Steady-State Kinetics of Enzyme-Catalyzed Hydrolysis of Echothiophate, a P–S Bonded Organophosphorus as Monitored by Spectrofluorimetry
Abstract
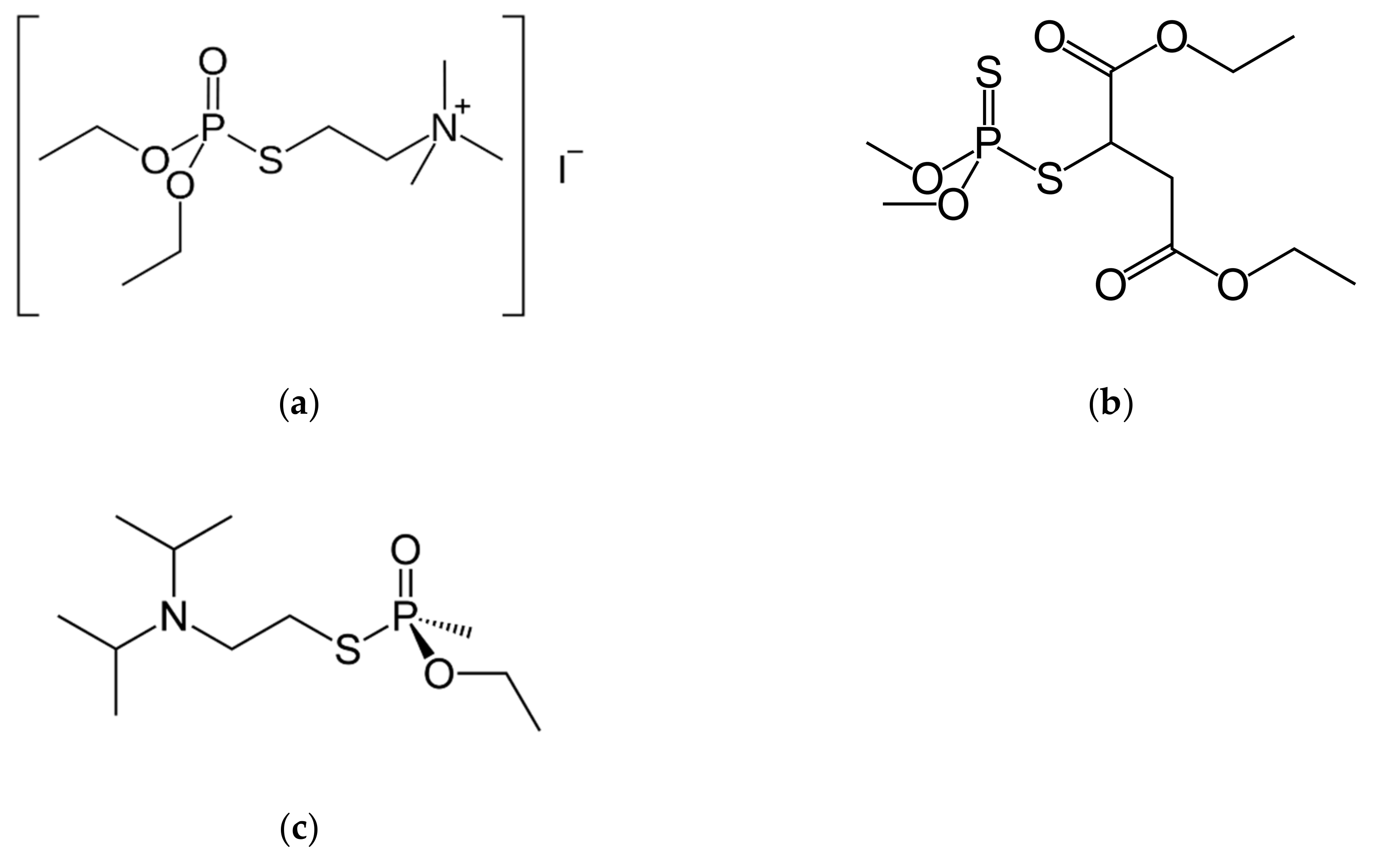
1. Introduction
2. Results and Discussion
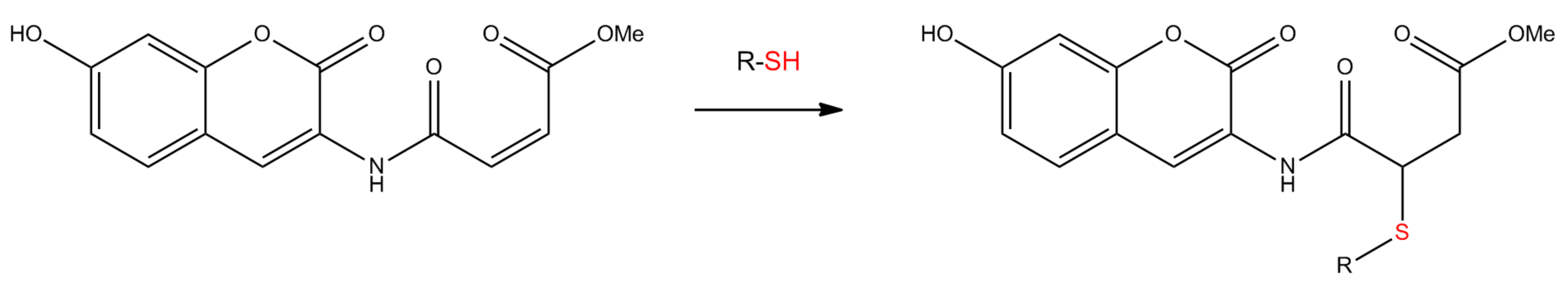
2.1. Interaction of Probe IV With Enzymes
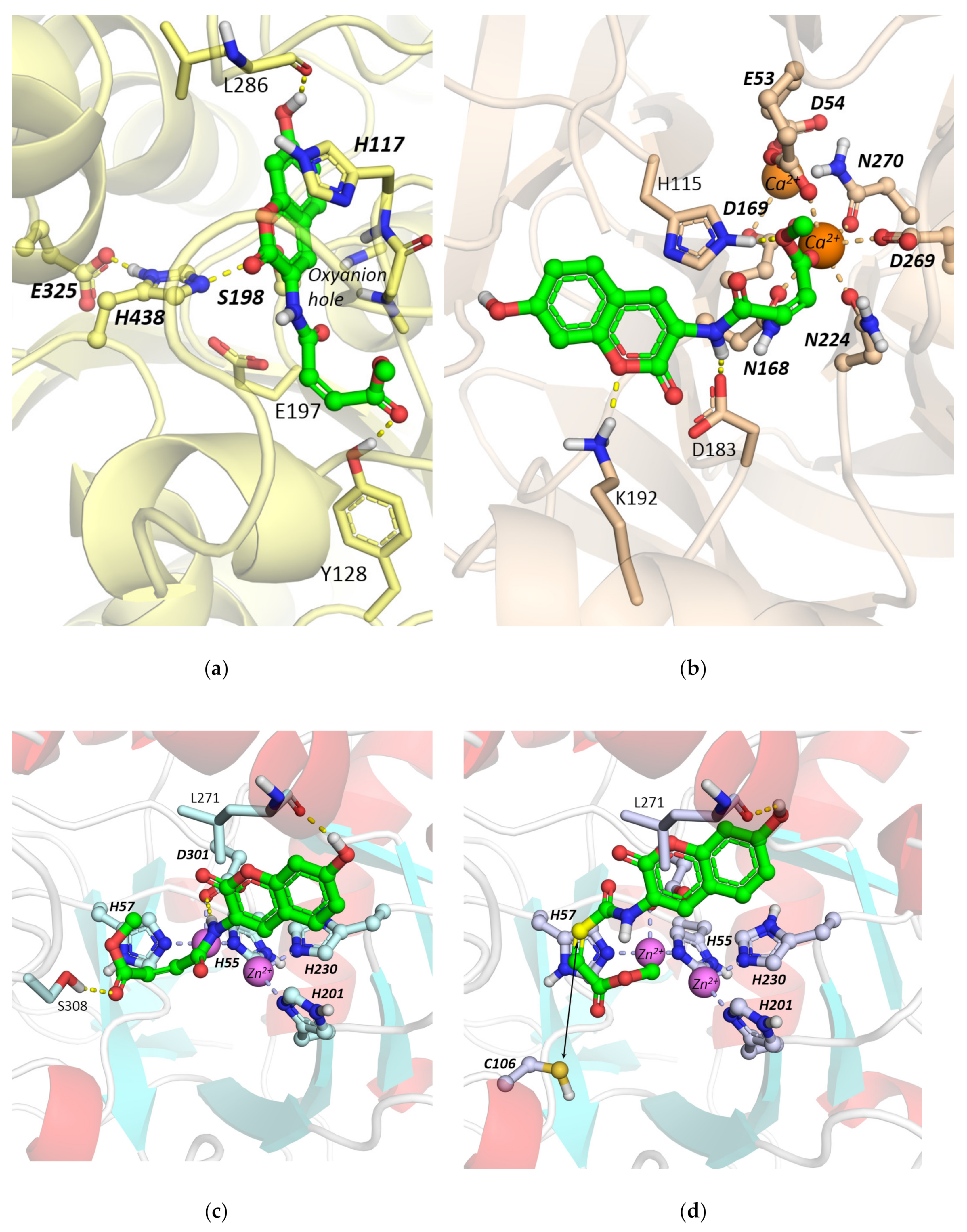
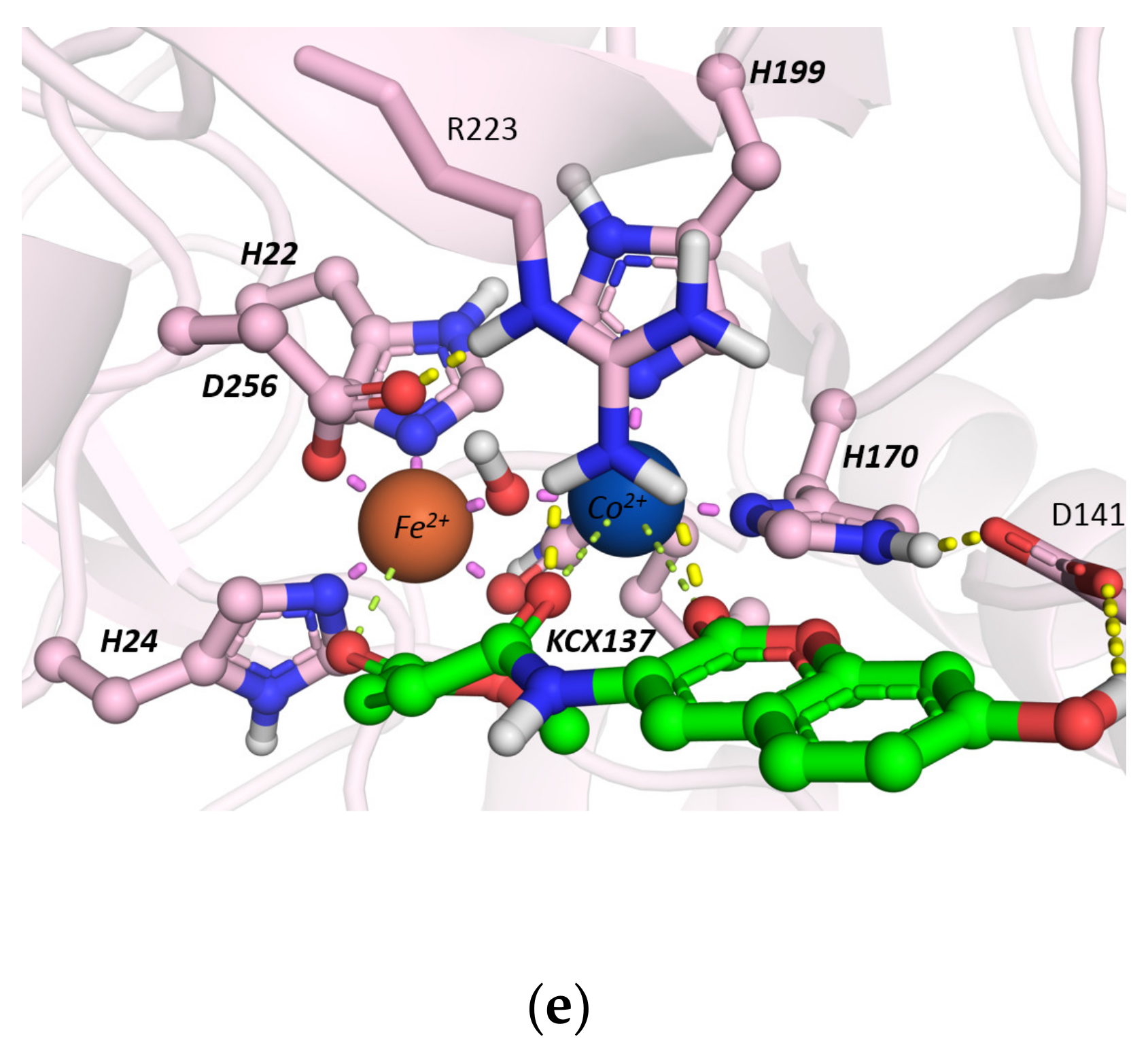
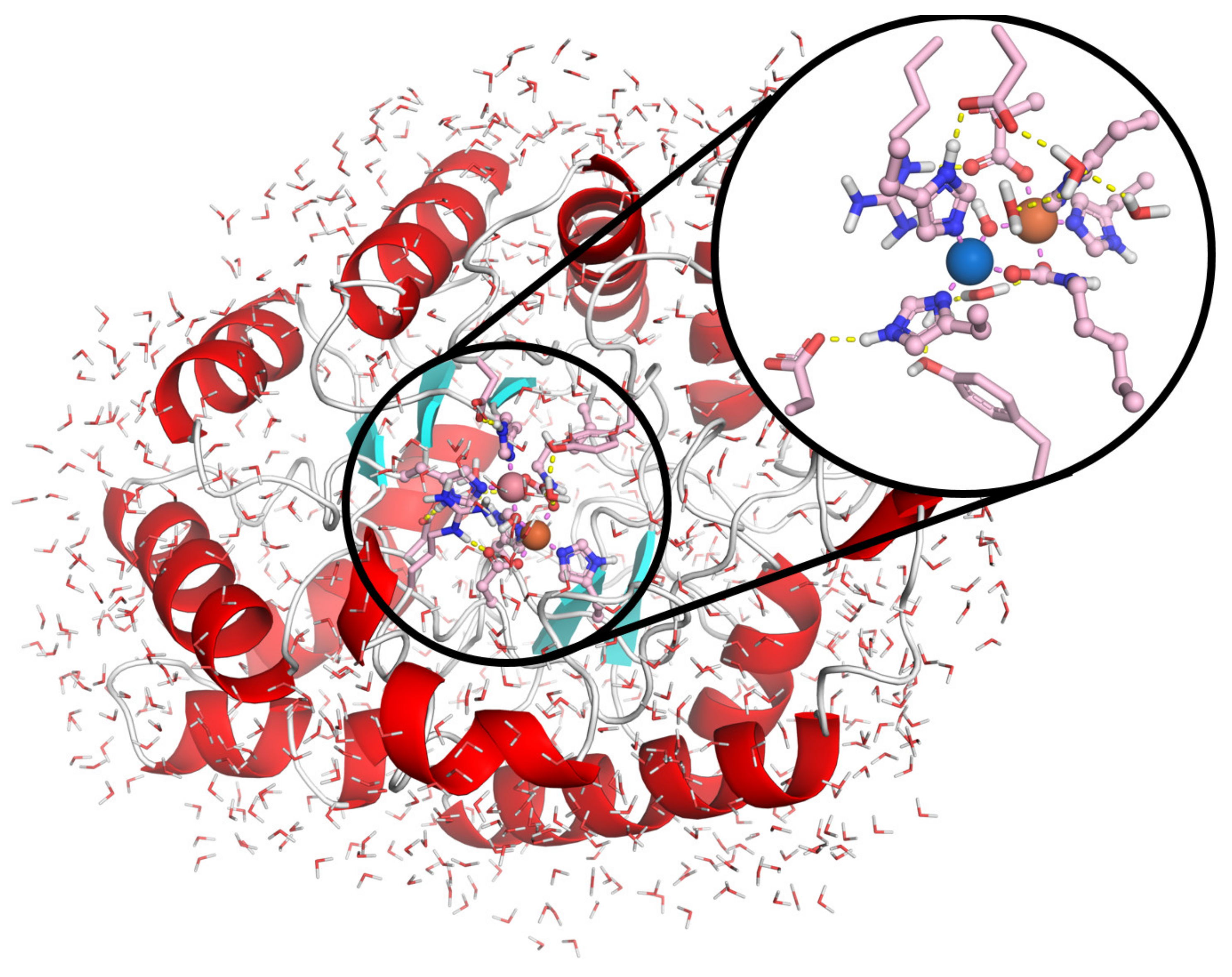
Molecular Docking Studies
2.2. Steady-State Kinetics of Enzyme-Catalyzed Hydrolysis of Echothiophate
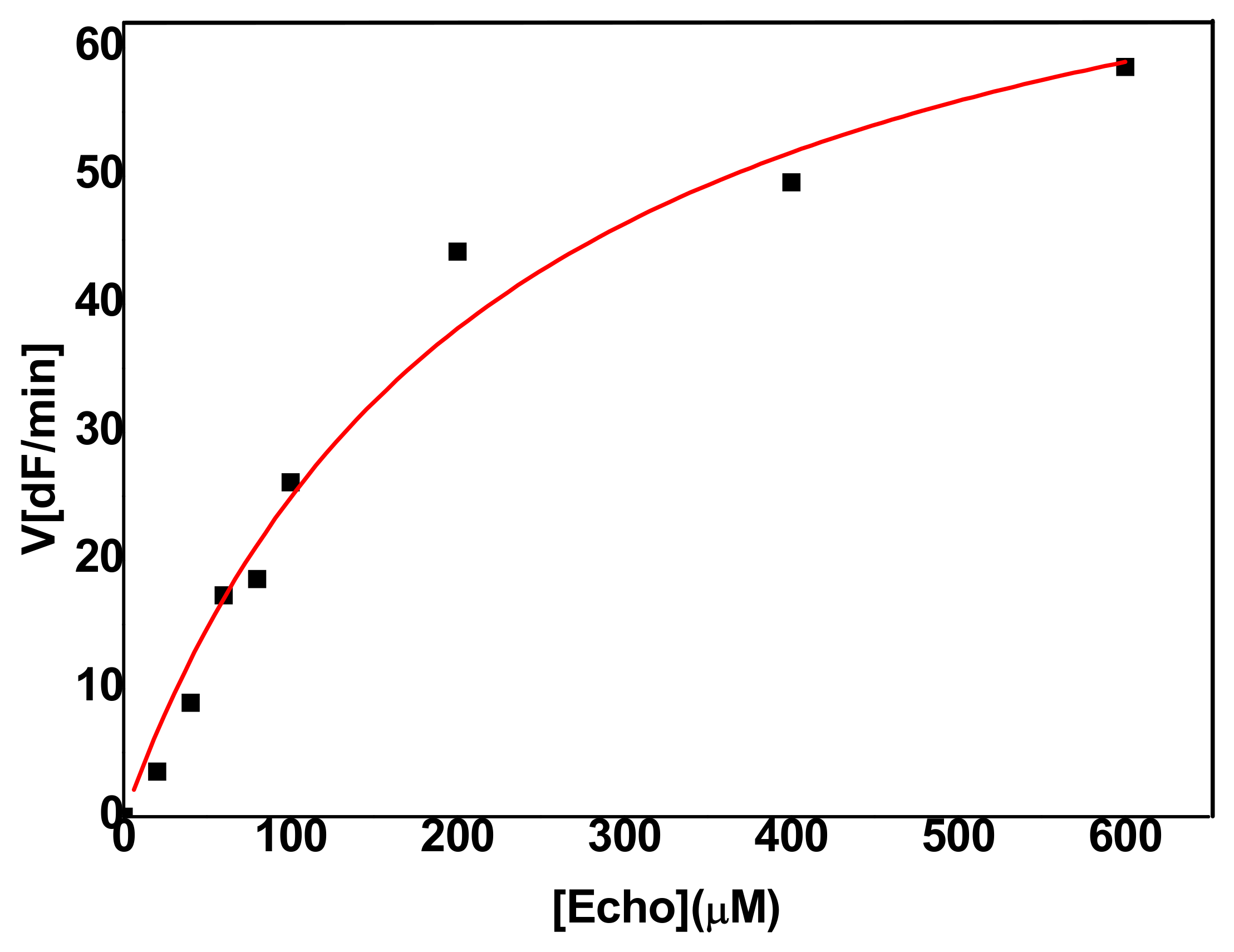
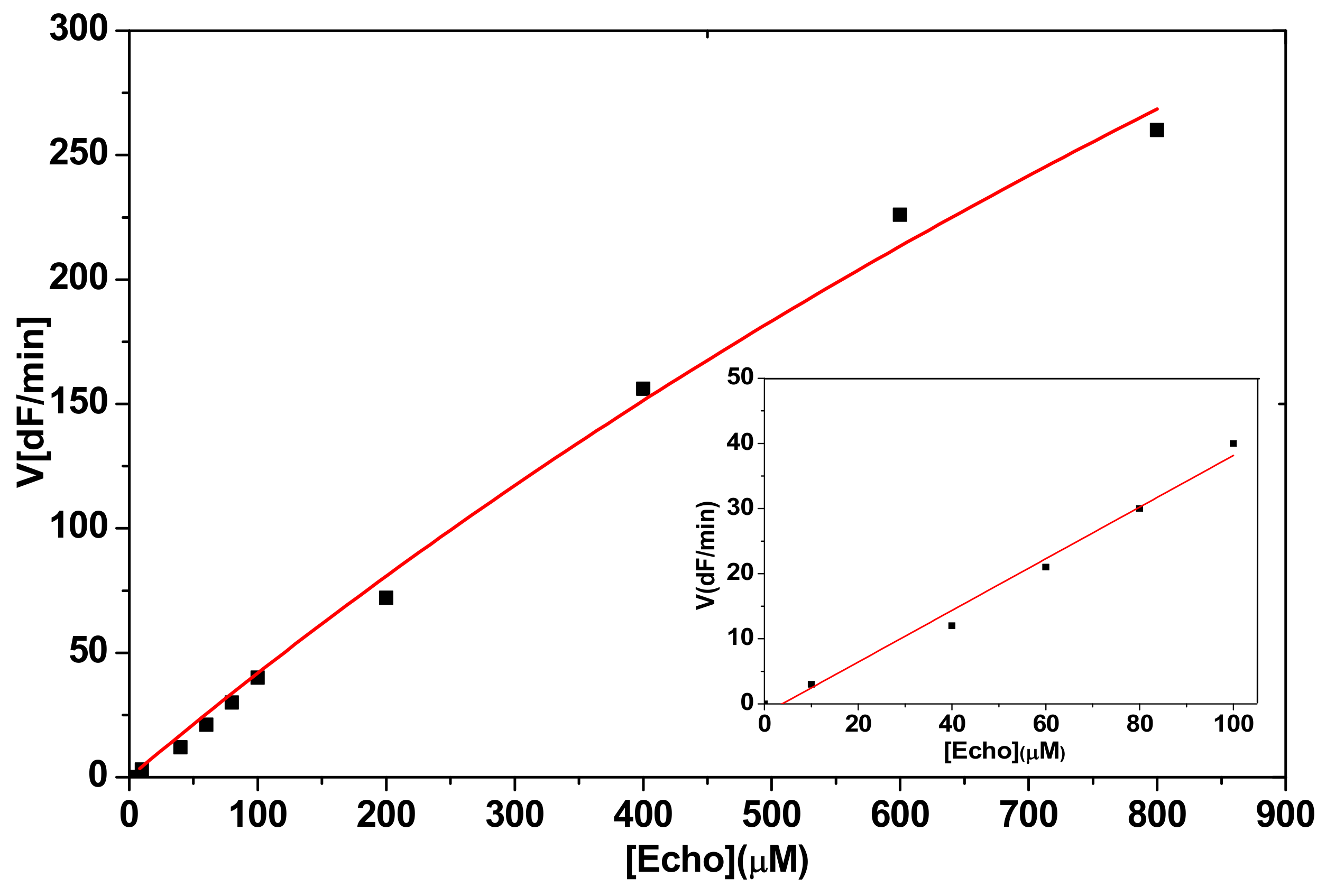
2.2.1. Hydrolysis of Echothiophate by the G117H Mutant of Human Butyrylcholinesterase
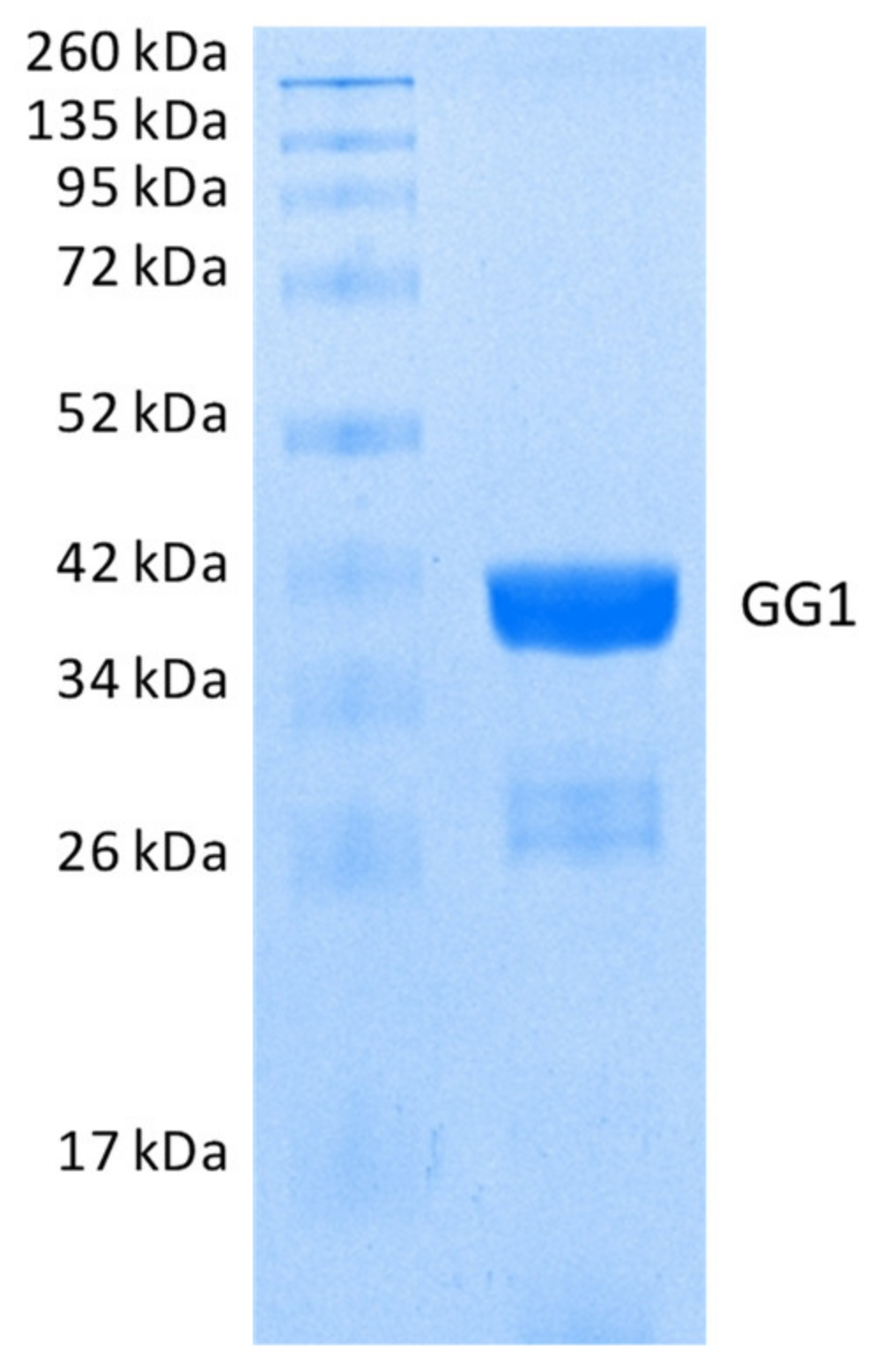
2.2.2. Hydrolysis of Echothiophate by the GG1 Mutant of Brevundimonas Diminuta PTE
3. Materials and Methods
3.1. Chemicals
3.2. Enzymes
3.3. Reaction of Probe IV With Thiols and Calibration
3.4. Steady-State Kinetic Study of Enzyme-Catalyzed Hydrolysis of Echothiophate
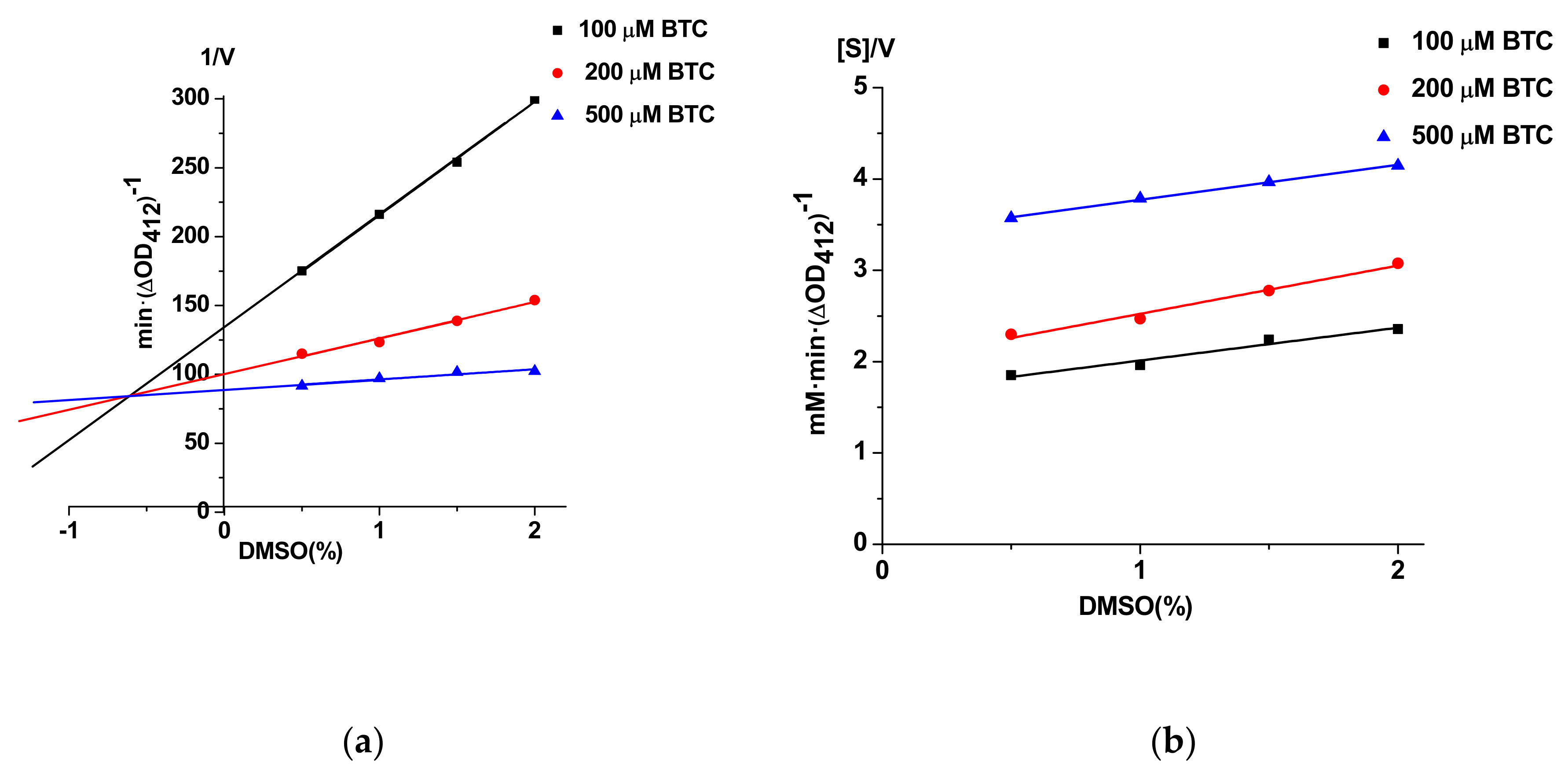
3.5. Possibility of Unwanted Interactions of DMSO and Probe IV With Enzymes
3.6. Molecular Modeling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef]
- Chowdhary, S.; Bhattacharyya, R.; Banerjee, D. Acute organophosphorus poisoning. Clin. Chim. Acta 2014, 431, 66–76. [Google Scholar] [CrossRef]
- Schmidt, K.G.; Horowitz, Y.; Buckman, G.; Segev, E.; Levinger, E.; Geyer, O. Lowering of IOP by echothiophate iodide in pseudophakic eyes with glaucoma. Curr. Eye. Res. 2010, 35, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Bonner, M.R.; Coble, J.; Blair, A.; Beane Freeman, L.E.; Hoppin, J.A.; Sandler, D.P.; Alavanja, M.C. Malathion exposure and the incidence of cancer in the agricultural health study. Am. J. Epidemiol. 2007, 166, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Idriss, S.; Levitt, J. Malathion for head lice and scabies: treatment and safety considerations. J. Drugs Dermatol. 2009, 8, 715–720. [Google Scholar] [PubMed]
- Reiter, G.; Muller, S.; Hill, I.; Weatherby, K.; Thiermann, H.; Worek, F.; Mikler, J. In vitro and in vivo toxicological studies of V nerve agents: Molecular and stereoselective aspects. Toxicol. Lett. 2015, 232, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Luschekina, S. Catalytic Bioscavengers: The second Generation of Bioscavenger-Based Medical Countermeasures. In Handbook of Toxicology of Chemical Warfare Agents, 3rd ed.; Gupta, R.D., Ed.; Academic Press: London, UK, 2020; in press. [Google Scholar]
- Bigley, A.N.; Raushel, F.M. The evolution of phosphotriesterase for decontamination and detoxification of organophosphorus chemical warfare agents. Chem. Biol. Interact. 2019, 308, 80–88. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Lockridge, O.; Blong, R.M.; Masson, P.; Froment, M.T.; Millard, C.B.; Broomfield, C.A. A single amino acid substitution, Gly117His, confers phosphotriesterase (organophosphorus acid anhydride hydrolase) activity on human butyrylcholinesterase. Biochemistry 1997, 36, 786–795. [Google Scholar] [CrossRef]
- Poyot, T.; Nachon, F.; Froment, M.T.; Loiodice, M.; Wieseler, S.; Schopfer, L.M.; Lockridge, O.; Masson, P. Mutant of Bungarus fasciatus acetylcholinesterase with low affinity and low hydrolase activity toward organophosphorus esters. Biochim. Biophys. Acta 2006, 1764, 1470–1478. [Google Scholar] [CrossRef]
- Mukhametgalieva, A.R.; Zueva, I.V.; Aglyamova, A.R.; Lushchekina, S.V.; Masson, P. A new sensitive spectrofluorimetric method for measurement of activity and kinetic study of cholinesterases. Biochim Biophys. Acta Proteins Proteom. 2020, 1868, 140270. [Google Scholar] [CrossRef] [PubMed]
- Latip, W.; Knight, V.F.; Abdul Halim, N.; Ong, K.K.; Mohd Kassim, N.A.; Wan Yunus, W.M.Z.; Mohd Noor, S.A.; Mohamad Ali, M.S. Microbial Phosphotriesterase: Structure, Function, and Biotechnological Applications. Catalysts 2019, 9, 671. [Google Scholar] [CrossRef]
- Jacquet, P.; Daude, D.; Bzdrenga, J.; Masson, P.; Elias, M.; Chabriere, E. Current and emerging strategies for organophosphate decontamination: special focus on hyperstable enzymes. Environ. Sci. Pollut. Res. Int. 2016, 23, 8200–8218. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, T.C.; de Castro, A.A.; Silva, D.R.; Cristina Silva, M.; Franca, T.C.C.; Bennion, B.J.; Kuca, K. Computational Enzymology and Organophosphorus Degrading Enzymes: Promising Approaches Toward Remediation Technologies of Warfare Agents and Pesticides. Curr. Med. Chem. 2016, 23, 1041–1061. [Google Scholar] [CrossRef]
- Bigley, A.N.; Desormeaux, E.; Xiang, D.F.; Bae, S.Y.; Harvey, S.P.; Raushel, F.M. Overcoming the Challenges of Enzyme Evolution To Adapt Phosphotriesterase for V-Agent Decontamination. Biochemistry 2019, 58, 2039–2053. [Google Scholar] [CrossRef] [PubMed]
- Despotovic, D.; Aharon, E.; Dubovetskyi, A.; Leader, H.; Ashani, Y.; Tawfik, D.S. A mixture of three engineered phosphotriesterases enables rapid detoxification of the entire spectrum of known threat nerve agents. Protein Eng. Des. Sel. 2019. [Google Scholar] [CrossRef]
- Cardozo, M.; de Almeida, J.; Cavalcante, S.F.A.; Salgado, J.R.S.; Goncalves, A.S.; Franca, T.C.C.; Kuca, K.; Bizzo, H.R. Biodegradation of Organophosphorus Compounds Predicted by Enzymatic Process Using Molecular Modelling and Observed in Soil Samples Through Analytical Techniques and Microbiological Analysis: A Comparison. Molecules 2019, 25, 58. [Google Scholar] [CrossRef]
- Worek, F.; Thiermann, H.; Wille, T. Catalytic bioscavengers in nerve agent poisoning: A promising approach? Toxicol. Lett. 2016, 244, 143–148. [Google Scholar] [CrossRef]
- Goldsmith, M.; Ashani, Y. Catalytic bioscavengers as countermeasures against organophosphate nerve agents. Chem. Biol. Interact. 2018, 292, 50–64. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, E.J.; Tsao, C.; Kasten, S.A.; Boeri, M.V.; Dao, T.L.; DeBus, S.J.; Cadieux, C.L.; Baker, C.A.; Otto, T.C.; et al. Nanoscavenger provides long-term prophylactic protection against nerve agents in rodents. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Job, L.; Kohler, A.; Escher, B.; Worek, F.; Skerra, A. A catalytic bioscavenger with improved stability and reduced susceptibility to oxidation for treatment of acute poisoning with neurotoxic organophosphorus compounds. Toxicology letters 2020, 321, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Masson, P.; Nachon, F.; Broomfield, C.A.; Lenz, D.E.; Verdier, L.; Schopfer, L.M.; Lockridge, O. A collaborative endeavor to design cholinesterase-based catalytic scavengers against toxic organophosphorus esters. Chem. Biol. Interact. 2008, 175, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Lushchekina, S.V.; Schopfer, L.M.; Grigorenko, B.L.; Nemukhin, A.V.; Varfolomeev, S.D.; Lockridge, O.; Masson, P. Optimization of Cholinesterase-Based Catalytic Bioscavengers Against Organophosphorus Agents. Front. Pharmacol. 2018, 9, 211. [Google Scholar] [CrossRef]
- Grigorenko, B.L.; Novichkova, D.A.; Lushchekina, S.V.; Zueva, I.V.; Schopfer, L.M.; Nemukhin, A.V.; Varfolomeev, S.D.; Lockridge, O.; Masson, P. Computer-designed active human butyrylcholinesterase double mutant with a new catalytic triad. Chem. Biol. Interact. 2019, 306, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, M.; Macek Hrvat, N.; Baumann, K.; Morasi Pipercic, S.; Makaric, S.; Tomic, S.; Jovic, O.; Hrenar, T.; Milicevic, A.; Jelic, D.; et al. A comprehensive evaluation of novel oximes in creation of butyrylcholinesterase-based nerve agent bioscavengers. Toxicol. Appl. Pharmacol. 2016, 310, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Baker, S.L.; Murata, H.; Harris, N.; Ji, W.; Amitai, G.; Matyjaszewski, K.; Russell, A.J. Tuning Butyrylcholinesterase Inactivation and Reactivation by Polymer-Based Protein Engineering. Adv. Sci. (Weinh) 2020, 7, 1901904. [Google Scholar] [CrossRef]
- Zorbaz, T.; Malinak, D.; Kuca, K.; Musilek, K.; Kovarik, Z. Butyrylcholinesterase inhibited by nerve agents is efficiently reactivated with chlorinated pyridinium oximes. Chem. Biol. Interact. 2019, 307, 16–20. [Google Scholar] [CrossRef]
- Goldsmith, M.; Aggarwal, N.; Ashani, Y.; Jubran, H.; Greisen, P.J.; Ovchinnikov, S.; Leader, H.; Baker, D.; Sussman, J.L.; Goldenzweig, A.; et al. Overcoming an optimization plateau in the directed evolution of highly efficient nerve agent bioscavengers. Protein Eng. Des. Sel. 2017, 30, 333–345. [Google Scholar] [CrossRef]
- Chabrière, E.; Daudé, D.; Elias, M. Nouvelles enzymes pte mutées. WO2019016468A1, 17 July 2019. [Google Scholar]
- Chen, Y.C. Beware of docking! Trends Pharmacol. Sci. 2015, 36, 78–95. [Google Scholar] [CrossRef]
- Xu, Y.; Cheng, S.; Sussman, J.L.; Silman, I.; Jiang, H. Computational Studies on Acetylcholinesterases. Molecules 2017, 22, 1324. [Google Scholar] [CrossRef] [PubMed]
- Lushchekina, S.V.; Makhaeva, G.F.; Novichkova, D.A.; Zueva, I.V.; Kovaleva, N.V.; Richardson, R.J. Supercomputer Modeling of Dual-Site Acetylcholinesterase (AChE) Inhibition. Supercomput. Front. Innov. 2018, 5. [Google Scholar] [CrossRef]
- Santos-Martins, D.; Forli, S.; Ramos, M.J.; Olson, A.J. AutoDock4(Zn): an improved AutoDock force field for small-molecule docking to zinc metalloproteins. J. Chem. Inf. Model. 2014, 54, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Balaz, S.; Shelver, W.H. A practical approach to docking of zinc metalloproteinase inhibitors. J. Mol. Graph. Model. 2004, 22, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Friboulet, A.; Rieger, F.; Goudou, D.; Amitai, G.; Taylor, P. Interaction of an organophosphate with a peripheral site on acetylcholinesterase. Biochemistry 1990, 29, 914–920. [Google Scholar] [CrossRef]
- Masson, P.; Legrand, P.; Bartels, C.F.; Froment, M.-T.; Schopfer, L.M.; Lockridge, O. Role of Aspartate 70 and Tryptophan 82 in binding of succinyldithiocholine to human butyrylcholinesterase. Biochemistry 1997, 36, 2266–2277. [Google Scholar] [CrossRef]
- Kumar, A.; Darreh-Shori, T. DMSO: A Mixed-Competitive Inhibitor of Human Acetylcholinesterase. ACS Chem. Neurosci. 2017, 8, 2618–2625. [Google Scholar] [CrossRef]
- Bigley, A.N.; Xu, C.; Henderson, T.J.; Harvey, S.P.; Raushel, F.M. Enzymatic neutralization of the chemical warfare agent VX: evolution of phosphotriesterase for phosphorothiolate hydrolysis. J. Am. Chem. Soc. 2013, 135, 10426–10432. [Google Scholar] [CrossRef]
- Reed, B.A.; Sabourin, C.L.; Lenz, D.E. Human butyrylcholinesterase efficacy against nerve agent exposure. J. Biochem. Mol. Toxicol. 2017, 31. [Google Scholar] [CrossRef]
- Cerasoli, D.M.; Armstrong, S.J.; Reeves, T.E.; Hodgins, S.M.; Kasten, S.A.; Lee-Stubbs, R.B.; Cadieux, C.L.; Otto, T.C.; Capacio, B.R.; Lenz, D.E. Butyrylcholinesterase, a stereospecific in vivo bioscavenger against nerve agent intoxication. Biochem. Pharmacol. 2020, 171, 113670. [Google Scholar] [CrossRef]
- Nachon, F.; Carletti, E.; Wandhammer, M.; Nicolet, Y.; Schopfer, L.M.; Masson, P.; Lockridge, O. X-ray crystallographic snapshots of reaction intermediates in the G117H mutant of human butyrylcholinesterase, a nerve agent target engineered into a catalytic bioscavenger. Biochem. J. 2011, 434, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewska, M.; Fenollar, F.; Villard, C.; Azza, S.; Roux, M.; Raoult, D. An immunoproteomic approach for identification of clinical biomarkers of Whipple’s disease. Proteomics Clin. Appl. 2008, 2, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Li, H.; Sun, L.; Liu, L.; Zhang, C.; Xi, Z. A highly sensitive fluorescence probe for fast thiol-quantification assay of glutathione reductase. Angew. Chem. Int. Ed. Engl. 2009, 48, 4034–4037. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, S.S.; Smirnov, I.V.; Stepanova, A.V.; Bobik, T.V.; Mokrushina, Y.A.; Ponomarenko, N.A.; Belogurov, A.A., Jr.; Rubtsova, M.P.; Kartseva, O.V.; Gomzikova, M.O.; et al. Microfluidic droplet platform for ultrahigh-throughput single-cell screening of biodiversity. Proc. Natl. Acad. Sci. USA 2017, 114, 2550–2555. [Google Scholar] [CrossRef] [PubMed]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J. 1974, 137, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Pajk, S.; Knez, D.; Kosak, U.; Zorovic, M.; Brazzolotto, X.; Coquelle, N.; Nachon, F.; Colletier, J.P.; Zivin, M.; Stojan, J.; et al. Development of potent reversible selective inhibitors of butyrylcholinesterase as fluorescent probes. J. Enzyme. Inhib. Med. Chem. 2020, 35, 498–505. [Google Scholar] [CrossRef]
- Bigley, A.N.; Raushel, F.M. Catalytic mechanisms for phosphotriesterases. Biochim. Biophys. Acta 2013, 1834, 443–453. [Google Scholar] [CrossRef]
- Chen, D.; Menche, G.; Power, T.D.; Sower, L.; Peterson, J.W.; Schein, C.H. Accounting for ligand-bound metal ions in docking small molecules on adenylyl cyclase toxins. Proteins 2007, 67, 593–605. [Google Scholar] [CrossRef]
- Nemukhin, A.V.; Grigorenko, B.L.; Lushchekina, S.V.; Varfolomeev, S.D. Quantum chemical modelling in the research of molecular mechanisms of enzymatic catalysis. Russ. Chem. Rev. 2012, 81, 1011–1025. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.J.; et al. General Atomic and Molecular Electronic-Structure System. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Löwdin, P.-O. On the Nonorthogonality Problem. In Adv. in Quantum Chem.; Per-Olov, L., Ed.; Academic Press: Cambridge, MA, USA, 1970; Volume 5, pp. 185–199. [Google Scholar]
- Benning, M.M.; Shim, H.; Raushel, F.M.; Holden, H.M. High resolution X-ray structures of different metal-substituted forms of phosphotriesterase from Pseudomonas diminuta. Biochem. 2001, 40, 2712–2722. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Aharoni, A.; Gaidukov, L.; Brumshtein, B.; Khersonsky, O.; Meged, R.; Dvir, H.; Ravelli, R.B.; McCarthy, A.; Toker, L.; et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat. Struct. Mol. Biol. 2004, 11, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Hiblot, J.; Gotthard, G.; Chabriere, E.; Elias, M. Characterisation of the organophosphate hydrolase catalytic activity of SsoPox. Sci. Rep. 2012, 2, 779. [Google Scholar] [CrossRef] [PubMed]
- Word, J.M.; Lovell, S.C.; Richardson, J.S.; Richardson, D.C. Asparagine and glutamine: using hydrogen atom contacts in the choice of side-chain amide orientation. J. Mol. Biol. 1999, 285, 1735–1747. [Google Scholar] [CrossRef]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; Van Dam, H.J.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; et al. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comput. Phys. Commun. 2010, 181, 1477–1489. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Voevodin, V.; Antonov, A.; Nikitenko, D.; Shvets, P.; Sobolev, S.; Sidorov, I.; Stefanov, K.; Voevodin, V.; Zhumatiy, S. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zueva, I.V.; Lushchekina, S.V.; Daudé, D.; Chabrière, E.; Masson, P. Steady-State Kinetics of Enzyme-Catalyzed Hydrolysis of Echothiophate, a P–S Bonded Organophosphorus as Monitored by Spectrofluorimetry. Molecules 2020, 25, 1371. https://doi.org/10.3390/molecules25061371
Zueva IV, Lushchekina SV, Daudé D, Chabrière E, Masson P. Steady-State Kinetics of Enzyme-Catalyzed Hydrolysis of Echothiophate, a P–S Bonded Organophosphorus as Monitored by Spectrofluorimetry. Molecules. 2020; 25(6):1371. https://doi.org/10.3390/molecules25061371
Chicago/Turabian StyleZueva, Irina V., Sofya V. Lushchekina, David Daudé, Eric Chabrière, and Patrick Masson. 2020. "Steady-State Kinetics of Enzyme-Catalyzed Hydrolysis of Echothiophate, a P–S Bonded Organophosphorus as Monitored by Spectrofluorimetry" Molecules 25, no. 6: 1371. https://doi.org/10.3390/molecules25061371
APA StyleZueva, I. V., Lushchekina, S. V., Daudé, D., Chabrière, E., & Masson, P. (2020). Steady-State Kinetics of Enzyme-Catalyzed Hydrolysis of Echothiophate, a P–S Bonded Organophosphorus as Monitored by Spectrofluorimetry. Molecules, 25(6), 1371. https://doi.org/10.3390/molecules25061371




