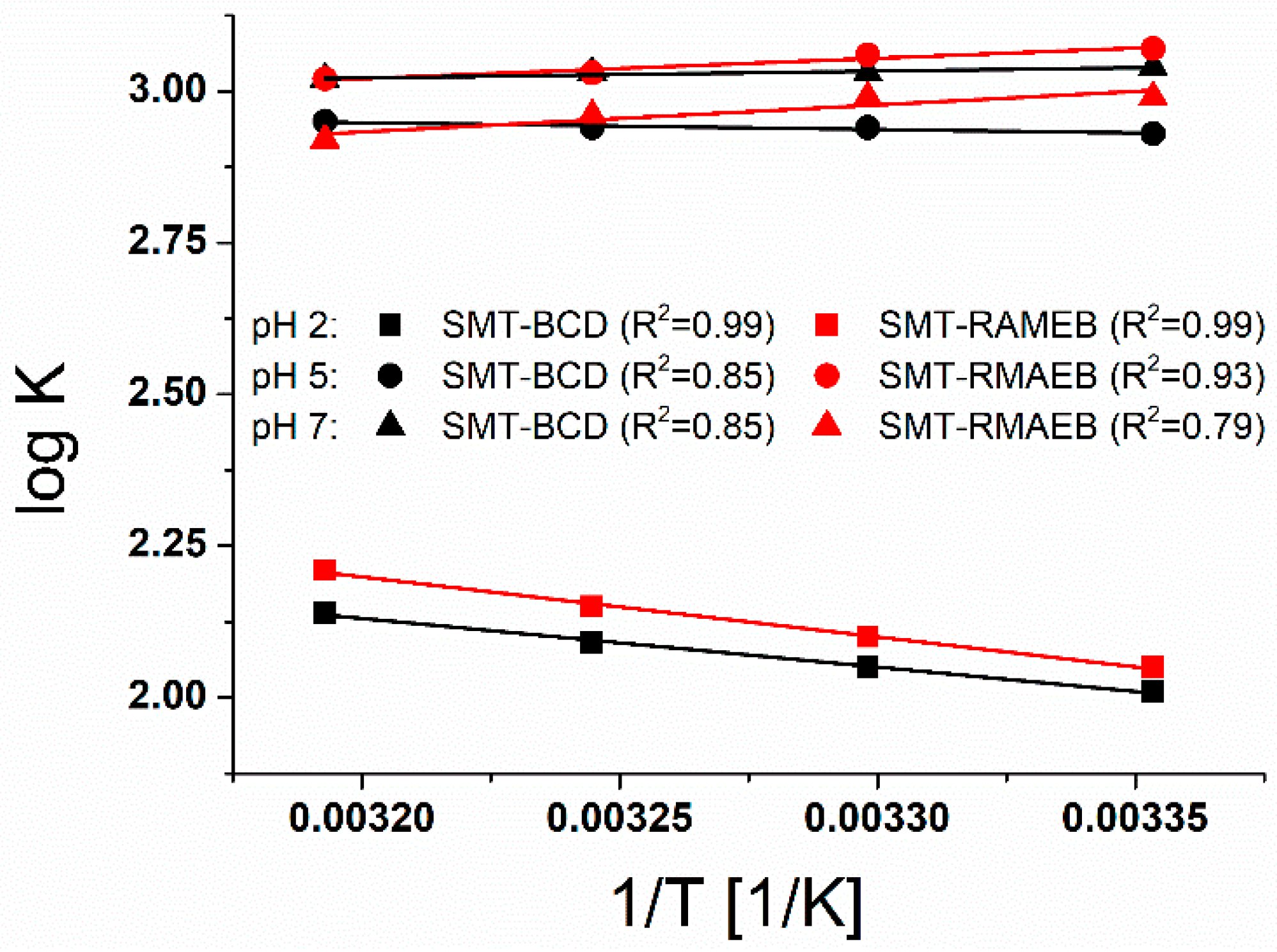
2.1. Temperature Dependence of the Association Constants of Sulfamethazine-CD Complexes at Different pH
2.2. Modeling Studies
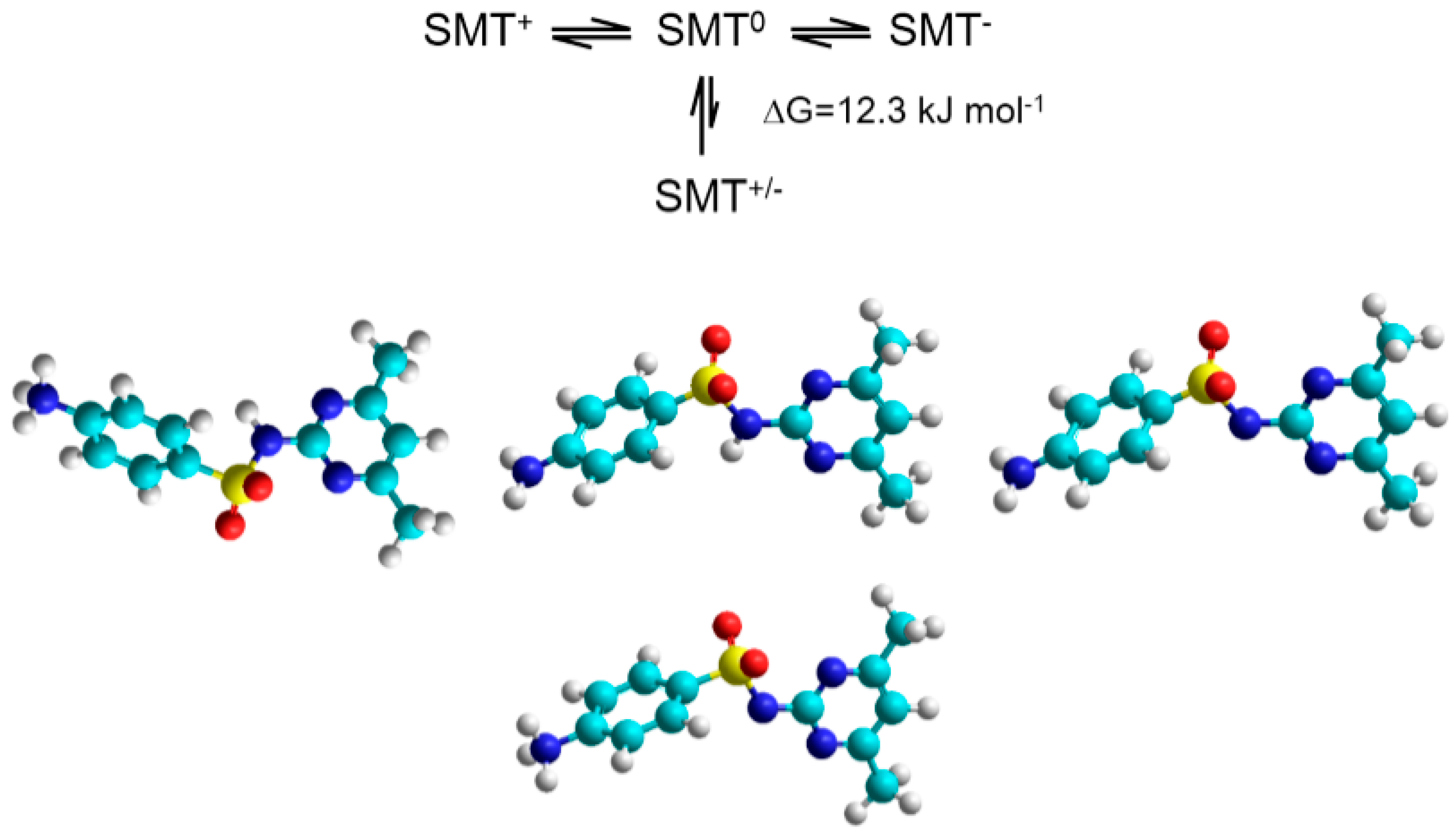
2.3. Driving Forces of the SMT-CD Complex Formations
3. Materials and Methods
3.1. Reagents
3.2. Fluorescence Spectroscopic Studies
3.3. Infrared Spectroscopy
3.4. Modeling Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Zhang, D.; Zhao, L.; Zhang, P.; Lu, X.; He, S. Extraction property of p-tert-butylsulfonylcalix[4]- arene possessing irradiation stability towards cesium(I) and strontium(II). Appl. Sci. 2016, 6, 212. [Google Scholar] [CrossRef]
- Galindo-Murillo, R.; Olmedo-Romero, A.; Cruz-Flores, E.; Petrar, P.M.; Kunsagi-Mate, S.; Barroso-Flores, J. Calix[n]arene-based drug carriers: A DFT study of their electronic interactions with a chemotherapeutic agent used against leukemia. Comput. Theor. Chem. 2004, 1035, 84. [Google Scholar] [CrossRef]
- Brancatelli, G.; Dalcanale, E.; Pinalli, R.; Geremia, S. Probing the structural determinants of amino acid recognition: X-Ray studies of crystalline ditopic host-guest complexes of the positively charged amino acids, Arg, Lys, and His with a cavitand molecule. Molecules 2018, 23, 3368. [Google Scholar] [CrossRef] [PubMed]
- Czibulya, Z.; Horvath, E.; Nagymihaly, Z.; Kollar, L.; Kunsagi-Mate, S. Competitive processes associated to the interaction of a cavitand derivative with caffeic acid. Supramol. Chem. 2016, 28, 582. [Google Scholar] [CrossRef]
- Hahm, E.; Kang, E.J.; Pham, X.-H.; Jeong, D.; Jeong, D.H.; Jung, S.; Jun, B.-H. Mono-6-deoxy-6-aminopropylamino-β-cyclodextrin on Ag-embedded SiO2 nanoparticle as a selectively capturing ligand to flavonoids. Nanomaterials 2019, 9, 1349. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Gazdag, Z.; Szente, L.; Meggyes, M.; Horváth, G.; Lemli, B.; Kunsági-Máté, S.; Kuzma, M.; Kőszegi, T. Antioxidant and antimicrobial properties of randomly methylated β cyclodextrin – captured essential oils. Food Chem. 2019, 278, 305. [Google Scholar] [CrossRef]
- Xing, M.; Yanli, Z. Biomedical applications of supramolecular systems based on host−guest interactions. Chem. Rev. 2015, 115, 7794. [Google Scholar] [CrossRef]
- Ryu, J.H.; Hong, D.J.; Lee, M. Aqueous self-assembly of aromatic rod building blocks. Chem. Commun. 2008, 1054, 1043. [Google Scholar] [CrossRef]
- Zang, W.; Jin, W.; Fukushima, T.; Saeki, A.; Seki, S.; Aida, T. Supramolecular linear heterojunction composed of graphite-like semiconducting nanotubular segments. Science 2011, 334, 340. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, K.; Roy, M.N. Study to explore the mechanism to form inclusion complexes of b-cyclodextrin with vitamin molecules. Sci. Rep. 2016, 6, 35764. [Google Scholar] [CrossRef]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of cyclodextrin/volatile inclusion complexes: A Review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef] [PubMed]
- Menezes, P.D.P.; Andrade, T.A.; Frank, L.A.; de Souza, E.P.B.S.S.; Trindade, G.D.G.G.; Trindade, I.A.S.; Serafini, M.R.; Guterres, S.S.; Araújo, A.A.S. Advances of nanosystems containing cyclodextrins and their applications in pharmaceuticals. Int. J. Pharm. 2019, 559, 312. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jiang, J.; Ma, J.; Zhou, Y.; von Gunten, U. Enhanced transformation of sulfonamide antibiotics by manganese(IV) oxide in the presence of model humic constituents. Water Res. 2019, 153, 200. [Google Scholar] [CrossRef] [PubMed]
- Le-Minh, N.; Stuetz, R.M.; Khan, S.J. Determination of six sulfonamide antibiotics, two metabolites and trimethoprim in wastewater by isotope dilution liquid chromatography/ tandem mass spectrometry. Talanta 2012, 89, 407. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, V.; Adrian, J.; Guiteras, J.; Marco, M.P.; Companyó, R. Validation of an enzyme-linked immunosorbent assay for detecting sulfonamides in feed resources. J. Agric. Food Chem. 2010, 58, 7526. [Google Scholar] [CrossRef]
- Boreen, A.L.; Arnold, W.A.; McNeill, K. Photochemical fate of sulfa drugs in the aquatic environment: Sulfa drugs containing five-membered heterocyclic groups. Env. Sci. Technol. 2004, 38, 3933. [Google Scholar] [CrossRef]
- Nesterenko, I.S.; Nokel, M.A.; Eremin, S.A. Immunochemical methods for the detection of sulfanylamide drugs. J. Anal. Chem. 2009, 64, 453. [Google Scholar] [CrossRef]
- Bani-Yaseen, A.D.; Mo’ala, A. Spectral, thermal, and molecular modeling studies on the encapsulation of selected sulfonamide drugs in b-cyclodextrin nano-cavity. Spectrochim. Acta A 2014, 131, 424. [Google Scholar] [CrossRef]
- Saha, S.; Roy, A.; Roy, M.N. Mechanistic investigation of inclusion complexes of a sulfa drug with a- and b-cyclodextrins. Ind. Eng. Chem. Res. 2017, 56, 11672. [Google Scholar] [CrossRef]
- Prabhu, A.A.M.; Venkatesh, G.; Rajendiran, N. Spectral characteristics of sulfa drugs: Effect of solvents, pH and β-cyclodextrin. J. Solution. Chem. 2010, 39, 1061. [Google Scholar] [CrossRef]
- Rajendiran, N.; Siva, S. Inclusion complex of sulfadimethoxine with cyclodextrins: Preparation and characterization. Carbohydr. Polym. 2014, 101, 828. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gaba, R.; Soni, S. Absorption and fluorescence spectral studies of the interaction of sulpha drugs with α-cyclodextrin. Phys. Chem. Liq. 2019, 57, 362. [Google Scholar] [CrossRef]
- Zoppi, A.; Delviro, A.; Aissa, V.; Longhi, M.R. Binding of sulfamethazine to b-cyclodextrin and methyl-b-cyclodextrin. AAPS Pharm. Sci. Tech. 2013, 14, 727. [Google Scholar] [CrossRef]
- Zoppi, A.; Quevedo, M.A.; Delviro, A.; Longhi, M.R. Complexation of sulfonamides with b-cyclodextrin studied by experimental and theoretical methods. J. Pharm. Sci. 2010, 99, 3166. [Google Scholar] [CrossRef]
- Morisue, M.; Ueno, I. Preferential solvation unveiled by anomalous conformational equilibration of porphyrin dimers: Nucleation growth of solvent−solvent segregation. J. Phys. Chem. B 2018, 122, 5251. [Google Scholar] [CrossRef]
- Wintgens, V.; Lorthioir, C.; Miskolczy, Z.; Amiel, C.; Biczók, L. Substituent effects on the inclusion of 1-alkyl-6-alkoxy-quinolinium in 4-sulfonatocalix[n]arenes. ACS Omega 2018, 3, 8631. [Google Scholar] [CrossRef]
- Nicolas, H.; Yuan, B.; Xu, J.; Zhang, X.; Schönhoff, M. pH-responsive host–guest complexation in pillar[6]arene-containing polyelectrolyte multilayer films. Polymers 2017, 9, 719. [Google Scholar] [CrossRef]
- Dan, Z.; Cao, H.; He, X.; Zhang, Z.; Zou, L.; Zeng, L.; Xu, Y.; Yin, Q.; Xu, M.; Zhong, D.; et al. A pH-responsive host-guest nanosystem loading succinobucol suppresses lung metastasis of breast cancer. Theranostics 2016, 6, 435. [Google Scholar] [CrossRef]
- Li, B.; Meng, Z.; Li, Q.; Huang, X.; Kang, Z.; Dong, H.; Chen, J.; Sun, J.; Dong, Y.; Li, J.; et al. A pH responsive complexation-based drug delivery system for oxaliplatin. Chem. Sci. 2017, 8, 4458. [Google Scholar] [CrossRef]
- Mohamed Ameen, H.; Kunsági-Máté, S.; Szente, L.; Lemli, B. Encapsulation of sulfamethazine by native and randomly methylated β-cyclodextrins: The role of the dipole properties of guests. Spectrochim. Acta A Spectrosc. 2020, 225, 117475. [Google Scholar] [CrossRef]
- Liu, M.; Guo, Q.; Shi, Y.; Cai, C.; Pei, W.; Yan, H.; Jia, H.; Han, J. Studies on pH and temperature dependence of inclusion complexes of bisdemethoxycurcumin with β-cyclodextrin derivatives. J. Mol. Struct 2019, 1179, 336. [Google Scholar] [CrossRef]
- Poór, M.; Matisz, G.; Kunsági-Máté, S.; Derdák, D.; Szente, L.; Lemli, B. Fluorescence spectroscopic investigation of the interaction of citrinin with native and chemically modified cyclodextrins. J. Lumin. 2016, 172, 23. [Google Scholar] [CrossRef]
- Texido, M.; Pignatello, J.J.; Beltran, J.L.; Granados, M.; Peccia, J. Speciation of the ionizable antibiotic sulfamethazine on black carbon (Biochar). Environ. Sci. Technol. 2011, 45, 100020. [Google Scholar] [CrossRef]
- Lazar, P.; Lee, Y.; Kim, S.; Chandrasekaran, M.; Lee, K.W. Molecular dynamics simulation study for ionic strength dependence of RNA-host factor interaction in Staphylococcus aureus Hfq. Bull. Korean Chem. Soc. 2010, 31, 1519. [Google Scholar] [CrossRef][Green Version]
- Liu, L.; Gou, Q.-X. The driving forces in the inclusion complexation of cyclodextrins. J. Incl. Phenom. Macro. 2002, 42, 1. [Google Scholar] [CrossRef]
- Terekhova, I.V.; Chislov, M.V.; Brusnikina, M.A.; Chibunova, E.S.; Volkova, T.V.; Zvereva, I.A.; Proshin, A.N. Thermodynamics and binding mode of novel structurally related 1,2,4-thiadiazole derivatives with native and modified cyclodextrins. Chem. Phys. Lett. 2017, 671, 28. [Google Scholar] [CrossRef]
- Dragan, A.I.; Read, C.M.; Crane-Robinson, C. Enthalpy-entropy compensation: The role of solvation. Eur. Biophys. J. 2017, 46, 301. [Google Scholar] [CrossRef]
- Pan, A.; Biswas, T.; Rakshit, A.K.; Moulik, S.P. Enthalpy-entropy compensation (EEC) effect: A revisit. J. Phys. Chem. B 2015, 119, 15876. [Google Scholar] [CrossRef]
- Pan, A.; Kar, T.; Rakshit, A.K.; Moulik, S.P. Enthalpy-entropy compensation (EEC) effect: Decisive role of free energy. J. Phys. Chem. B 2016, 120, 10531. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, J. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875. [Google Scholar] [CrossRef]
- Kunsági-Máté, S.; Iwata, K. Effect of cluster formation of solvent molecules on the preferential solvatation of anthracene in binary alcoholic solutions. Chem. Phys. Lett. 2009, 473, 284. [Google Scholar] [CrossRef]
- Kunsági-Máté, S.; Iwata, K. Electron density dependent composition of the solvation shell of phenol derivatives in binary solutions of water and ethanol. J. Solut. Chem. 2013, 42, 165. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743. [Google Scholar] [CrossRef] [PubMed]
- Poór, M.; Bálint, M.; Hetényi, C.; Gődér, B.; Kunsági-Máté, S.; Kőszegi, T.; Lemli, B. Investigation of non-covalent interactions of aflatoxins (B1, B2, G1, G2 and M1) with serum albumin. Toxins 2017, 9, 339. [Google Scholar] [CrossRef]
- Poór, M.; Boda, G.; Kunsági-Máté, S.; Needs, P.W.; Kroon, P.A.; Lemli, B. Fluorescence spectroscopic evaluation of the interactions of quercetin, isorhamnetin, and quercetin-3’-sulfate with different albumins. J. Lumin. 2018, 194, 156. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739. [Google Scholar] [CrossRef]
- HyperChem, Hypercube Inc. 2007. Available online: http://www.hyper.com/ (accessed on 12 December 2019).
- Faisal, Z.; Kunsági-Máté, S.; Lemli, B.; Szente, L.; Bergmann, D.; Humpf, H.-U.; Poór, M. Interaction of dihydrocitrinone with native and chemically modified cyclodextrins. Molecules 2019, 24, 1328. [Google Scholar] [CrossRef]
- Fliszár-Nyúl, E.; Lemli, B.; Kunsági-Máté, S.; Szente, L.; Poór, M. Interactions of mycotoxin alternariol with cyclodextrins and its removal from aqueous solution by beta-cyclodextrin bead polymer. Biomolecules 2019, 9, 428. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds BCD and RAMEB are available from CycloLab Ltd. (L.S.). |
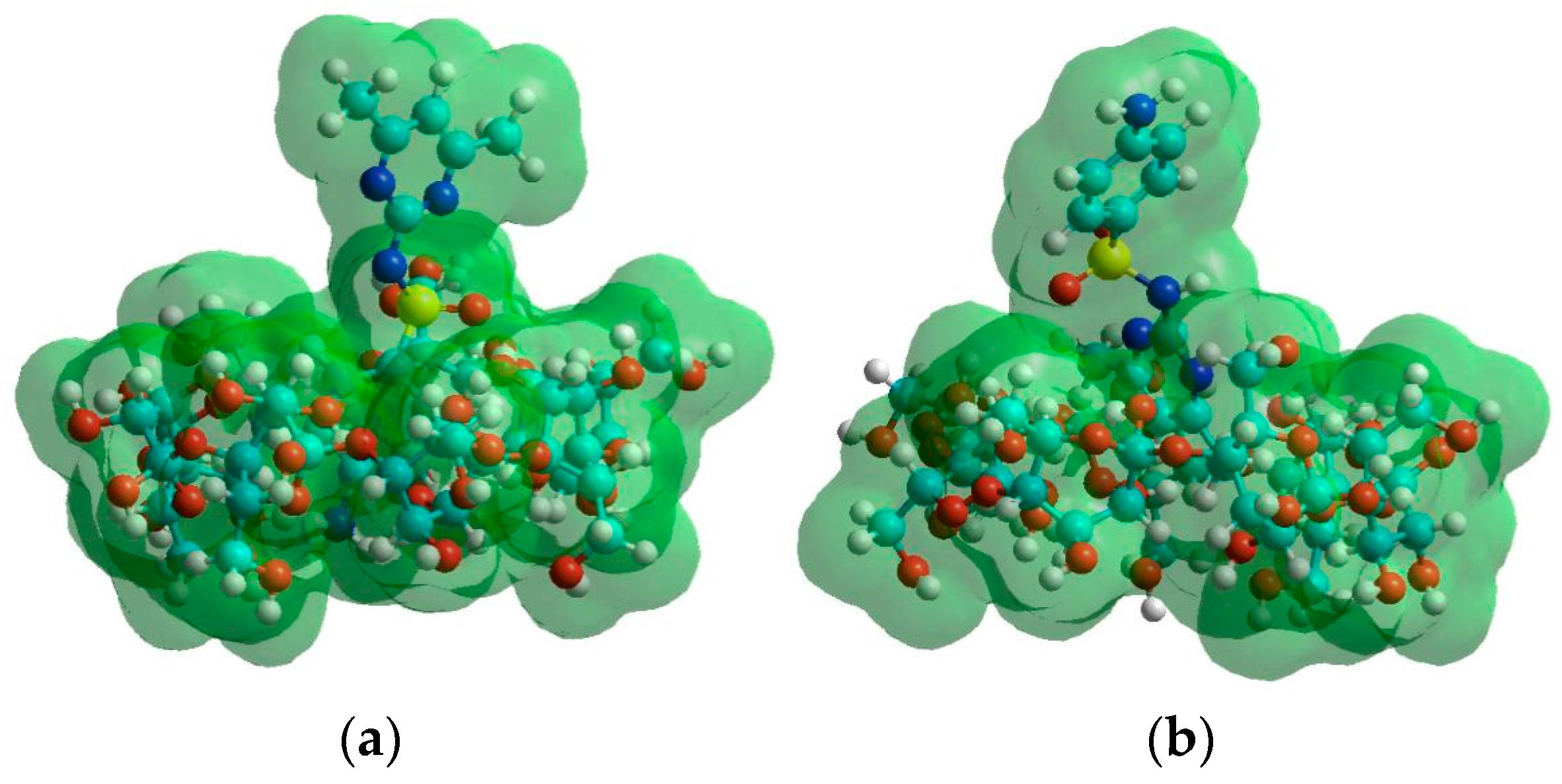
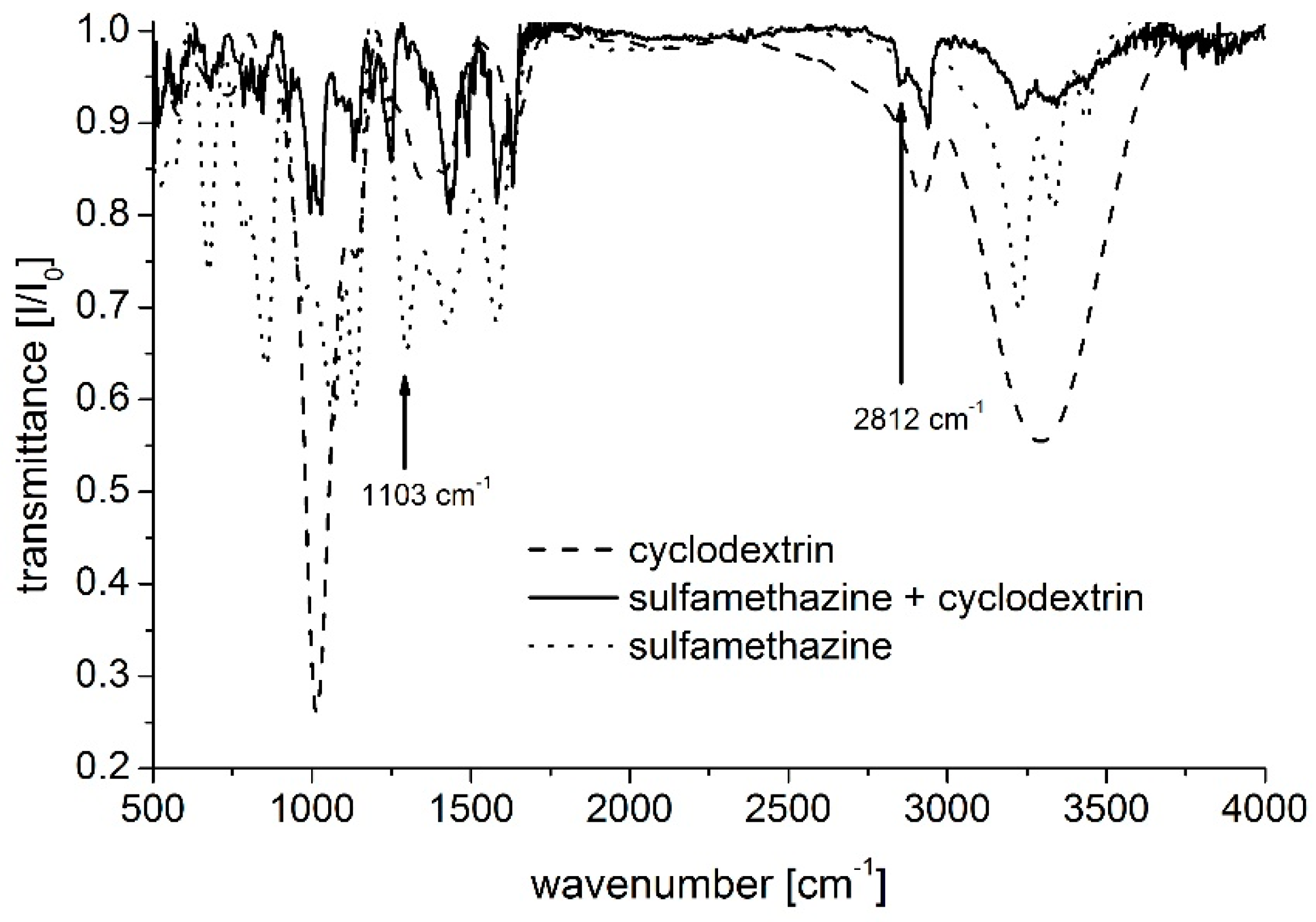
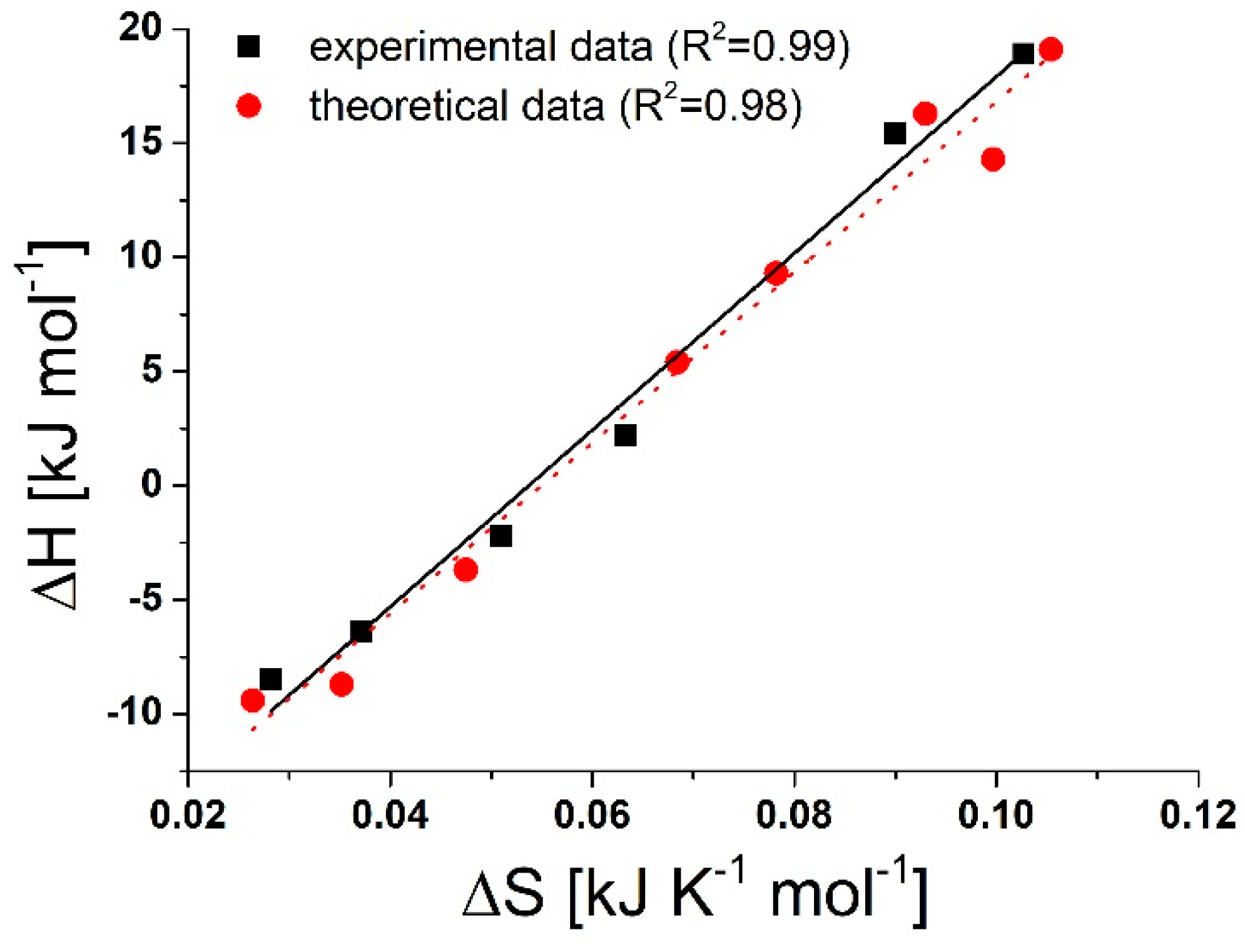
| Host Species | pH | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 5 | 7 | |||||||
| ΔH | ΔS | ΔG298K | ΔH | ΔS | ΔG298K | ΔH | ΔS | ΔG298K | |
| BCD | 15.4 ± 0.8 | 90.0 ± 2.5 | −11.4 ± 1.5 | 2.2 ± 0.5 | 63.3 ± 1.7 | −16.7 ± 1.0 | −2.2 ± 0.5 | 51.0 ± 1.7 | −17.3 ± 1.0 |
| RAMEB | 18.9 ± 0.8 | 102.7 ± 2.6 | −11.7 ± 1.6 | −6.4 ± 1.0 | 37.2 ± 3.3 | −17.5 ± 2.0 | −8.5 ± 1.2 | 28.8 ± 3.8 | −17.1 ± 2.3 |
| Host Specie | Host Simulated as | Guest’s Charges | |||||||
|---|---|---|---|---|---|---|---|---|---|
| +1 (Cationic) | 0 (Nonionic) | 0 (Zwitterionic) | −1 (Anionic) | ||||||
| ΔH | ΔS | ΔH | ΔS | ΔH | ΔS | ΔH | ΔS | ||
| BCD | 0 BCD | 16.3 | 93.0 | 9.3 | 78.2 | 5.4 | 68.4 | −3.7 | 47.5 |
| RAMEB | −1 BCD | 19.1 | 105.4 | 14.3 | 99.7 | −8.7 | 35.2 | −9.4 | 26.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Ameen, H.; Kunsági-Máté, S.; Bognár, B.; Szente, L.; Poór, M.; Lemli, B. Thermodynamic Characterization of the Interaction between the Antimicrobial Drug Sulfamethazine and Two Selected Cyclodextrins. Molecules 2019, 24, 4565. https://doi.org/10.3390/molecules24244565
Mohamed Ameen H, Kunsági-Máté S, Bognár B, Szente L, Poór M, Lemli B. Thermodynamic Characterization of the Interaction between the Antimicrobial Drug Sulfamethazine and Two Selected Cyclodextrins. Molecules. 2019; 24(24):4565. https://doi.org/10.3390/molecules24244565
Chicago/Turabian StyleMohamed Ameen, Hiba, Sándor Kunsági-Máté, Balázs Bognár, Lajos Szente, Miklós Poór, and Beáta Lemli. 2019. "Thermodynamic Characterization of the Interaction between the Antimicrobial Drug Sulfamethazine and Two Selected Cyclodextrins" Molecules 24, no. 24: 4565. https://doi.org/10.3390/molecules24244565
APA StyleMohamed Ameen, H., Kunsági-Máté, S., Bognár, B., Szente, L., Poór, M., & Lemli, B. (2019). Thermodynamic Characterization of the Interaction between the Antimicrobial Drug Sulfamethazine and Two Selected Cyclodextrins. Molecules, 24(24), 4565. https://doi.org/10.3390/molecules24244565