Fine Characterization of the Macromolecular Structure of Huainan Coal Using XRD, FTIR, 13C-CP/MAS NMR, SEM, and AFM Techniques
Abstract
1. Introduction
2. Results and Discussion
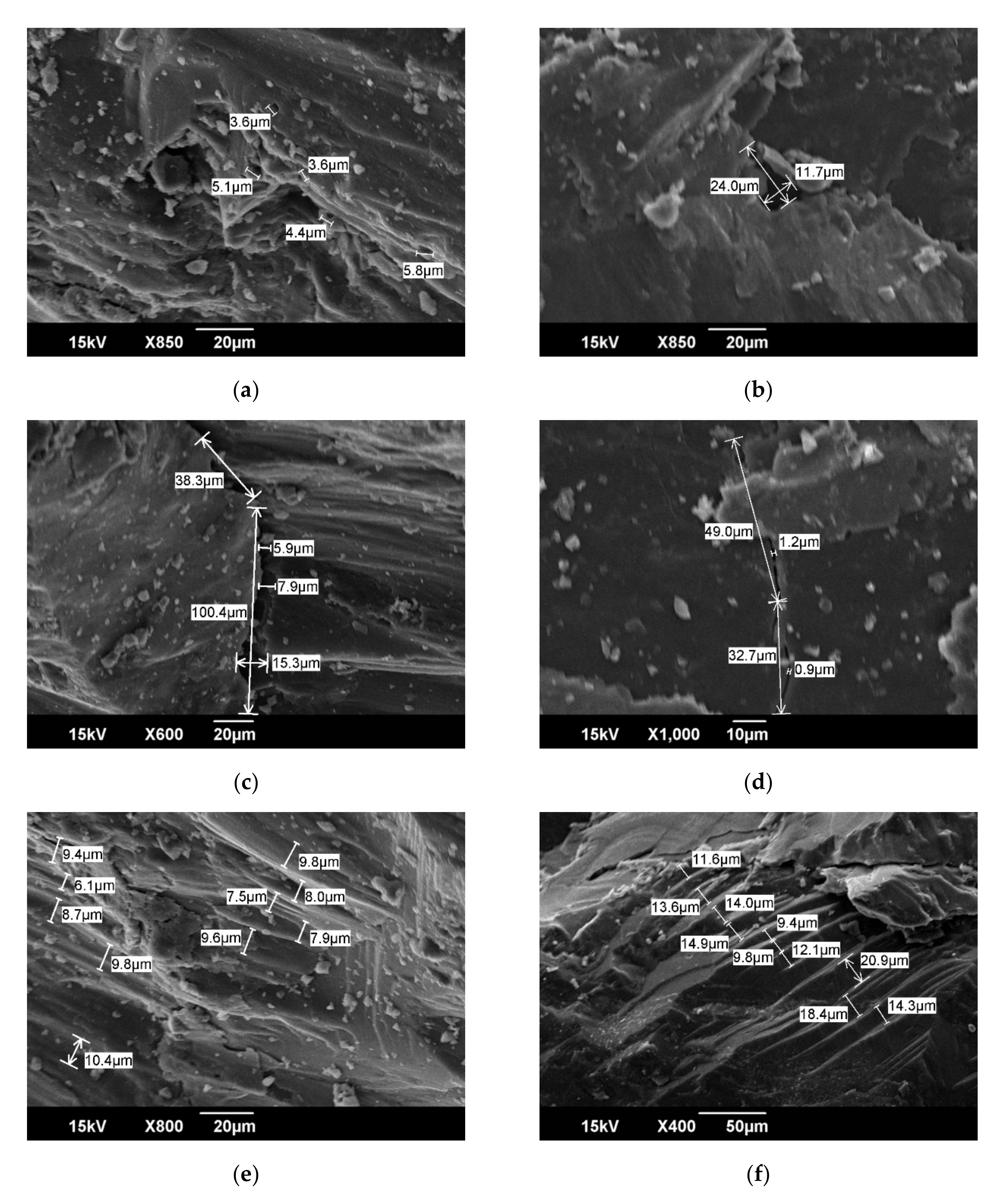
2.1. SEM Characteristics of Original HNC
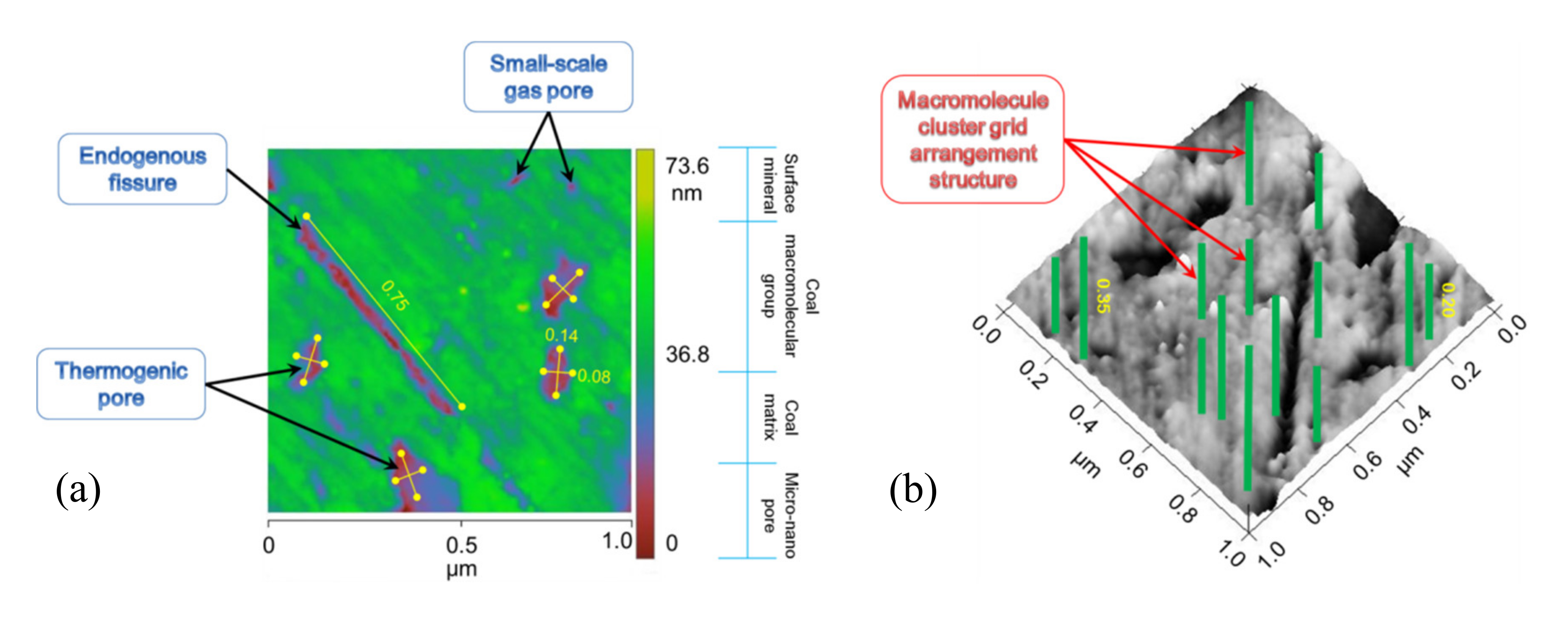
2.2. AFM Characteristics of Original HNC
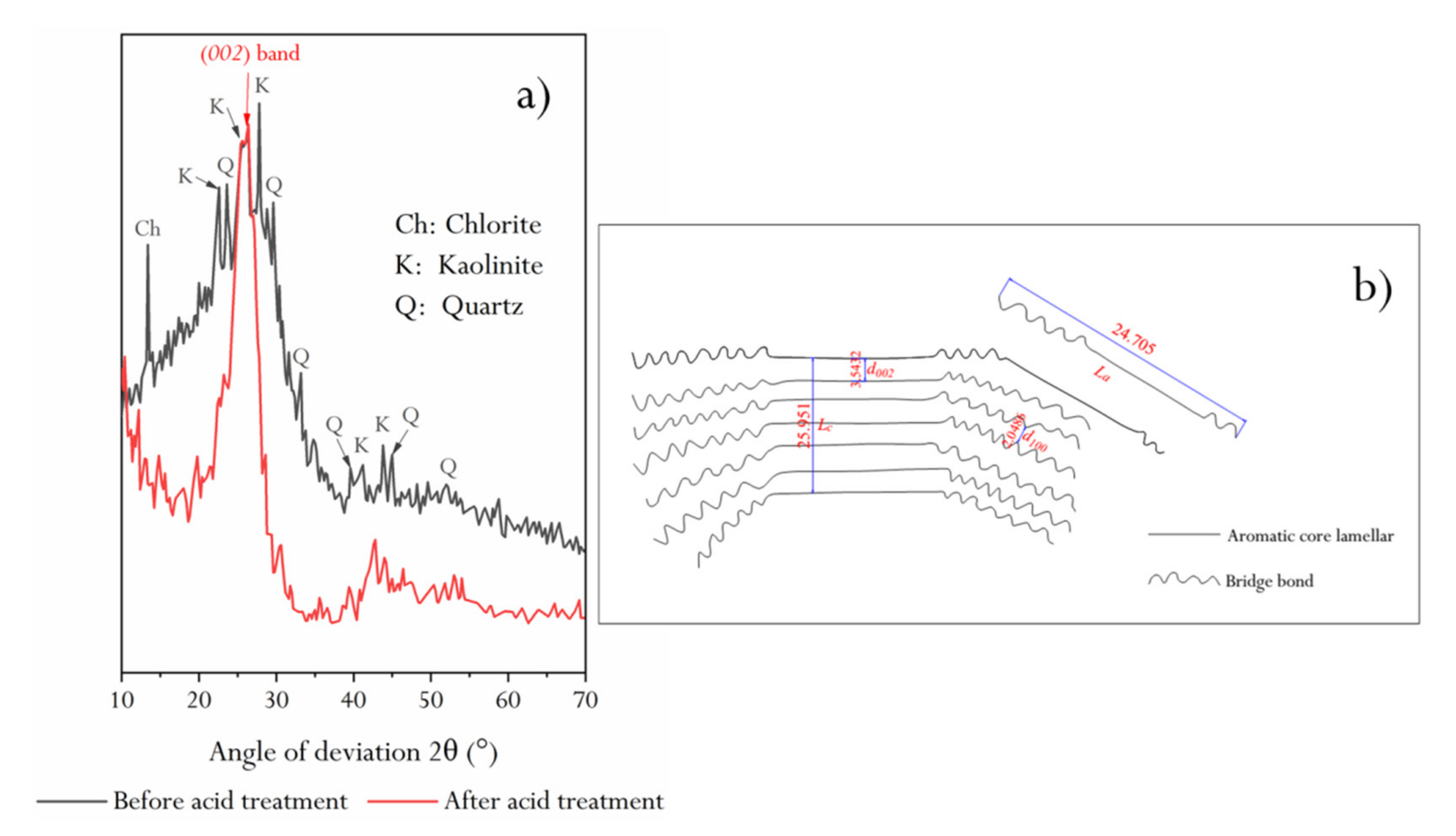
2.3. XRD Characteristics of HNC before and after Acid Treatment
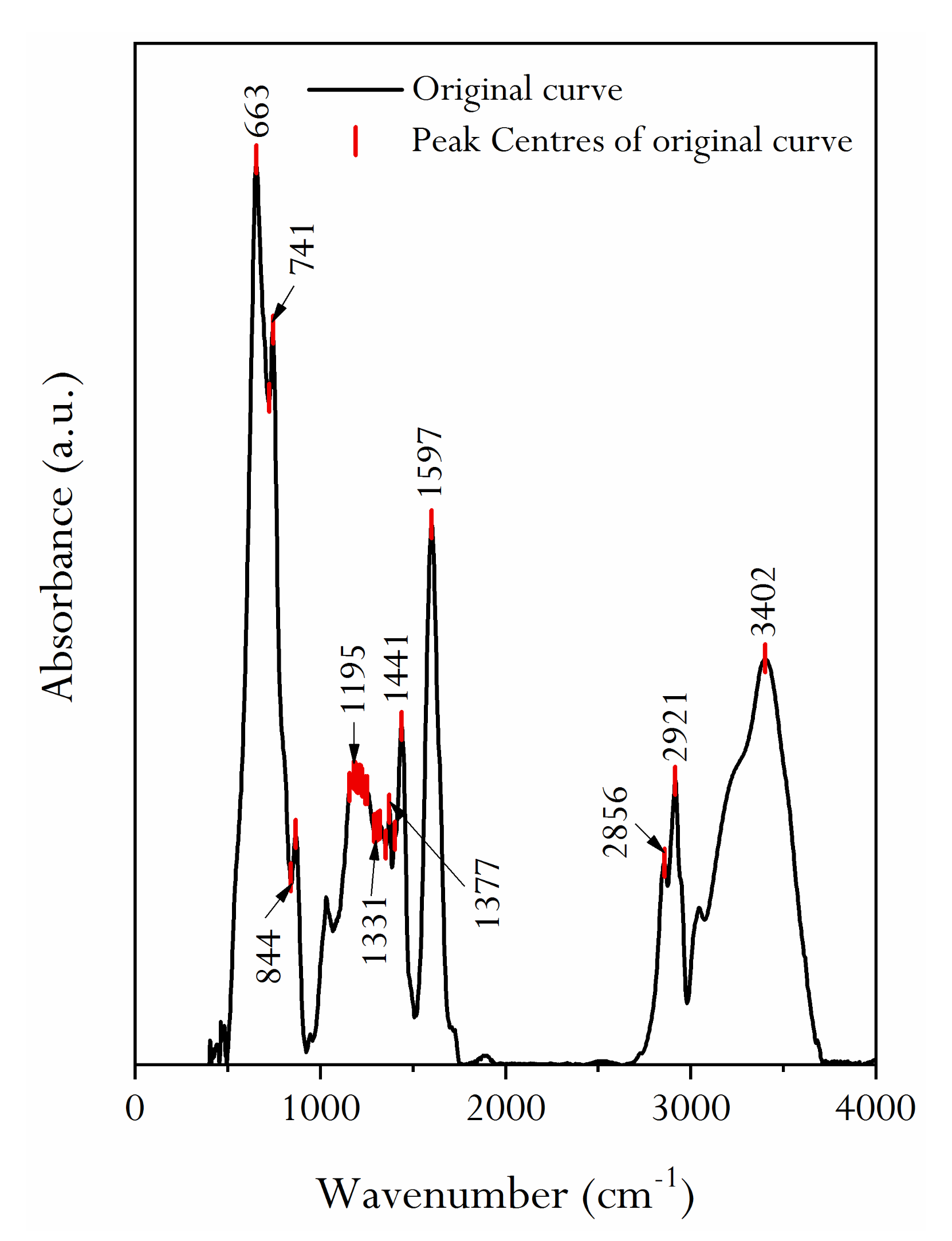
2.4. Infrared Spectral Characteristics of HNC and Its Oxygen-Containing Functional Groups
2.4.1. FTIR Characteristics of HNC after Acid Treatment
2.4.2. Calculation of Oxygen-Containing Functional Group Content
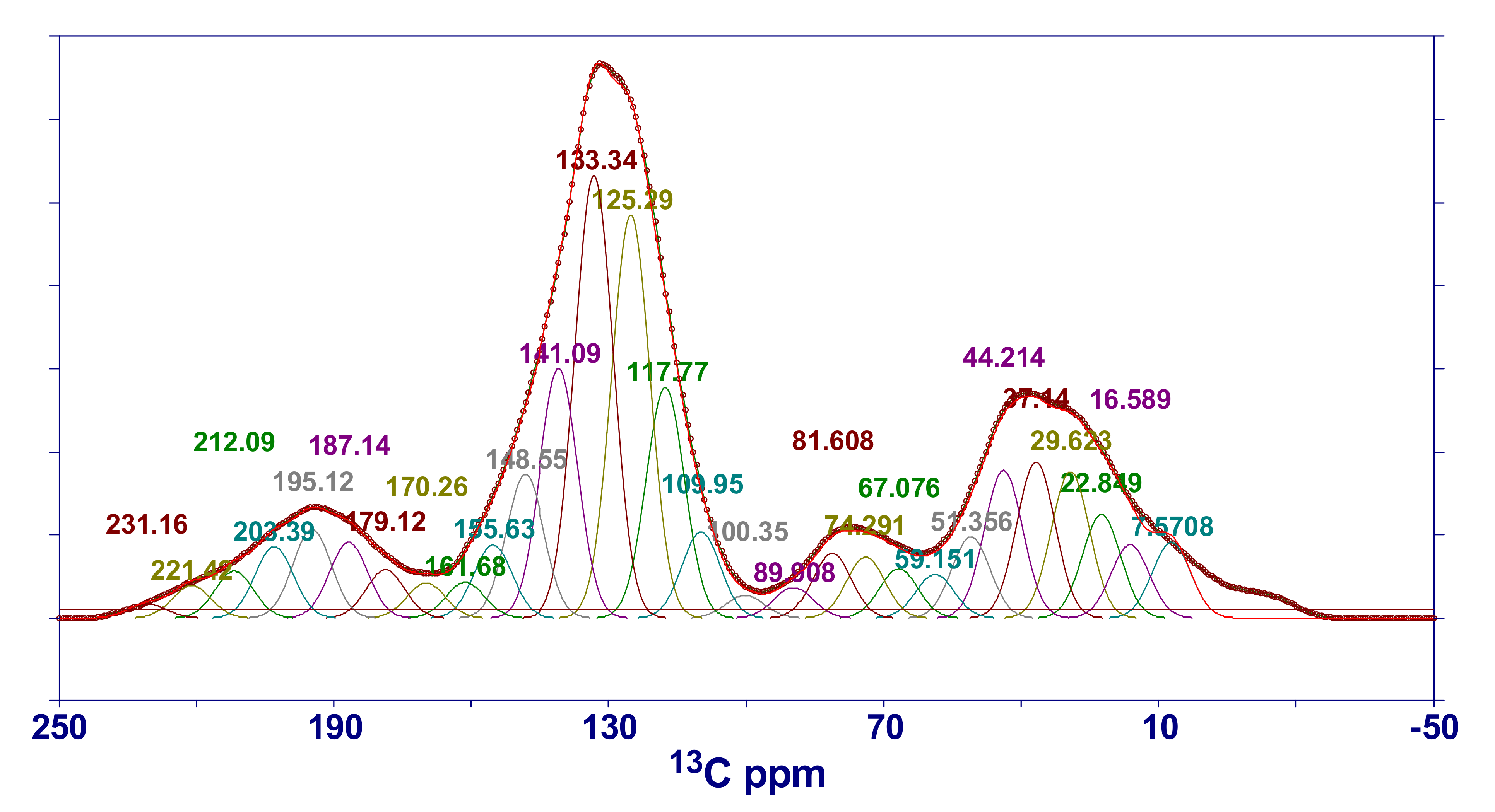
2.4.3. NMR Characteristics of HNC
3. Materials and Methods
3.1. Sample Collection
3.2. Proximate Analysis and Ultimate Analysis
3.3. Demineralization of Samples
3.4. XRD
3.5. FTIR
3.6. 13C-CP/MAS NMR
3.7. SEM
3.8. AFM
3.9. Data Processing and Calculation Procedure
3.9.1. XRD
3.9.2. FTIR
3.9.3. 13C NMR
3.9.4. SEM
3.9.5. AFM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xue, Y.P. The Global Energy Governance Structure and China-Europe Energy Relations. In China-EU Relations; Springer: Singapore, Singapore, 2017; pp. 155–165. [Google Scholar]
- Levine, D.G.; Schlosberg, R.H.; Silbernagel, B.G. Understanding the chemistry and physics of coal structure (A Review). Proc. Natl. Acad. Sci. USA 1982, 79, 3365–3370. [Google Scholar] [CrossRef]
- Shi, Q.; Xu, C.M.; Zhang, Y.H.; Liu, X.X.; Cui, D.C.; Guo, X.F.; Long, Y.H.; Wang, R. Molecular composition of low temperature coal tar and its transformation in the hydrogenation-refining process. Sci. Sin. 2018, 48, 397–410. [Google Scholar] [CrossRef]
- Ni, H.X.; Xu, C.M.; Wang, R.; Guo, X.F.; Long, Y.H.; Ma, C.; Yan, L.L.; Liu, X.X.; Shi, Q. Composition and Transformation of Sulfur-, Oxygen-, and Nitrogen-containing Compounds in the Hydrotreating Process of a Low Temperature Coal Tar. Energy Fuels 2018, 32, 3077–3084. [Google Scholar] [CrossRef]
- Meyers, R.A. Coal Structure; Academic Press: New York, NY, USA, 1982; p. 87. [Google Scholar]
- Poutsma, M.L. A Review of Thermolysis Studies of Model Compounds Relevant to Processing of Coal. No. ORNL/TM-10637; Oak Ridge National Lab: Oak Ridge, TN, USA, 1987. [Google Scholar]
- Arenillas, A.; Pevida, C.; Rubiera, F.; Palacios, J.M.; Navarret, R.; Denoyel, R.; Rouquerol, J.; Pis, J.J. Surface characterisation of synthetic coal chars made from model compounds. Carbon 2004, 42, 1345–1350. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Shi, S.D.; Li, Y.W. Coal liquefaction technologies—Development in China and challenges in chemical reaction engineering. Chem. Eng. Sci. 2010, 65, 12–17. [Google Scholar] [CrossRef]
- Solomon, P.R.; Fletcher, T.H.; Pugmire, R.J. Progress in coal pyrolysis. Fuel 1993, 72, 587–597. [Google Scholar] [CrossRef]
- Zhao, S.L.; Pudasainee, D.; Duan, Y.F.; Gupta, R. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog. Energy Combust. Sci. 2019, 73, 26–64. [Google Scholar] [CrossRef]
- You, L.; Xing, B.L.; Zheng, M.K.; Yi, G.Y.; Huang, G.X.; Zhang, C.X.; Yuan, R.F.; Chen, Z.F.; Cao, Y.J. Hydrothermal Synthesis of Ultra-Light Coal-Based Graphene Oxide Aerogel for Efficient Removal of Dyes from Aqueous Solutions. Nanomaterials 2018, 8, 670. [Google Scholar]
- He, X.J.; Li, X.J.; Ma, H.; Han, J.F.; Zhang, H.; Yu, C.; Xiao, N.; Qiu, J.S. ZnO template strategy for the synthesis of 3D interconnected graphene nanocapsules from coal tar pitch as supercapacitor electrode materials. J. Power Sources 2017, 340, 183–191. [Google Scholar] [CrossRef]
- Silva, L.F.; Crissien, T.J.; Tutikian, B.F.; Sampaio, C.H. Rare Earth Elements and carbon nanotubes in coal mine around spontaneous combustions. J. Clean. Prod. 2020, 120068. [Google Scholar] [CrossRef]
- Gredilla, A.; de Vallejuelo, S.F.O.; Rodriguez-Iruretagoiena, A.; Gomez, L.; Oliveira, M.L.S.; Arana, G.; Diego, A.D.; Madariaga, J.M.; Silva, L.F.O. Evidence of mercury sequestration by carbon nanotubes and nanominerals present in agricultural soils from a coal fired power plant exhaust. J. Hazard. Mater. 2019, 378, 120747. [Google Scholar] [CrossRef]
- Vu, C.M.; Nguyen, L.T.; Phuc, B.T.; Tung, N.H.; Nguyen, D.D. Enhanced mode I interlaminar fracture toughness and mechanical properties of carbon fiber-filled vinyl ester resin-based composite by using both coal fly ash and nano-/micro-glass fiber. Nguyen Polym. Bull. 2020, 77, 357–374. [Google Scholar]
- Xie, X.X.; He, X.J.; Zhang, H.F.; Wei, F.; Xiao, N.; Qiu, J.H. Interconnected sheet-like porous carbons from coal tar by a confined soft-template strategy for supercapacitors. Chem. Eng. J. 2018, 350, 49–56. [Google Scholar] [CrossRef]
- Lisse, C.M.; Dennerl, K.; Englhauser, J.; Englhauser, J.; Harden, M. Discovery of X-ray and extreme ultraviolet emission from comet C/Hyakutake. Science 1996, 274, 205–209. [Google Scholar] [CrossRef]
- Lewis, H. Ultra- fine structure of coals and coke. Nature 1994, 153, 697. [Google Scholar]
- Song, D.Y.; Yang, C.B.; Zhang, X.K.; Su, X.B.; Zhang, X. Structure of the organic crystallite unit in coal as determined by X-ray diffraction. Min. Sci. Technol. 2011, 21, 667–671. [Google Scholar] [CrossRef]
- Huntjens, F.; Krevelen, D.V. Chemical structure and properties of coal: II. Reflectance. Fuel 1954, 33, 88–103. [Google Scholar]
- Georgakopoulos and Andreas, Study of Low Rank Greek Coals Using FTIR Spectroscopy. Energy Sources 2003, 25, 995–1005. [CrossRef]
- Başaran, Y.; Denizli, A.; Sakintuna, B.; Taralp, A.; Yürüm, Y. Bio-Liquefaction/Solubilization of Low-Rank Turkish Lignites and Characterization of the Products. Energy Fuels 2003, 17, 1068–1074. [Google Scholar] [CrossRef]
- Chen, Y.M.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Bandyopadhyay, D. Study of kinetics of iron minerals in coal by Fe-57 Mossbauer and FT-IR spectroscopy during natural burning. Hyperfine Interact. 2005, 163, 167–176. [Google Scholar] [CrossRef]
- Gezici, O.; Demir, I.; Demircan, A.; Ünlü, N.; Karaarslan, M. Subtractive-FTIR spectroscopy to characterize organic matter in lignite samples from different depths. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 63–69. [Google Scholar] [CrossRef]
- Davis, A.; Kuehn, D.W.; Starsinic, M.; Coleman, M.M.; Kuehn, D.W.; Davis, A. Concerning the application of FT-IR to the study of coal: A critical assessment of band assignments and the application of spectral analysis programs. Appl. Spectrosc. 1981, 35, 475–485. [Google Scholar]
- Sullivan, M.J.; Maciel, G.E. Structural resolution in the carbon-13 nuchear magnetic resonance spectrometric analysis of coal by cross polarization and magic-angle spinning. Anal. Chem. 1982, 54, 1606–1615. [Google Scholar] [CrossRef]
- Wang, J.P.; Li, G.Y.; Guo, R.; Li, A.Q.; Liang, Y.H. Theoretical and experimental insight into coal structure: Establishing a chemical model for Yuzhou lignite. Energy Fuels 2017, 31, 124–132. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, G.Y.; Zhao, Y.Y.; Zhao, M.H.; Tang, J.W. Construction of the molecular structure model of the Shengli lignite using TG-GC/MS and FTIR spectrometry data. Fuel 2017, 203, 924–931. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, M.; Singh, M.P. SEM and reflected light petrography: A case study on natural cokes from seam XIV, Jharia coalfield, India. Fuel 2013, 112, 502–512. [Google Scholar] [CrossRef]
- Wang, S.; Baxter, L.; Fonseca, F. Biomass fly ash in concrete: SEM, EDX and ESEM analysis. Fuel 2008, 87, 372–379. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM-EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Niu, Q.H.; Pan, J.N.; Cao, L.W.; Ji, Z.M.; Wang, H.C.; Wang, K.; Wang, Z.Z. The evolution and formation mechanisms of closed pores in coal. Fuel 2017, 200, 555–563. [Google Scholar] [CrossRef]
- Golubev, Y.A.; Kovaleva, O.V.; Yushkin, N.P. Observations and morphological analysis of supermolecular structure of natural bitumens by atomic force microscopy. Fuel 2008, 87, 32–38. [Google Scholar] [CrossRef]
- Lawrie, G.A.; Gentle, I.R.; Fong, C.; Glikson, M. Atomic force microscopy studies of bowen basin coal macerals. Fuel 1997, 76, 1519–1526. [Google Scholar] [CrossRef]
- Gay, P.; Hirsch, P.B.; Kelly, A. X-ray studies of polycrystalline metals deformed by rolling. III. The physical interpretation of the experimental results. Proc. Roy Soc. London A 1954, 226, 143–159. [Google Scholar] [CrossRef]
- Ibara, J.V.; Munoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–760. [Google Scholar] [CrossRef]
- Trewhella, M.J.; Poplett, I.J.; Grint, A. Structure of Green River oil shale kerogen: Determination using solid state 13C NMR spectroscopy. Fuel 1986, 65, 541–546. [Google Scholar] [CrossRef]
- Solum, A.M.; Pugmire, R.J.; Grant, D.M. N-15 CPMAS NMR of the Argonne premium coals. Energy Fuels 1997, 11, 491–494. [Google Scholar] [CrossRef]
- Wilson, M.A.; Pugmire, R.J.; Karas, J.; Alemany, L.B.; Woolfenden, W.R.; Grant, D.M.; Given, P.H. Carbon distribution in coals and coal macerals by cross polarization magic angle spinning carbon-13 nuclear magnetic resonance spectrometry. Anal. Chem. 1984, 56, 933–943. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Coal; Elsevier: Amsterdam, The Netherlands, 1981. [Google Scholar]
- Vandenbroucke, M.; Largeau, C. Kerogen origin, evolution and structure. Org. Geochem. 2007, 38, 719–833. [Google Scholar] [CrossRef]
- Wu, D.; Liu, G.J.; Sun, R.Y.; Chen, S.C. Influences of magmatic intrusion on the macromolecular and pore structures of coal: Evidences from Raman spectroscopy and atomic force microscopy. Fuel 2014, 119, 191–201. [Google Scholar]
Sample Availability: HNC samples for this study can be obtained from Dun Wu. |

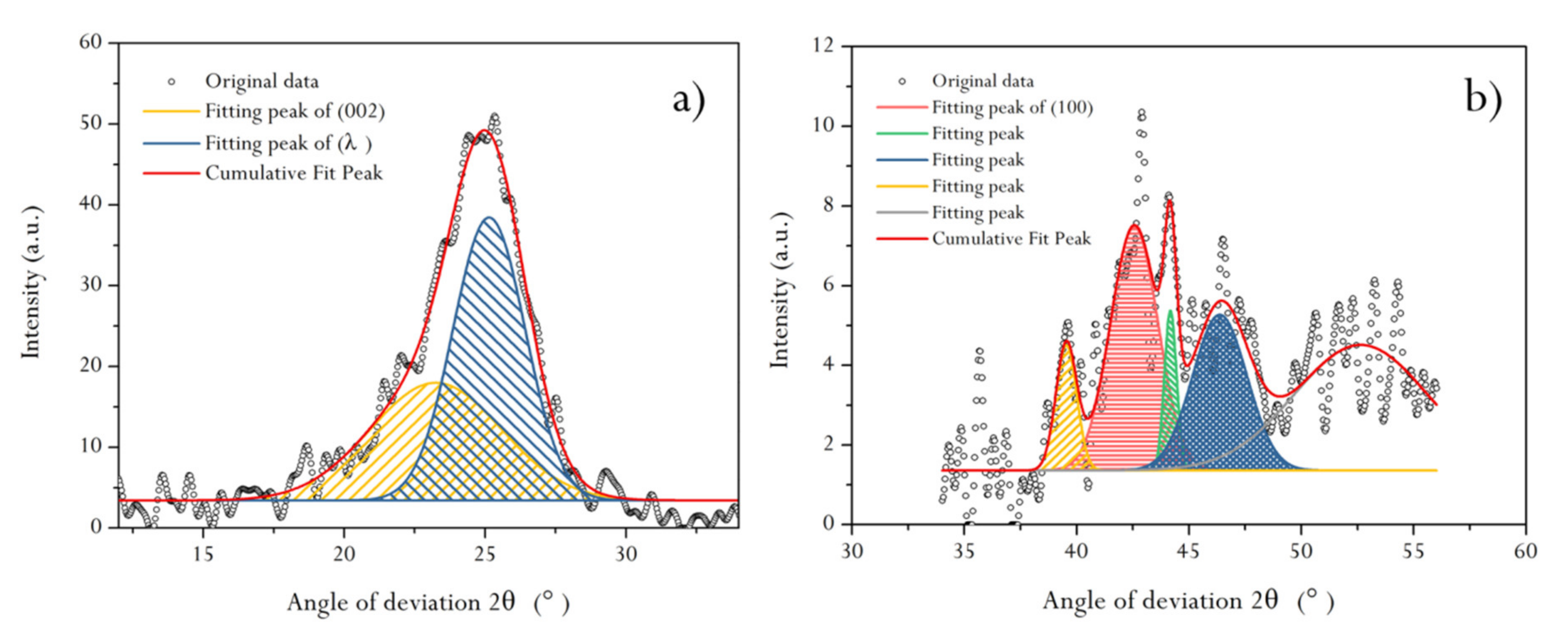

| Band Interval | 2θ002 (°) | θ002 (°) | Cosθ002 | K1 | λ (Å) | β002 (°) | β002 (rad) | Lc (Å) | d002 (Å) | d100 (Å) | La/Lc | Nave |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10°–35° | 25.11 | 12.556 | 0.976 | 0.94 | 1.54056 | 3.121 | 0.0545 | 25.951 | 3.5432 | 2.0486 | 1.0504 | 7.3242 |
| Band interval | 2θ100 (°) | θ100 (°) | cosθ100 | K2 | λ (Å) | β100 (°) | β100 (rad) | La (Å) | ||||
| 35°–55° | 44.17 | 22.086 | 0.926 | 1.84 | 1.54056 | 7.095 | 0.1238 | 24.705 |
| Spectral Interval (cm−1) | Fitting Peak Number | Peak Position (cm−1) | FWHM (cm−1) | Fitting Peak Area | Assignment | Absorption Intensity | ||
|---|---|---|---|---|---|---|---|---|
| 1000–1800 | 1 | 1603 | 85.95 | 5.522 | aromatic nucleus (C=C) | S | ||
| 2 | 1493 | 26.10 | 0.152 | (C–C)ar stretching | W | |||
| 3 | 1441 | 46.28 | 1.817 | aliphatic chains (CH3, CH2) | M | |||
| 4 | 1401 | 30.85 | 0.347 | CH3− | W | |||
| 5 | 1373 | 16.66 | 0.153 | CH3−Ar, R | W | |||
| 6 | 1344 | 102.54 | 2.501 | CH3−C=O | W | |||
| 7 | 1238 | 113.49 | 3.260 | C−O−C | M | |||
| 8 | 1161 | 73.89 | 1.200 | Hydroxybenzene | M | |||
| 9 | 1032 | 31.05 | 0.147 | Alkyl ether | W | |||
| 10 | 1070 | 212.45 | 3.267 | C−O−C | W | |||
| 2800–3000 | 1 | 2834 | 25.89 | 0.155 | R2CH2 | M | ||
| 2 | 2859 | 36.24 | 0.546 | R2CH2 | M | |||
| 3 | 2891 | 26.43 | 0.293 | R3CH | S | |||
| 4 | 2918 | 34.68 | 0.869 | R3CH | S | |||
| 5 | 2951 | 23.61 | 0.250 | RCH3 | M | |||
| 6 | 2979 | 15.41 | 0.035 | RCH | W | |||
| Analysis Object | Content | |
|---|---|---|
| Proximate analysis (%) | Mad | 1.37 |
| Aad | 18.26 | |
| Vdaf | 33.82 | |
| Ultimate analysis (%) | Cdaf | 83.3 |
| Hdaf | 5.27 | |
| Odaf | 9.85 | |
| Ndaf | 1.23 | |
| Sdaf | 0.35 | |
| OCFGs | XC | 0.53748 |
| XH | 0.40804 | |
| XO | 0.04766 | |
| XN | 0.00680 | |
| Z–O– | 3.957 | |
| Z–C=O | 1.028 | |
| Z–OH | 0.512 | |
| Z–COOH | 0.189 | |
| Chemical Shift Interval | Assignment | Peak Position of Fitting Peak | Peak Area of Fitting Peak (%) | Peak Position of Fitting Peak | Peak Area of Fitting Peak (%) | ||
|---|---|---|---|---|---|---|---|
| 16 | R–CH3 | 7.6 | 0.024 | 117.8 | 0.074 | ||
| 20 | Ar–CH3 | 16.6 | 0.024 | 125.3 | 0.129 | ||
| 23 | CH2–CH3 | 22.8 | 0.033 | 133.3 | 0.141 | ||
| 33 | CH2 | 29.6 | 0.047 | 141.1 | 0.080 | ||
| 36–50 | ≡CH | 37.1 | 0.050 | 148.5 | 0.046 | ||
| 50–60 | O–CH3, O–CH2 | 44.2 | 0.047 | 155.6 | 0.023 | ||
| 60–70 | O–CH | 51.4 | 0.025 | 161.7 | 0.011 | ||
| 75–90 | R–O–R | 59.2 | 0.014 | 170.3 | 0.011 | ||
| 100–129 | Ar–H | 67.1 | 0.016 | 179.1 | 0.015 | ||
| 129–137 | Bridgehead (C–C) | 74.3 | 0.019 | 187.1 | 0.024 | ||
| 137–148 | Ar–C | 81.6 | 0.021 | 195.1 | 0.028 | ||
| 148–165 | Ar–O | 89.9 | 0.010 | 203.4 | 0.023 | ||
| 165–190 | COOH | 100.4 | 0.007 | 212.1 | 0.015 | ||
| 190–220 | C=O | 110.0 | 0.028 | 221.4 | 0.010 | ||
| 231.2 | 0.004 | ||||||
| Structural Parameters | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calculated value | 0.768 | 0.050 | 0.718 | 0.248 | 0.470 | 0.023 | 0.126 | 0.099 | 0.2315 | 0.2312 | 0.0003 | 0.049 | 0.143 | 0.038 | 0.160 | 0.620 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Zhang, H.; Hu, G.; Zhang, W. Fine Characterization of the Macromolecular Structure of Huainan Coal Using XRD, FTIR, 13C-CP/MAS NMR, SEM, and AFM Techniques. Molecules 2020, 25, 2661. https://doi.org/10.3390/molecules25112661
Wu D, Zhang H, Hu G, Zhang W. Fine Characterization of the Macromolecular Structure of Huainan Coal Using XRD, FTIR, 13C-CP/MAS NMR, SEM, and AFM Techniques. Molecules. 2020; 25(11):2661. https://doi.org/10.3390/molecules25112661
Chicago/Turabian StyleWu, Dun, Hui Zhang, Guangqing Hu, and Wenyong Zhang. 2020. "Fine Characterization of the Macromolecular Structure of Huainan Coal Using XRD, FTIR, 13C-CP/MAS NMR, SEM, and AFM Techniques" Molecules 25, no. 11: 2661. https://doi.org/10.3390/molecules25112661
APA StyleWu, D., Zhang, H., Hu, G., & Zhang, W. (2020). Fine Characterization of the Macromolecular Structure of Huainan Coal Using XRD, FTIR, 13C-CP/MAS NMR, SEM, and AFM Techniques. Molecules, 25(11), 2661. https://doi.org/10.3390/molecules25112661




