Effect of Palmitic Acid on Exosome-Mediated Secretion and Invasive Motility in Prostate Cancer Cells
Abstract
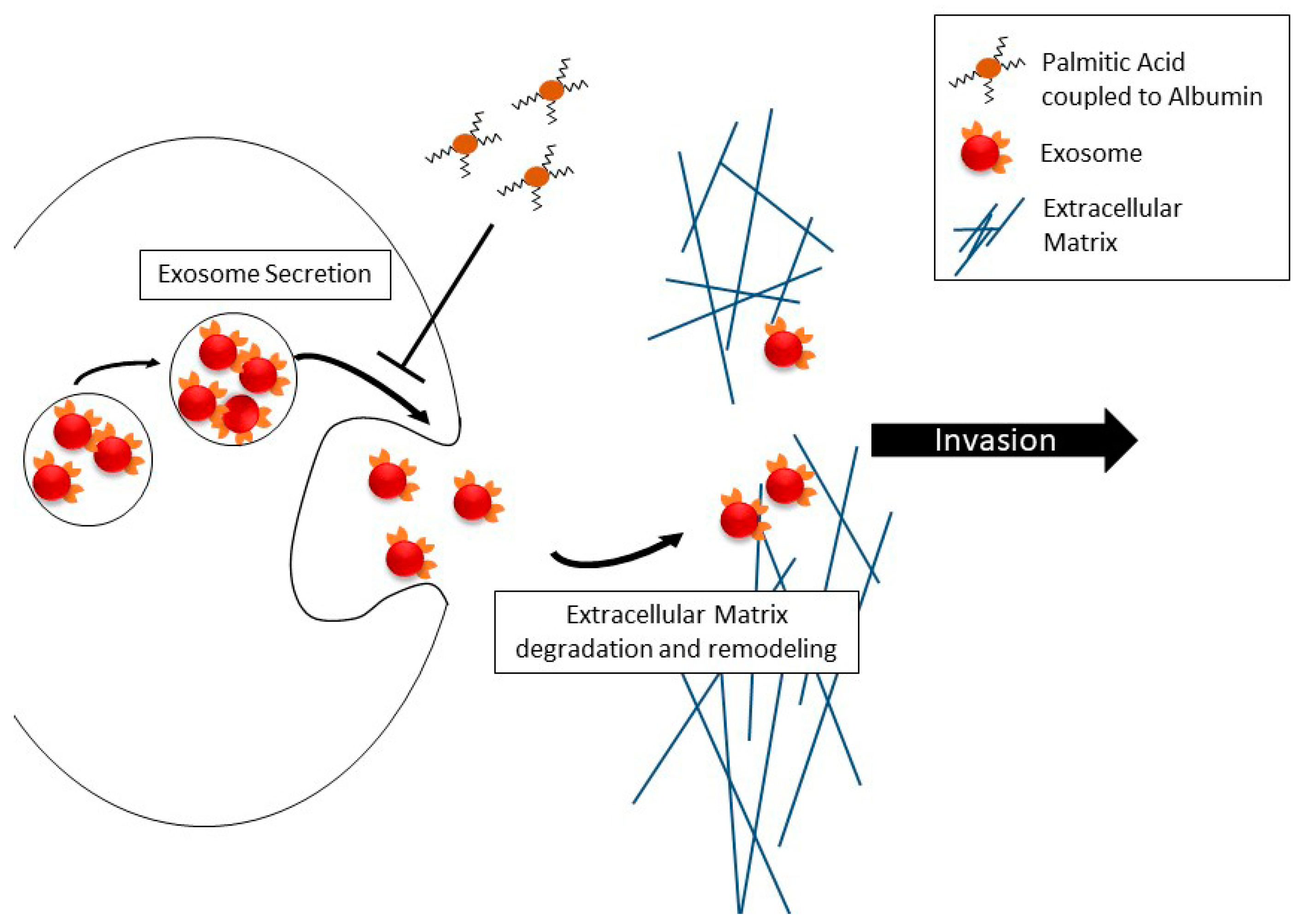
:1. Introduction
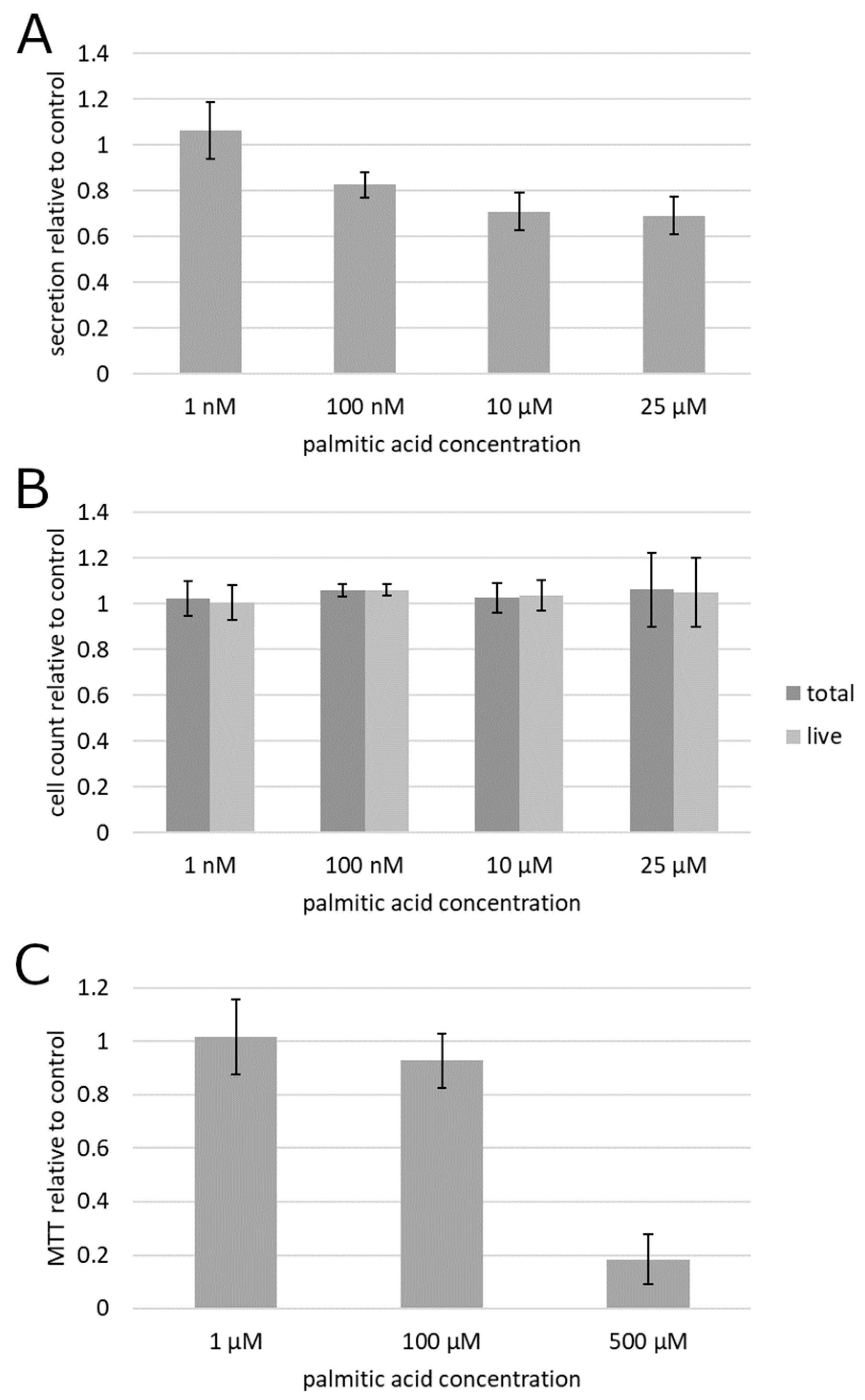
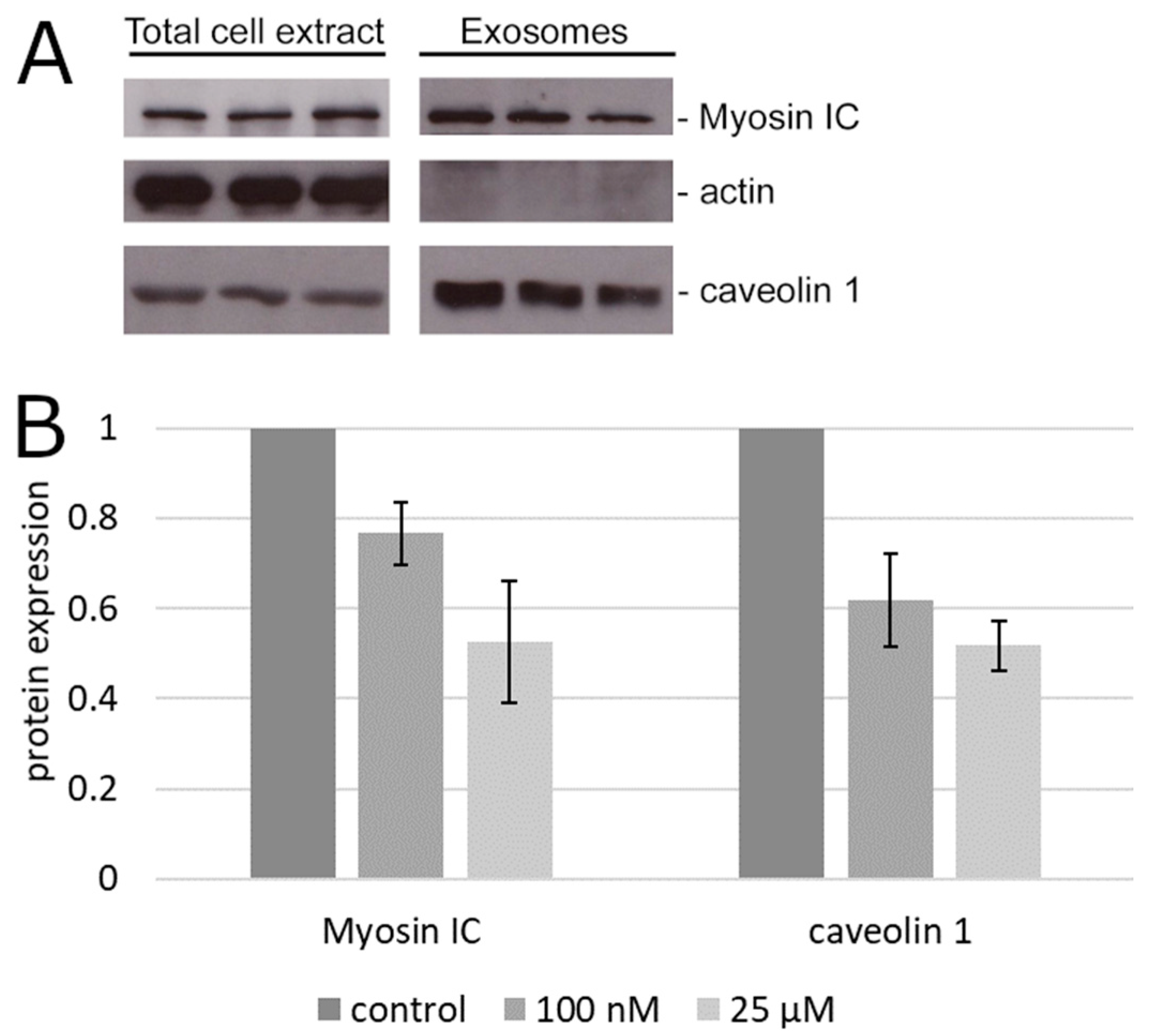
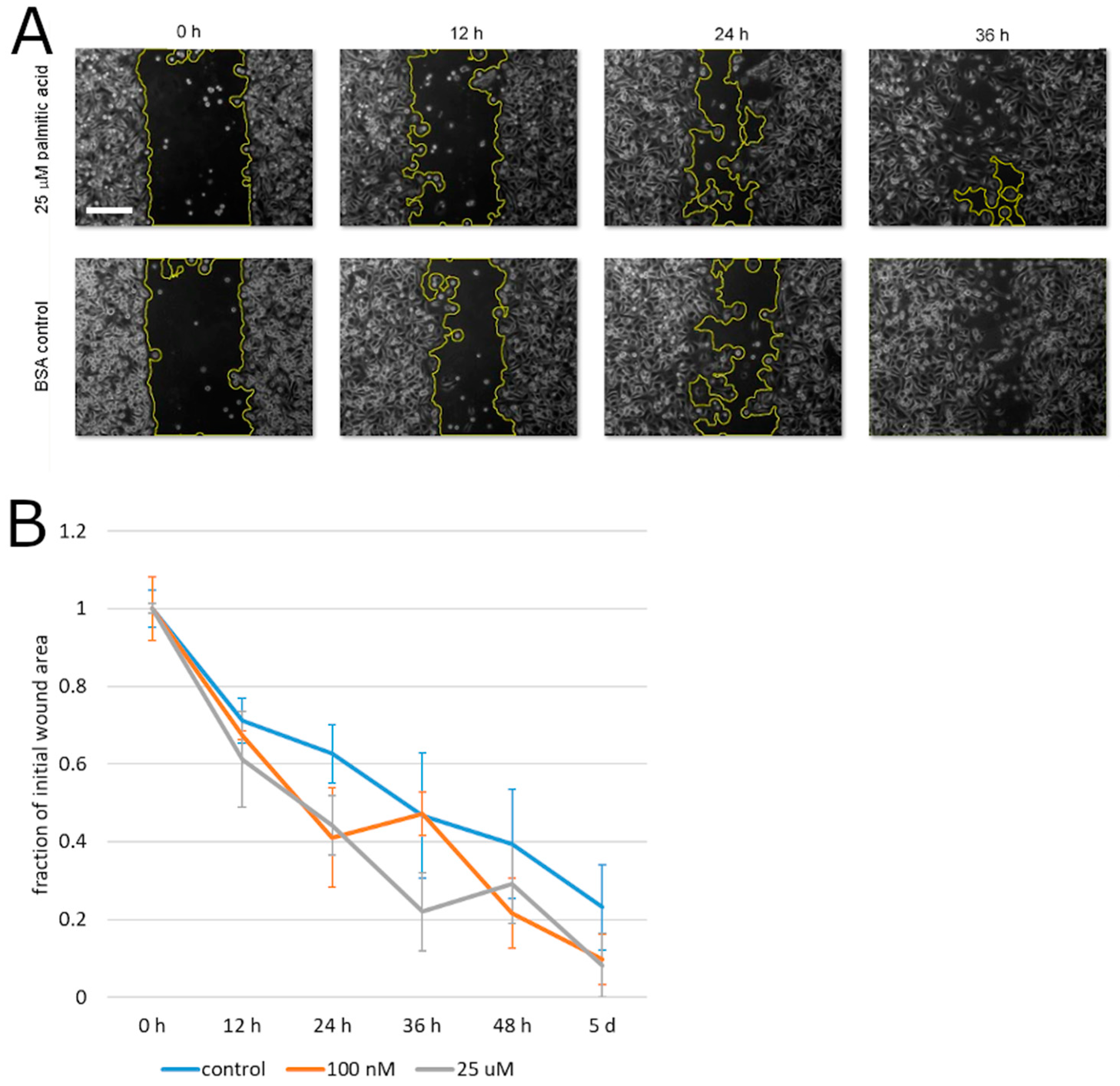
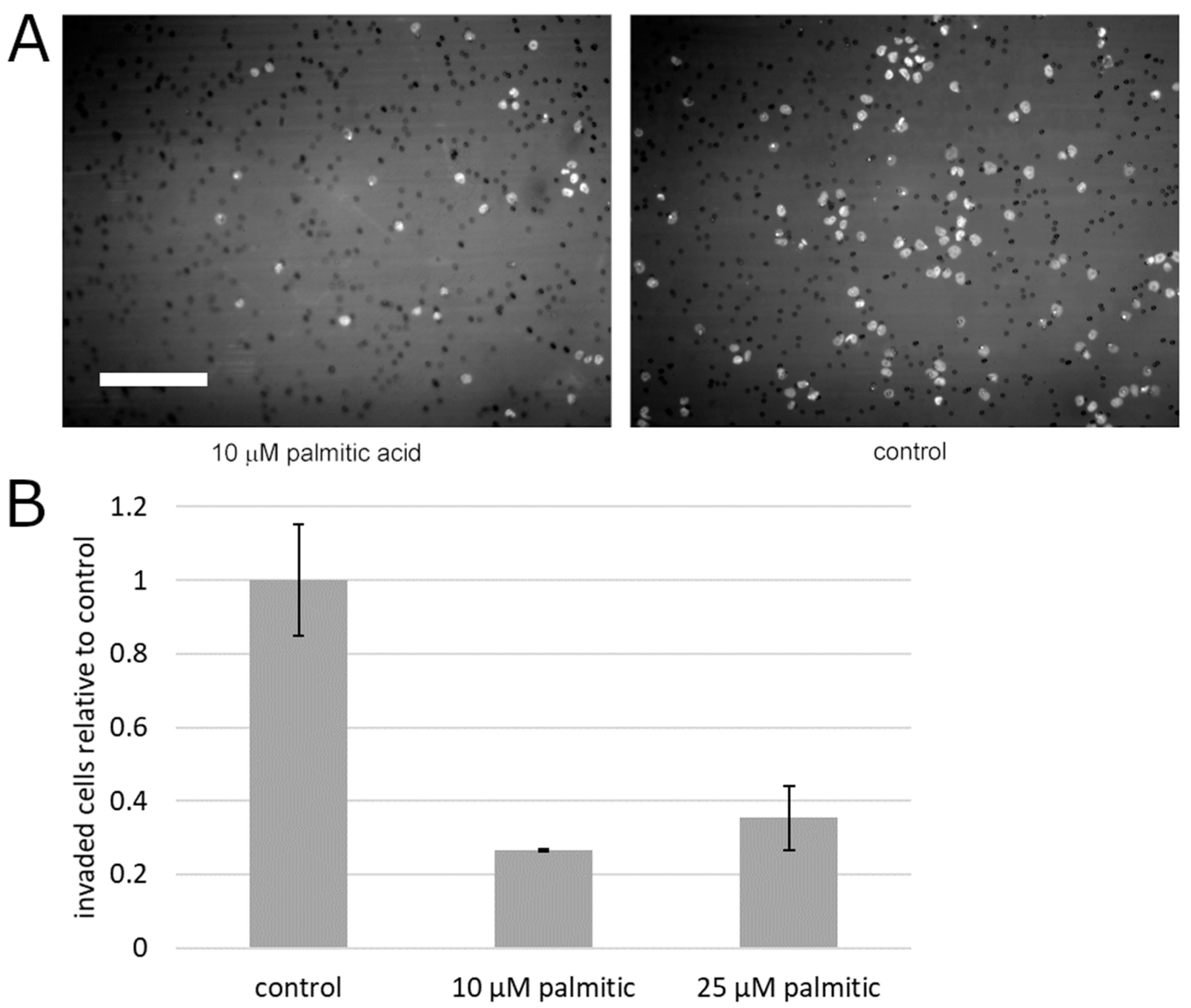
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Cell Counting
4.2. Treatment with Palmitic Acid
4.3. Exosome Separation and Quantification
4.4. Immunoblotting
4.5. Invasion Assay
4.6. Migration Assay
4.7. 3-Dimethylthiazolyl-2,5-diphenyltetrazolium (MTT) Assay
Author Contributions
Funding
Conflicts of Interest
References
- Kurahashi, N.; Inoue, M.; Iwasaki, M.; Sasazuki, S.; Tsugane, A.S. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol. Biomark. Prev. 2008, 17, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Bassett, J.K.; Severi, G.; Hodge, A.M.; MacInnis, R.J.; Gibson, R.A.; Hopper, J.L.; English, D.R.; Giles, G.G. Plasma phospholipid fatty acids, dietary fatty acids and prostate cancer risk. Int. J. Cancer 2013, 133, 1882–1891. [Google Scholar] [CrossRef]
- Di Sebastiano, K.M.; Mourtzakis, M. The role of dietary fat throughout the prostate cancer trajectory. Nutrients 2014, 6, 6095–6109. [Google Scholar] [CrossRef] [PubMed]
- Maly, I.V.; Hofmann, W.A. Fatty acids and calcium regulation in prostate cancer. Nutrients 2018, 10, 788. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Swanson, G.P.; Basler, J.W. Prognostic factors for failure after prostatectomy. J. Cancer 2010, 2, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Swanson, G.P.; Chen, W.; Trevathan, S.; Hermans, M. Long-term follow-up after prostatectomy for prostate cancer and the need for active monitoring. Prostate Cancer 2020, 2020, 7196189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.H.; Aronson, W.; Freedland, S.J. Nutrition, dietary interventions and prostate cancer: The latest evidence. BMC Med. 2015, 13, 3. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.H.; Aronson, W.; Freedland, S.J. An update of research evidence on nutrition and prostate cancer. Urol. Oncol. 2019, 37, 387–401. [Google Scholar] [CrossRef]
- Cho, H.J.; Kwon, G.T.; Park, H.; Song, H.; Lee, K.W.; Kim, J.I.; Park, J.H. A high-fat diet containing lard accelerates prostate cancer progression and reduces survival rate in mice: Possible contribution of adipose tissue-derived cytokines. Nutrients 2015, 7, 2539–2561. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.N.; Han, J.; Abdelkader, T.S.; Kim, T.H.; Lee, J.M.; Song, J.; Kim, K.S.; Park, J.H.; Park, J.H. High animal fat intake enhances prostate cancer progression and reduces glutathione peroxidase 3 expression in early stages of TRAMP mice. Prostate 2014, 74, 1266–1277. [Google Scholar] [CrossRef]
- Shankar, E.; Bhaskaran, N.; MacLennan, G.T.; Liu, G.; Daneshgari, F.; Gupta, S. Inflammatory signaling involved in high-fat diet induced prostate diseases. J. Urol. Res. 2015, 2, 1018. [Google Scholar]
- Li, Y.; Shi, B.; Dong, F.; Zhu, X.; Liu, B.; Liu, Y. Effects of inflammatory responses, apoptosis, and STAT3/NF-kappaB- and Nrf2-mediated oxidative stress on benign prostatic hyperplasia induced by a high-fat diet. Aging (Albany NY) 2019, 11, 5570–5578. [Google Scholar]
- Soto-Alarcon, S.A.; Ortiz, M.; Orellana, P.; Echeverria, F.; Bustamante, A.; Espinosa, A.; Illesca, P.; Gonzalez-Manan, D.; Valenzuela, R.; Videla, L.A. Docosahexaenoic acid and hydroxytyrosol co-administration fully prevents liver steatosis and related parameters in mice subjected to high-fat diet: A molecular approach. Biofactors 2019, 45, 930–943. [Google Scholar] [CrossRef] [PubMed]
- Illesca, P.; Valenzuela, R.; Espinosa, A.; Echeverria, F.; Soto-Alarcon, S.; Ortiz, M.; Videla, L.A. Hydroxytyrosol supplementation ameliorates the metabolic disturbances in white adipose tissue from mice fed a high-fat diet through recovery of transcription factors Nrf2, SREBP-1c, PPAR-gamma and NF-kappaB. Biomed. Pharmacother. 2019, 109, 2472–2481. [Google Scholar] [CrossRef]
- Deep, G.; Jain, A.; Kumar, A.; Agarwal, C.; Kim, S.; Leevy, W.M.; Agarwal, R. Exosomes secreted by prostate cancer cells under hypoxia promote matrix metalloproteinases activity at pre-metastatic niches. Mol. Carcinog. 2020, 59, 323–332. [Google Scholar] [CrossRef]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef] [Green Version]
- Schlaepfer, I.R.; Nambiar, D.K.; Ramteke, A.; Kumar, R.; Dhar, D.; Agarwal, C.; Bergman, B.; Graner, M.; Maroni, P.; Singh, R.P.; et al. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget 2015, 6, 22836–22856. [Google Scholar] [CrossRef]
- Llorente, A.; de Marco, M.C.; Alonso, M.A. Caveolin-1 and MAL are located on prostasomes secreted by the prostate cancer PC-3 cell line. J. Cell Sci. 2004, 117, 5343–5351. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, R.; Klatte, T.; Lucca, I.; Mbeutcha, A.; Seitz, C.; Karakiewicz, P.I.; Fajkovic, H.; Sun, M.; Lotan, Y.; Scherr, D.S.; et al. Prognostic value of Caveolin-1 in patients treated with radical prostatectomy: A multicentric validation study. BJU Int. 2016, 118, 243–249. [Google Scholar] [CrossRef] [Green Version]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.P.; Goh, B.C. Exosome-mediated metastasis: From epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar] [PubMed]
- Maly, I.V.; Domaradzki, T.M.; Gosy, V.A.; Hofmann, W.A. Myosin isoform expressed in metastatic prostate cancer stimulates cell invasion. Sci. Rep. 2017, 7, 8476. [Google Scholar] [PubMed] [Green Version]
- Ihnatovych, I.; Sielski, N.L.; Hofmann, W.A. Selective expression of myosin IC Isoform A in mouse and human cell lines and mouse prostate cancer tissues. PLoS ONE 2014, 9, e108609. [Google Scholar] [CrossRef] [Green Version]
- Ihnatovych, I.; Migocka-Patrzalek, M.; Dukh, M.; Hofmann, W.A. Identification and characterization of a novel myosin Ic isoform that localizes to the nucleus. Cytoskeleton (Hoboken) 2012, 69, 555–565. [Google Scholar] [PubMed]
- Record, M.; Silvente-Poirot, S.; Poirot, M.; Wakelam, M.J.O. Extracellular vesicles: Lipids as key components of their biogenesis and functions. J. Lipid Res. 2018, 59, 1316–1324. [Google Scholar] [PubMed] [Green Version]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J. Biol. Chem. 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [Green Version]
- Saidova, A.A.; Potashnikova, D.M.; Tvorogova, A.V.; Maly, I.V.; Hofmann, W.A.; Vorobjev, I.A. Specific and reliable detection of Myosin 1C isoform A by RTqPCR in prostate cancer cells. PeerJ 2018, 6, e5970. [Google Scholar]
- Yli-Jama, P.; Meyer, H.E.; Ringstad, J.; Pedersen, J.I. Serum free fatty acid pattern and risk of myocardial infarction: A case-control study. J. Intern. Med. 2002, 251, 19–28. [Google Scholar]
- Landim, B.C.; de Jesus, M.M.; Bosque, B.P.; Zanon, R.G.; da Silva, C.V.; Goes, R.M.; Ribeiro, D.L. Stimulating effect of palmitate and insulin on cell migration and proliferation in PNT1A and PC3 prostate cells: Counteracting role of metformin. Prostate 2018, 78, 731–742. [Google Scholar]
- Echeverria, F.; Valenzuela, R.; Espinosa, A.; Bustamante, A.; Alvarez, D.; Gonzalez-Manan, D.; Ortiz, M.; Soto-Alarcon, S.A.; Videla, L.A. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem. 2019, 63, 35–43. [Google Scholar] [CrossRef]
- Bijnsdorp, I.V.; Geldof, A.A.; Lavaei, M.; Piersma, S.R.; van Moorselaar, R.J.; Jimenez, C.R. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J. Extracell. Vesicles 2013, 2, 22097. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.; Rani, S.; O’Brien, K.; O’Neill, A.; Prencipe, M.; Sheikh, R.; Webb, G.; McDermott, R.; Watson, W.; Crown, J.; et al. Docetaxel-resistance in prostate cancer: Evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS ONE 2012, 7, e50999. [Google Scholar] [CrossRef] [PubMed]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol. 1979, 17, 16–23. [Google Scholar] [PubMed]
- Marshall, J. Transwell((R)) invasion assays. Methods Mol. Biol. 2011, 769, 97–110. [Google Scholar] [PubMed]
- Boyden, S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962, 115, 453–466. [Google Scholar] [CrossRef] [Green Version]
- Todaro, G.J.; Lazar, G.K.; Green, H. The initiation of cell division in a contact-inhibited mammalian cell line. J. Cell Physiol. 1965, 66, 325–333. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [Green Version]
- van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Bratton, B.A.; Maly, I.V.; Hofmann, W.A. Effect of polyunsaturated fatty acids on proliferation and survival of prostate cancer cells. PLoS ONE 2019, 14, e0219822. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maly, I.V.; Hofmann, W.A. Effect of Palmitic Acid on Exosome-Mediated Secretion and Invasive Motility in Prostate Cancer Cells. Molecules 2020, 25, 2722. https://doi.org/10.3390/molecules25122722
Maly IV, Hofmann WA. Effect of Palmitic Acid on Exosome-Mediated Secretion and Invasive Motility in Prostate Cancer Cells. Molecules. 2020; 25(12):2722. https://doi.org/10.3390/molecules25122722
Chicago/Turabian StyleMaly, Ivan V., and Wilma A. Hofmann. 2020. "Effect of Palmitic Acid on Exosome-Mediated Secretion and Invasive Motility in Prostate Cancer Cells" Molecules 25, no. 12: 2722. https://doi.org/10.3390/molecules25122722
APA StyleMaly, I. V., & Hofmann, W. A. (2020). Effect of Palmitic Acid on Exosome-Mediated Secretion and Invasive Motility in Prostate Cancer Cells. Molecules, 25(12), 2722. https://doi.org/10.3390/molecules25122722





