Theoretical Study of the Structures of 4-(2,3,5,6-Tetrafluoropyridyl)Diphenylphosphine Oxide and Tris(Pentafluorophenyl)Phosphine Oxide: Why Does the Crystal Structure of (Tetrafluoropyridyl)Diphenylphosphine Oxide Have Two Different P=O Bond Lengths?
Abstract
1. Introduction
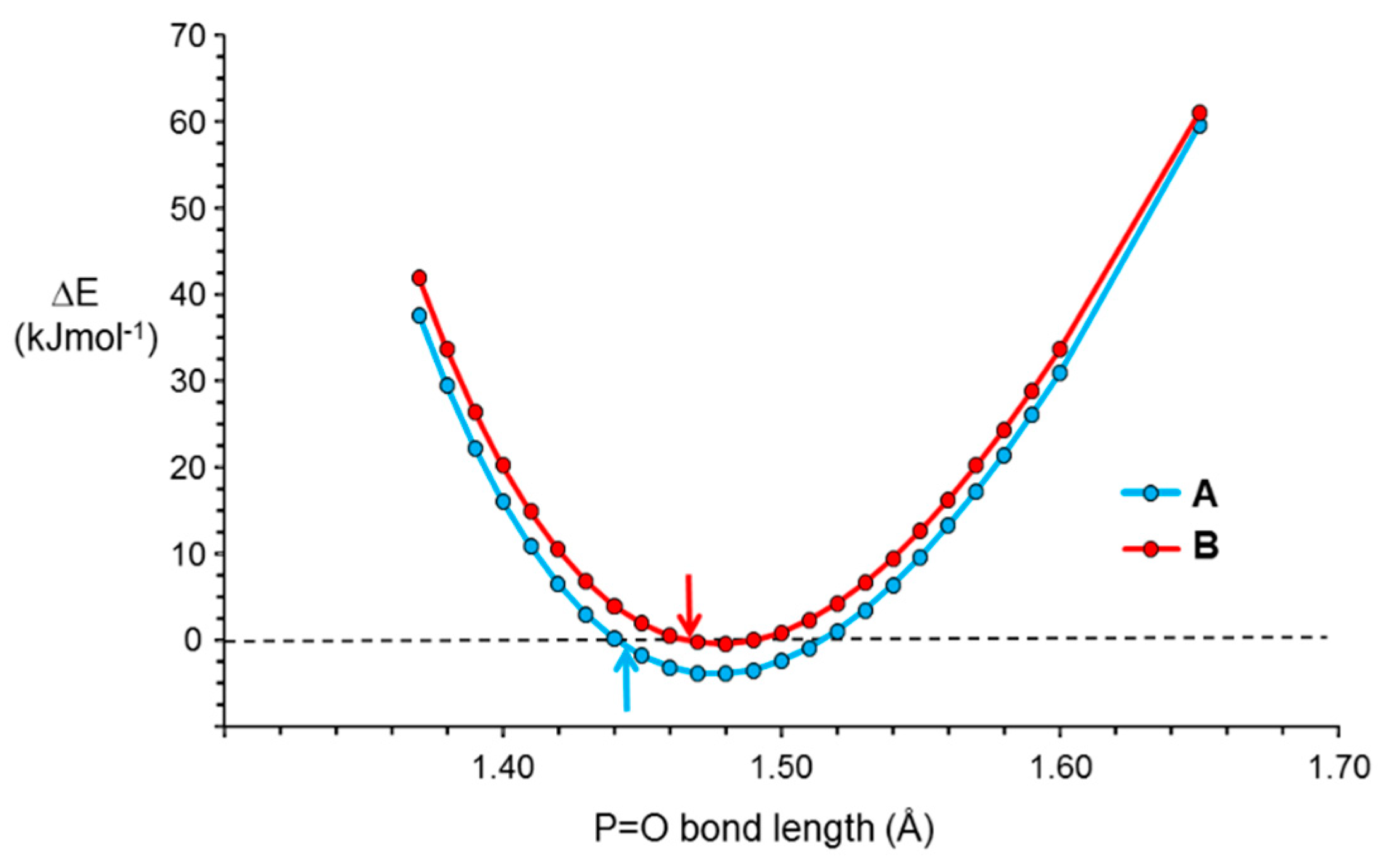
2. Results and Discussion
3. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bellabarba, R.M.; Nieuwenhuyzen, M.; Saunders, G.C. Intramolecular Dehydrofluorinative Coupling of the Asymmetric Diphosphine Ph2PCH2CH2PPh(C5F4N-4) and Pentamethylcyclopentadienyl Ligands in a Rhodium Complex. Organometallics 2003, 22, 1802–1810. [Google Scholar] [CrossRef]
- Jean, Y.; Lledos, A.; Burdett, J.K.; Hoffmann, R. Bond-stretch isomerism in transition-metal complexes. J. Am. Chem. Soc. 1998, 110, 4506–4516. [Google Scholar] [CrossRef]
- Parkin, G. Do bond-stretch isomers really exist? Acc. Chem. Res. 1992, 25, 455–460. [Google Scholar] [CrossRef]
- Steed, K.M.; Steed, J.W. Packing Problems: High Z′ Crystal Structures and Their Relationship to Cocrystals, Inclusion Compounds, and Polymorphism. Chem. Rev. 2015, 115, 2895–2933. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Lynch, D.E.; Byriel, K.A.; Kennard, C.H.L. Molecular cocrystals of carboxylic acids Part 24: Cocrystals involving triphenylphosphine oxide: Structures of the unique adduct hydrates of triphenylphosphine oxide with adamantane carboxylic acid and terephthalic acid, and the anhydrous adduct with o-phthalic acid. Z. Kristallogr. 1997, 212, 130–134. [Google Scholar]
- Pietrusiewicz, K.M.; Kataev, V.E.; Patsanovsky, I.I.; Ermolaeva, L.V.; Ishmaeva, E.A.; Kataev, A.V.; Wieczorek, W.; Zygo, K. Molecular structure, polarity and conformation analysis of 1, 3, 5-tris (diphenylphosphinoxidemethylene) benzene. Zh. Obshch. Khim. 1998, 68, 1510–1518. [Google Scholar]
- Genov, D.G.; Kresinski, R.A.; Tebby, J.C. Conformational Analysis of 2-Substituted Alkylphosphoryl Compounds. 4. Substituent Effect on the Nature of the Hydrogen Bonding in (2-Hydroxyalkyl)phosphoryl Systems. J. Org. Chem 1998, 63, 2574–2585. [Google Scholar] [CrossRef]
- Priya, S.; Balakrishna, M.S.; Mobin, S.M. Reactions of aminophosphines and aminobis(phosphines) with aldehydes and ketones: Coordination complexes of the resultant aminobis(alkylphosphineoxides) with cobalt, uranium, thorium and gadolinium salts: Crystal and molecular structures of Ph2P(O)CH(C6H4OH-o)N(H)Ph, Ph2P(O)CH(OH)C6H4OH-o and Ph2P(O)N(H)Ph. Polyhedron 2005, 24, 1641–1650. [Google Scholar]
- Ng, S.W. A second monoclinic modification of triphenylphosphine oxide hemihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o1431. [Google Scholar] [CrossRef]
- Hilliard, C.R.; Kharel, S.; Cluff, K.J.; Bhuvanesh, N.; Gladysz, J.A.; Blumel, J. Structures and Unexpected Dynamic Properties of Phosphine Oxides Adsorbed on Silica Surfaces. Chem. Eur. J. 2014, 20, 17292–17295. [Google Scholar] [CrossRef]
- Camps, P.; Colet, G.; Font-Bardia, M.; Muñoz-Torrero, V.; Solans, X.; Vázquez, S. Straightforward regio- and stereo-selective synthesis of t-2-[(diphenylphosphinoyl)methyl]-c-3-(disubstitutedphosphinoyl)-r-1-cyclopentanols. Tetrahedron 2002, 58, 3473–3484. [Google Scholar] [CrossRef]
- Müller, P.; Fuhr, O.; Doring, M. New phosphorus-containing quinone derivatives II: Tri- and tetraphosphorylated quinone derivatives. Heteroat. Chem. 2013, 24, 252–262. [Google Scholar] [CrossRef]
- Salem, H.; Schmitt, M.; Herrlich, U.; Kuhnel, E.; Brill, M.; Nagele, P.; Bogado, A.L.; Rominger, F.; Hofmann, P. Bulky N-Phosphinomethyl-Functionalized N-Heterocyclic Carbene Chelate Ligands: Synthesis, Molecular Geometry, Electronic Structure, and Their Ruthenium Alkylidene Complexes. Organometallics 2013, 32, 29–46. [Google Scholar] [CrossRef]
- Hsiao, Y.-C.; Chiang, W.-Y.; Weng, C.-M.; Hong, F.-E. Preparation and characterization of cobalt-containing P,N-ligands and an unusual palladium complex ion pair: Their applications in amination reactions. Tetrahedron 2008, 64, 9507–9514. [Google Scholar] [CrossRef]
- Patra, S.K.; Whittell, G.R.; Nagiah, S.; Ho, C.-L.; Wong, W.-Y.; Manners, I. Photocontrolled Living Anionic Polymerization of Phosphorus-Bridged [1]Ferrocenophanes: A Route to Well-Defined Polyferrocenylphosphine (PFP) Homopolymers and Block Copolymers. Chem. Eur. J. 2010, 16, 3240–3250. [Google Scholar] [CrossRef] [PubMed]
- Naae, D.G.; Lin, T.-W. Solid-gas reactions of diphenyl-F-phenyl phosphine oxide with methylamine and ammonia. J. Fluor. Chem. 1979, 13, 473–486. [Google Scholar] [CrossRef]
- Nicholson, B.K.; Thwaite, S.E. Tris(pentafluorophenyl)phosphine oxide. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, o1700–o1701. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in crystals. Cryst. Eng. Commun. 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Takahashi, O.; Kohno, Y.; Nishio, M. Relevance of Weak Hydrogen Bonds in the Conformation of Organic Compounds and Bioconjugates: Evidence from recent Experimental Data and High-Level ab Initio MO Calculations. Chem. Rev. 2010, 110, 6049–6076. [Google Scholar] [CrossRef] [PubMed]
- Mooibroek, T.J.; Gamesz, P.; Reedijk, J. Lone pair–π interactions: A new supramolecular bond? Cryst. Eng. Commun. 2008, 10, 1501–1515. [Google Scholar] [CrossRef]
- Singh, S.K.; Das, A. The n → π* interaction: A rapidly emerging non-covalent interaction. Phys. Chem. Chem. Phys. 2015, 17, 9596–9612. [Google Scholar] [CrossRef]
- Echeverria, J. The n → π interaction in Metal Complexes. Chem. Commun. 2018, 54, 3061–3064. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiners, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Seddon, K.R. The Hydrogen Bond and Crystal Engineering. Chem. Soc. Rev. 1993, 93, 397–407. [Google Scholar] [CrossRef]
- Desiraju, G.R. A Bond by Any Other Name. Angew. Chem. Int. Ed. 2001, 50, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.2; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Mathematical, Physical and Chemical Tables. In International Tables for Crystallography; Wilson, A.J.C., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1995; vol. C, pp. 685–706. [Google Scholar]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Contreras-Garcia, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-Garcia, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |









| Distance or Angle | Molecule A | Molecule B | Calculated |
|---|---|---|---|
| P=O | 1.441(3) | 1.466(2) | 1.478 |
| P–C(11) | 1.792(4) | 1.788(3) | 1.805 |
| P–C(21) | 1.798(4) | 1.798(3) | 1.807 |
| P–C(31) | 1.832(3) | 1.836(4) | 1.851 |
| O=P–C(11) | 112.2(2) | 112.7(1) | 112.9 |
| O=P–C(21) | 113.2(2) | 114.0(1) | 115.2 |
| O=P–C(31) | 113.0(2) | 110.3(1) | 110.8 |
| O=P–C(31)–C(32) | −130.1(3) | 128.3(3) | −112.8 |
| O=P–C(31)–C(36) | 45.9(3) | −48.9(3) | 63.8 |
| O=P–C(11) –C(12) | −128.3(1) | −26.9(3) | 22.2 |
| O=P–C(11) –C(16) | 51.0(3) | 149.6(3) | −156.6 |
| O∙∙∙C(16A/12B) | 3.138(5) | 3.063(4) | 3.053 |
| O∙∙∙H | 2.614 | ||
| O∙∙∙H–C(16A/12B) | 103.5 | ||
| O=P–C(21)–C(22) | 31.8(3) | −44.1(3) | 24.6 |
| O=P–C(21)–C(26) | −147.4(3) | 136.2(3) | −156.2 |
| O∙∙∙C(22) | 3.109(5) | 3.181(5) | 3.121 |
| O∙∙∙H | 2.693 | ||
| O∙∙∙H–C(22) | 103.2 | ||
| C(11A)∙∙∙C(26A) / C(16B)∙∙∙C(21B) | 3.168(4) | 3.131(6) | 3.219 |
| C–H∙∙∙C6H5plane a | 2.283 |
| Distance or Angle | Molecule A | Molecule B | Calculated |
|---|---|---|---|
| P=O | 1.467(2) | 1.467(2) | 1.466 |
| P–C | 1.812(2) | 1.811(2) | 1.822 |
| 1.821(3) | 1.813(2) | 1.822 | |
| 1.826(2) | 1.821(2) | 1.833 | |
| O=P–C | 111.5(1) | 110.4(1) | 111.2 |
| 112.5(1) | 114.0(1) | 113.1 | |
| 115.1(1) | 114.3(1) | 115.0 | |
| O=P–C–C | −1.3(2), 177.4(2) | 2.5(2), −173.6(2) | 3.3, −173.0 |
| −50.0(2), 131.8(2) | 48.4(2), −136.9(2) | 48.8, −130.9 | |
| −56.6(2), 116.4(2) | 57.8(2), −114.6(2) | 61.9, −110.1 | |
| O∙∙∙F | 2.800(2) | 2.758(2) | 2.787 |
| Within Chains | |||
| O(1A)···C5F4Nplane 1 | 2.787(5) | O(1B)···C5F4Nplane | 2.920(5) |
| O(1A)···C5F4Ncent. 2 | 2.818(5) | O(1B)···C5F4Ncent. | 2.945(5) |
| P=O(1A)···C5F4Ncent. | 141.3(2) | P=O(1B)···C5F4Ncent. | 136.9(1) |
| O(1A)···C(26B) | 3.363(5) | O(1B)···C(12A) | 3.331(4) |
| P=O(1A)···C(26B) | 120.0(2) | P=O(1B)···C(12A) | 120.7(1) |
| O(1A)···C(26B)–C(21B) | 128.6(2) | O(1B)···C(12A)–C(11A) | 129.2(2) |
| O(1A)···C(26B)–C(25B) | 109.7(2) | O(1B)···C(12A)–C(13A) | 111.2(2) |
| C(13A)···C6H5plane | 3.685(6) | C(25B)···C6H5plane | 3.810(6) |
| C(13A)···C6H5cent. | 4.220(6) | C(25B)···C6H5cent. | 4.068(6) |
| C(12A)–C(13A)···C6H5cent. | 104.3(2) | C(24B)–C(25B)···C6H5cent. | 133.6(3) |
| C(14A)–C(13A)···C6H5cent. | 131.2(3) | C(26B)–C(25B)···C6H5cent. | 105.7(2) |
| Between Chains | |||
| O(1A)···C(15A) | 3.661(4) | O(1B)···C(23A) | 3.432(5) |
| P=O(1A)···C(15A) | 125.2(2) | P=O(1B)···C(23A) | 122.9(1) |
| O(1A)···C(15A)–C(14A) | 124.5(3) | O(1B)···C(23A)–C(22A) | 119.5(3) |
| O(1A)···C(15A)–C(16A) | 113.9(2) | O(1B)···C(23A)–C(24A) | 119.3(3) |
| Compound or Model Structure | Energy kJ mol−1 | |
|---|---|---|
| A → B | B → A | |
| 1 | −70 | −71 |
| I | −28 | −29 |
| II | −23 | −21 |
| III | −24 | −23 |
| 21 | −99 | −100 |
| IV1 | −38 | −38 |
| V1 | −60 | −58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lane, J.R.; Saunders, G.C. Theoretical Study of the Structures of 4-(2,3,5,6-Tetrafluoropyridyl)Diphenylphosphine Oxide and Tris(Pentafluorophenyl)Phosphine Oxide: Why Does the Crystal Structure of (Tetrafluoropyridyl)Diphenylphosphine Oxide Have Two Different P=O Bond Lengths? Molecules 2020, 25, 2778. https://doi.org/10.3390/molecules25122778
Lane JR, Saunders GC. Theoretical Study of the Structures of 4-(2,3,5,6-Tetrafluoropyridyl)Diphenylphosphine Oxide and Tris(Pentafluorophenyl)Phosphine Oxide: Why Does the Crystal Structure of (Tetrafluoropyridyl)Diphenylphosphine Oxide Have Two Different P=O Bond Lengths? Molecules. 2020; 25(12):2778. https://doi.org/10.3390/molecules25122778
Chicago/Turabian StyleLane, Joseph R., and Graham C. Saunders. 2020. "Theoretical Study of the Structures of 4-(2,3,5,6-Tetrafluoropyridyl)Diphenylphosphine Oxide and Tris(Pentafluorophenyl)Phosphine Oxide: Why Does the Crystal Structure of (Tetrafluoropyridyl)Diphenylphosphine Oxide Have Two Different P=O Bond Lengths?" Molecules 25, no. 12: 2778. https://doi.org/10.3390/molecules25122778
APA StyleLane, J. R., & Saunders, G. C. (2020). Theoretical Study of the Structures of 4-(2,3,5,6-Tetrafluoropyridyl)Diphenylphosphine Oxide and Tris(Pentafluorophenyl)Phosphine Oxide: Why Does the Crystal Structure of (Tetrafluoropyridyl)Diphenylphosphine Oxide Have Two Different P=O Bond Lengths? Molecules, 25(12), 2778. https://doi.org/10.3390/molecules25122778





