Signature Ions in MS/MS Spectra for Dansyl-Aminohexyl-QQIV Adducts on Lysine
Abstract
1. Introduction
2. Results
2.1. Plasma Proteins in the Partially Purified Human Butyrylcholinesterase Preparation
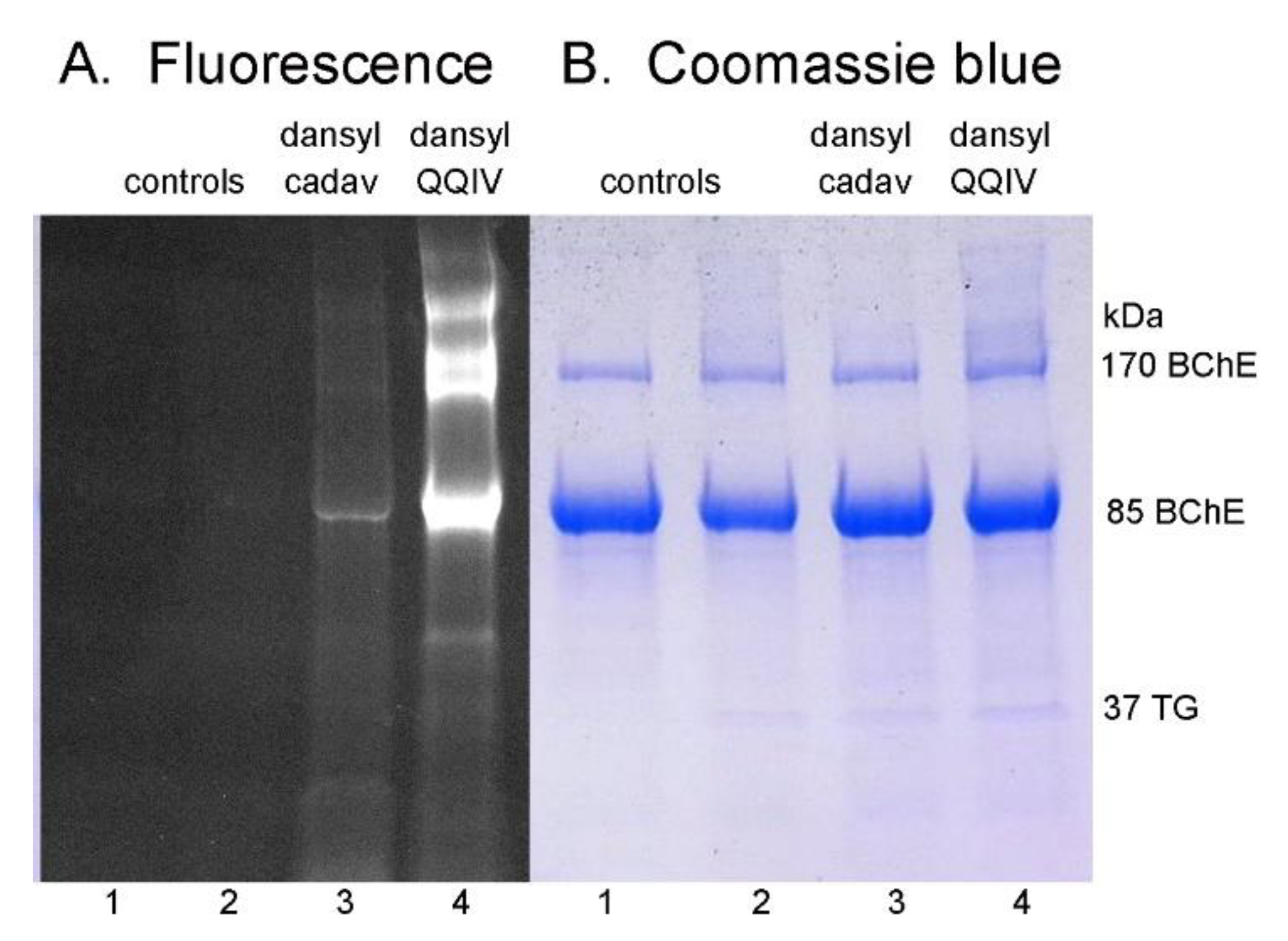
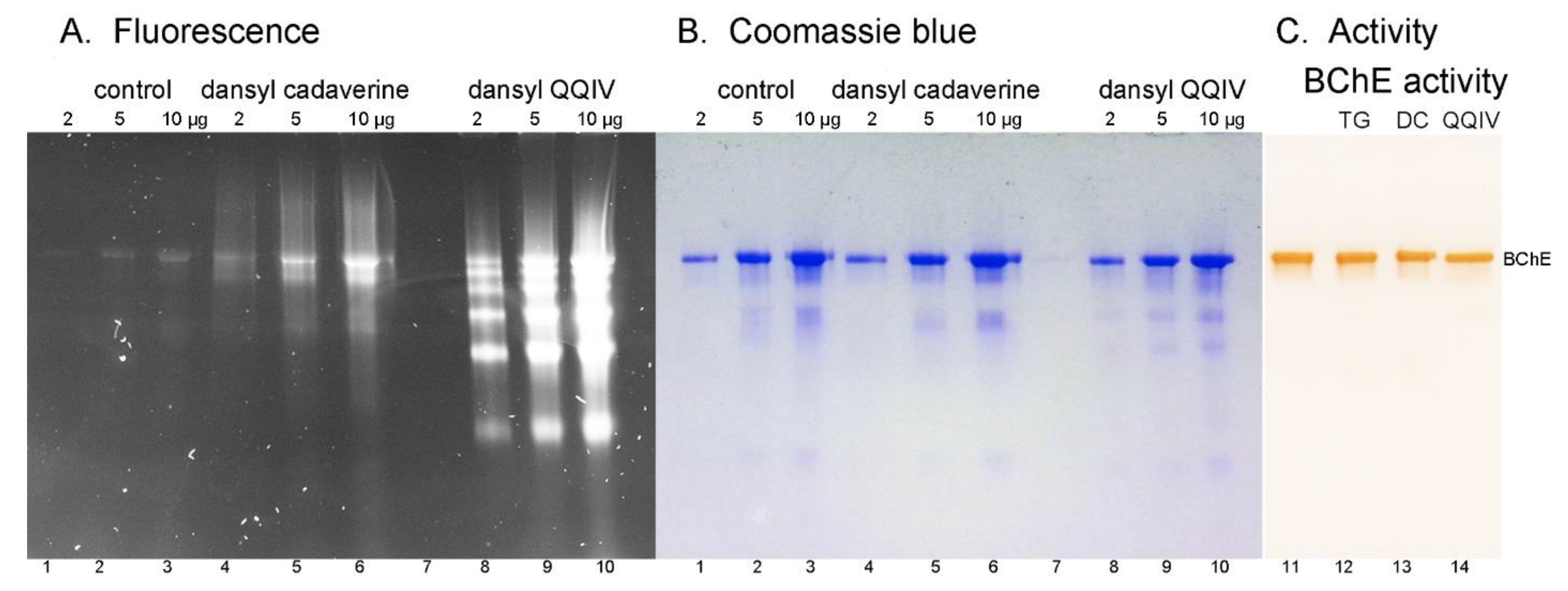
2.2. Sodium Dodecyl Sulfate (SDS) Gel Electrophoresis of Proteins Labeled by DansylQQIV or Dansyl Cadaverine
2.3. Nondenaturing Gel Electrophoresis
2.4. Butyrylcholinesterase Activity
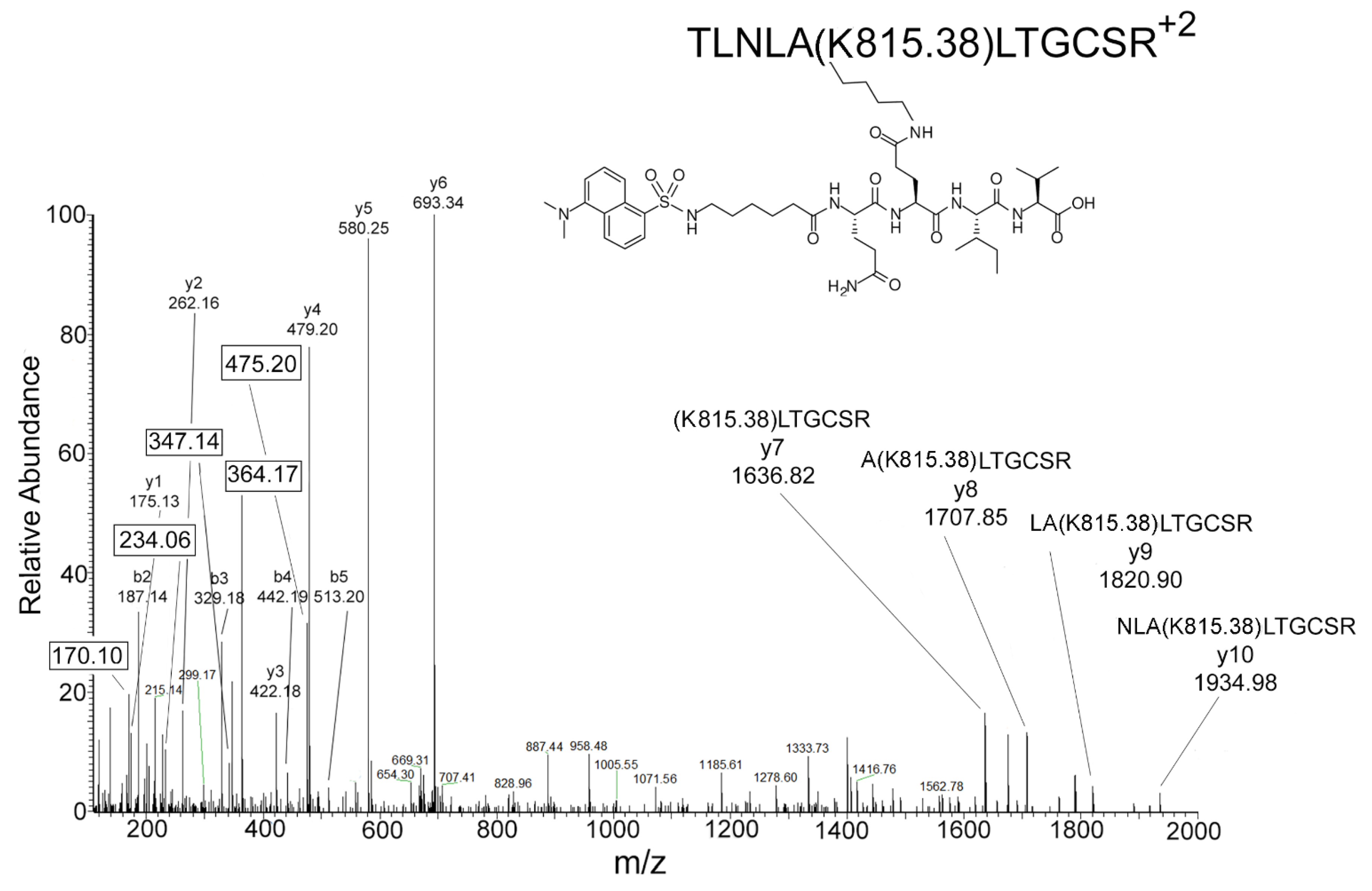
2.5. Mass Spectrometry Results for DansylQQIV Adducts on Human Butyrylcholinesterase
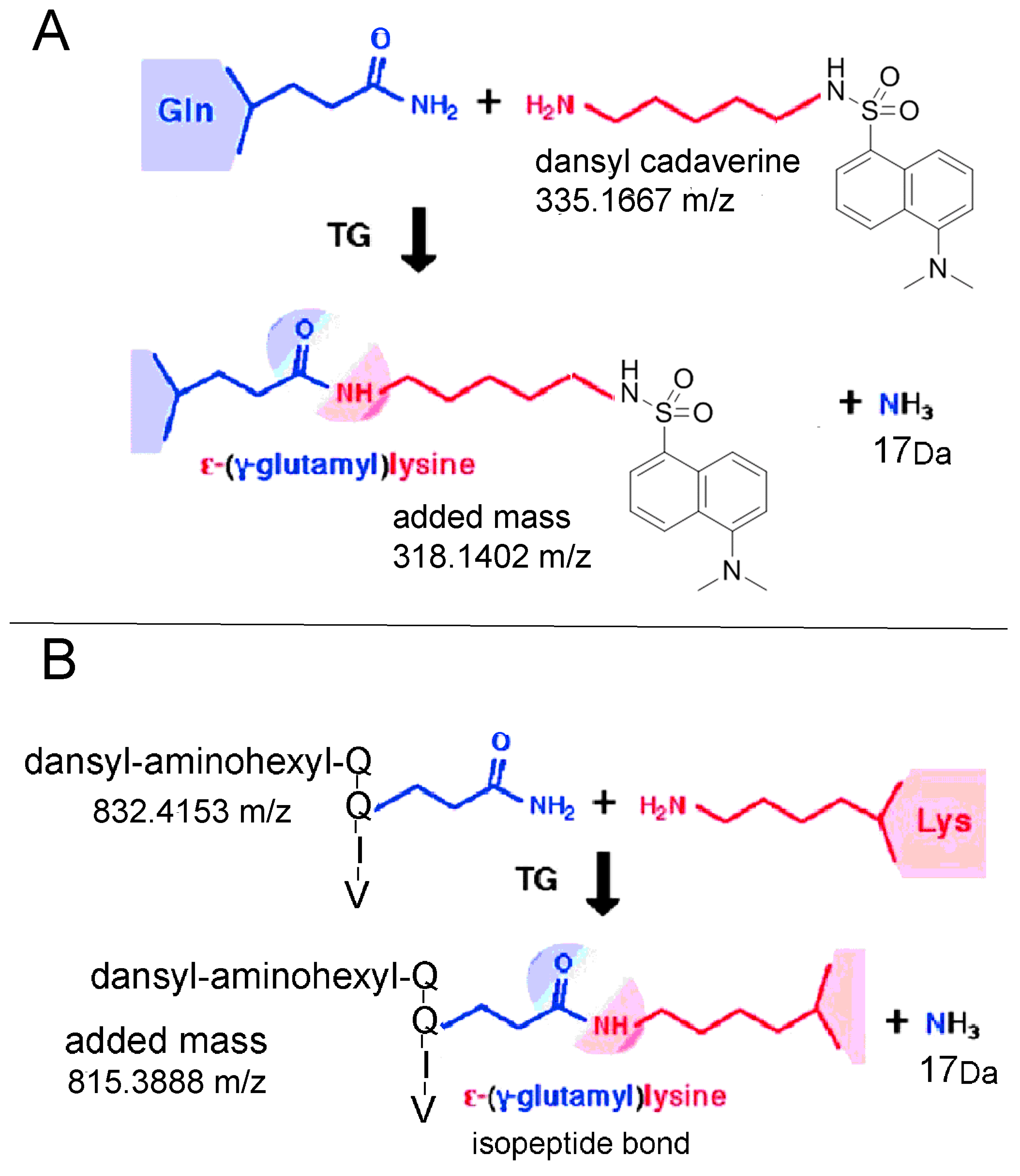
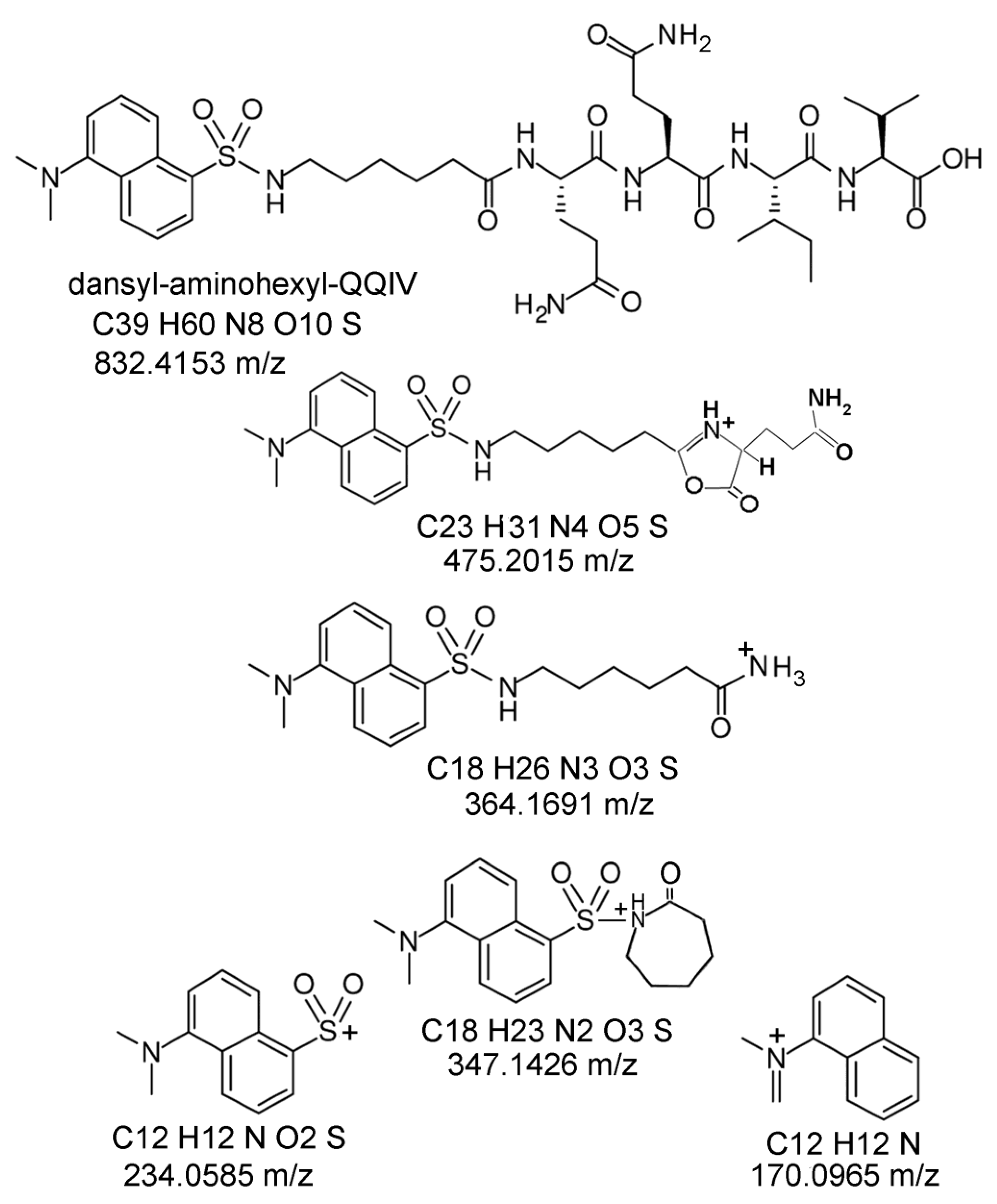
2.6. Proposed Structures of the Signature ions Associated with Fragmentation of DansylQQIV
2.7. Second Q in DansylQQIV is the Preferred Glutamine Donor
2.8. Mass Spectrometry Results for DansylQQIV and Dansyl QQ Adducts on Plasma Proteins Other than Butyrylcholinesterase
2.9. Dansyl Cadaverine Adducts on Glutamine
3. Discussion
3.1. Value of Signature ions
3.2. Search Parameter Settings Make A Huge Difference
3.3. Signature Ions Do Not Derive from Free DansylQQIV
3.4. More Intense Fluorescent Bands for DansylQQIV Adducts than For Dansyl Cadaverine Adducts
3.5. No Loss of Butyrylcholinesterase Activity for Fluorescent Adducts Produced by the Action of Bacterial Transglutaminase
4. Materials and Methods
4.1. Materials
4.2. Transglutaminase-Catalyzed Incorporation of DansylQQIV and Dansyl Cadaverine into Human Plasma Proteins
4.3. Butyrylcholinesterase Activity Assay
4.4. Fluorescence of DansylQQIV and Dansyl-cadaverine-labeled Proteins on Polyacrylamide Gels
4.5. Sample Preparation for Liquid Chromatography Tandem Mass Spectrometry
4.6. Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) on the Orbitrap Fusion Lumos Mass Spectrometer
4.7. Protein Prospector Search for DansylQQIV and Dansyl Cadaverine Modified Peptides
4.8. Proteome Discoverer Search for DansylQQIV Modified Peptides
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorand, L.; Iismaa, S.E. Transglutaminase diseases: From biochemistry to the bedside. FASEB J. 2018, 33, 3–12. [Google Scholar] [CrossRef]
- Folk, J.E.; Cole, P.W. Mechanism of action of guinea pig liver transglutaminase. I. Purification and properties of the enzyme: Identification of a functional cysteine essential for activity. J. Boil. Chem. 1966, 241, 5518–5525. [Google Scholar]
- Lorand, L.; Lockridge, O.M.; Campbell, L.K.; Myhrman, R.; Bruner-Lorand, J. Transamidating enzymes. II. A continuous fluorescent method suited for automating measurements of factor XIII in plasma. Anal. Biochem. 1971, 44, 221–231. [Google Scholar] [PubMed]
- Lorand, L.; Velasco, P.T.; Murthy, S.N.; Wilson, J.; Parameswaran, K.N. Isolation of transglutaminase-reactive sequences from complex biological systems: A prominent lysine donor sequence in bovine lens. Proc. Natl. Acad. Sci. USA 1992, 89, 11161–11163. [Google Scholar] [CrossRef]
- Murthy, S.N.P.; Wilson, J.H.; Lukas, T.J.; Kuret, J.; Lorand, L. Cross-linking sites of the human tau protein, probed by reactions with human transglutaminase. J. Neurochem. 1998, 71, 2607–2614. [Google Scholar] [CrossRef] [PubMed]
- Siefring, G.E.; Apostol, A.B.; Velasco, P.T.; Lorand, L. Enzymatic basis for the Ca2+-induced cross-linking of membrane proteins in intact human erythrocytes. Biochemistry 1978, 17, 2598–2604. [Google Scholar] [PubMed]
- Lorand, L.; Stern, A.; Velasco, P.T. Novel Inhibitors Against the Transglutaminase-catalysed Crosslinking of Lens Proteins. Exp. Eye Res. 1998, 66, 531–536. [Google Scholar] [CrossRef]
- Myhrman, R.; Bruner-Lorand, J. Lobster muscle transpeptidase. Methods Enzymol. 1970, 19, 765–770. [Google Scholar] [CrossRef]
- Folk, J.E.; Finlayson, J.S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Protein Fold. Cell 1977, 31, 1–133. [Google Scholar]
- Biberoglu, K.; Schopfer, L.M.; Tacal, O.; Lockridge, O. Characteristic fragment ions associated with dansyl cadaverine and biotin cadaverine adducts on glutamine. Anal. Biochem. 2020, 600, 113718. [Google Scholar] [CrossRef] [PubMed]
- Mindt, T.L.; Jungi, V.; Wyss, S.; Friedli, A.; Pla, G.; Novak-Hofer, I.; Grünberg, J.; Schibli, R. Modification of Different IgG1 Antibodies via Glutamine and Lysine using Bacterial and Human Tissue Transglutaminase. Bioconjugate Chem. 2008, 19, 271–278. [Google Scholar] [CrossRef]
- Pasternack, R.; Laurent, H.-P.; Rüth, T.; Kaiser, A.; Schön, N.; Fuchsbauer, H.-L. A Fluorescent Substrate of Transglutaminase for Detection and Characterization of Glutamine Acceptor Compounds. Anal. Biochem. 1997, 249, 54–60. [Google Scholar] [CrossRef] [PubMed]
- El-Hofi, M.; Ismail, A.; Nour, M.; Ibrahim, O. Isolation, purification and characterisation of transglutaminase from rosemary (Rosmarinus officinalis L.) leaves. Acta Sci. Pol. Technol. Aliment. 2014, 13, 267–278. [Google Scholar] [PubMed]
- DeLano, W.L. PyMol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- Schlosser, A.; Lehmann, W.D. ChemInform Abstract: Five-Membered Ring Formation in Unimolecular Reactions of Peptides: A Key Structural Element Controlling Low-Energy Collision-Induced Dissociation of Peptides. J. Mass Spectrom. 2010, 32, 1382–1390. [Google Scholar] [CrossRef]
- Polce, M.J.; Ren, D.; Wesdemiotis, C. Wesdemiotis, Dissociation of the peptide bond in protonated peptides. J. Mass. Spectrom. 2000, 35, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, I.; Bloemberg, G.V.; Noreen, S.; Thomas-Oates, J.; Lugtenberg, B.J.J. Increased Uptake of Putrescine in the Rhizosphere Inhibits Competitive Root Colonization by Pseudomonas fluorescens Strain WCS365. Mol. Plant.-Microbe Interact. 2001, 14, 1096–1104. [Google Scholar] [CrossRef]
- Gaydess, A.; Duysen, E.; Li, Y.; Gilman, V.; Kabanov, A.; Lockridge, O.; Bronich, T. Visualization of exogenous delivery of nanoformulated butyrylcholinesterase to the central nervous system. Chem. Interact. 2010, 187, 295–298. [Google Scholar] [CrossRef][Green Version]
- Jeger, S.; Zimmermann, K.; Blanc, A.; Grünberg, J.; Honer, M.; Hunziker, P.; Struthers, H.; Schibli, R. Site-Specific and Stoichiometric Modification of Antibodies by Bacterial Transglutaminase. Angew. Chem. Int. Ed. 2010, 49, 9995–9997. [Google Scholar] [CrossRef]
- Bechtold, U.; Otterbach, J.T.; Pasternack, R.; Fuchsbauer, H. Enzymic preparation of protein G-peroxidase conjugates catalysed by transglutaminase. J. Biochem. 2000, 127, 239–245. [Google Scholar] [CrossRef]
- Schopfer, L.M.; Lockridge, O.; David, E.; Hinrichs, S.H. Purification of human butyrylcholinesterase from frozen Cohn fraction IV-4 by ion exchange and Hupresin affinity chromatography. PLoS ONE 2019, 14, e0209795. [Google Scholar] [CrossRef]
- Li, B.; Sedlacek, M.; Manoharan, I.; Boopathy, R.; Duysen, E.; Masson, P.; Lockridge, O. Butyrylcholinesterase, paraoxonase, and albumin esterase, but not carboxylesterase, are present in human plasma. Biochem. Pharmacol. 2005, 70, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; MacLean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Protein | Accession # | DansylQQIV Lysine | Dansyl QQ Lysine | Dansyl Cadaverine Glutamine |
|---|---|---|---|---|
| Butyrylcholinesterase | P06276 | K276, K342, K376, K383, K436, K455, K556 | K276, K342, K376, K383, K436, K455, K556 | Q75, Q200, Q204, Q251, Q344, Q379, Q408, Q588 |
| Albumin | P02768 | None | None | Q614 |
| Haptoglobin | P00738 | K291 | None | Q400 |
| Immunoglobulin heavy constant alpha 1 | P01876 | K200 | K212 | Q266, Q283, Q287 |
| Alpha-1-antitrypsin | P01009 | None | None | None |
| Hemopexin | P02790 | None | K91, K466 | Q133, Q155, Q390 |
| Haptoglobin-related protein | P00739 | K233 | None | None |
| Apolipoprotein A-1 | P02647 | K120 | K47, K131 | Q56, Q87, Q93, Q129, Q141, Q151, Q162, Q240 |
| Serotransferrin | P02787 | None | None | None |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schopfer, L.M.; Lockridge, O. Signature Ions in MS/MS Spectra for Dansyl-Aminohexyl-QQIV Adducts on Lysine. Molecules 2020, 25, 2659. https://doi.org/10.3390/molecules25112659
Schopfer LM, Lockridge O. Signature Ions in MS/MS Spectra for Dansyl-Aminohexyl-QQIV Adducts on Lysine. Molecules. 2020; 25(11):2659. https://doi.org/10.3390/molecules25112659
Chicago/Turabian StyleSchopfer, Lawrence M., and Oksana Lockridge. 2020. "Signature Ions in MS/MS Spectra for Dansyl-Aminohexyl-QQIV Adducts on Lysine" Molecules 25, no. 11: 2659. https://doi.org/10.3390/molecules25112659
APA StyleSchopfer, L. M., & Lockridge, O. (2020). Signature Ions in MS/MS Spectra for Dansyl-Aminohexyl-QQIV Adducts on Lysine. Molecules, 25(11), 2659. https://doi.org/10.3390/molecules25112659







