Comparison of Various Chromatographic Systems for Identification of Vortioxetine in Bulk Drug Substance, Human Serum, Saliva, and Urine Samples by HPLC-DAD and LC-QTOF-MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of Chromatographic Systems
2.1.1. Analysis of Vortioxetine on Octadecyl Stationary Phase
2.1.2. Analysis of Vortioxetine on Phenyl Stationary Phase
2.1.3. Analysis of Vortioxetine by HILIC
2.1.4. Analysis of Vortioxetine by Ion-Exchange Chromatography
2.2. Determination of the Lipophilicity of Vortioxetine
2.3. Optimization of Sample Pre-Treatment Method
2.4. Analysis of Vortioxetine in Serum, Urine, and Saliva Samples
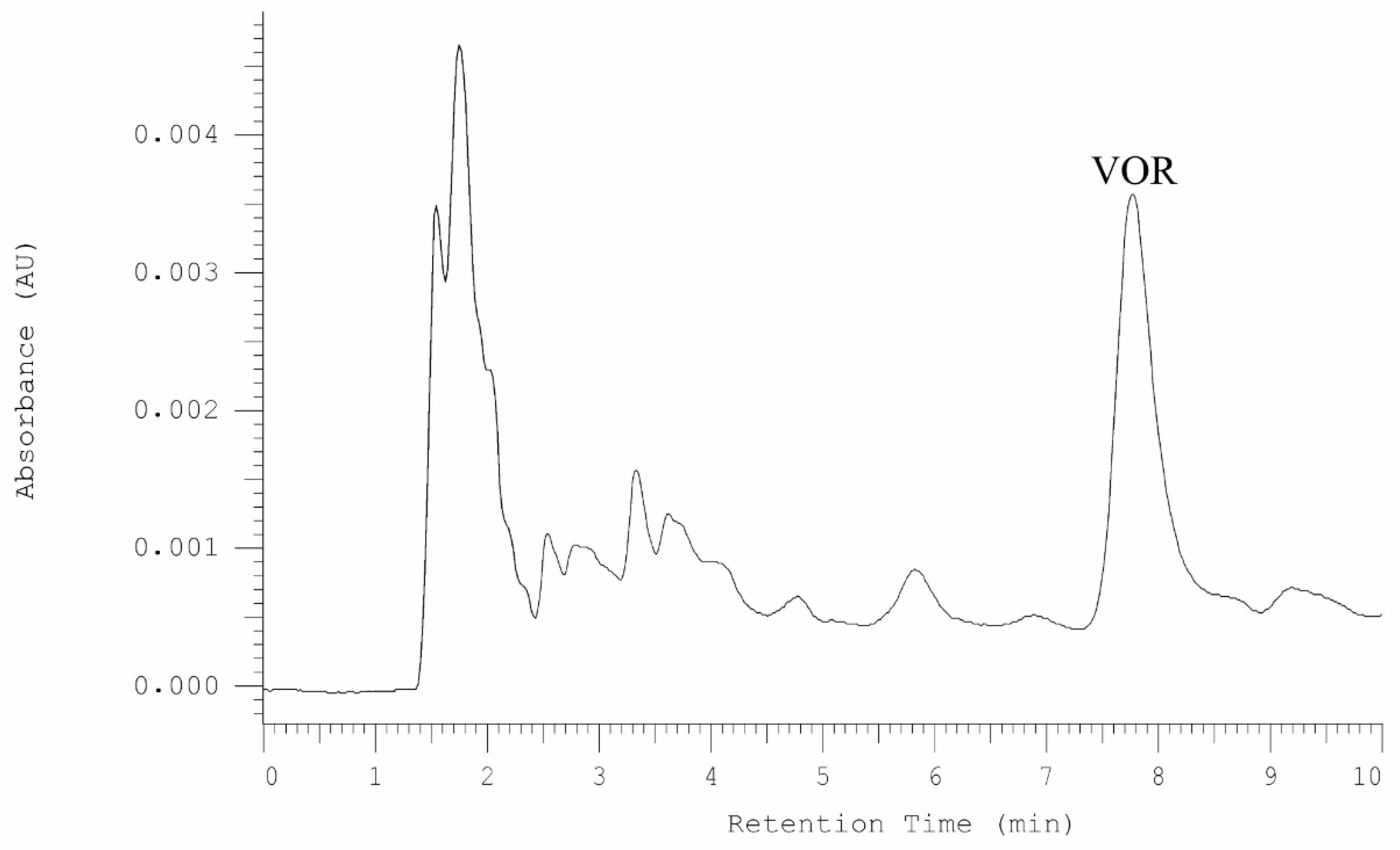
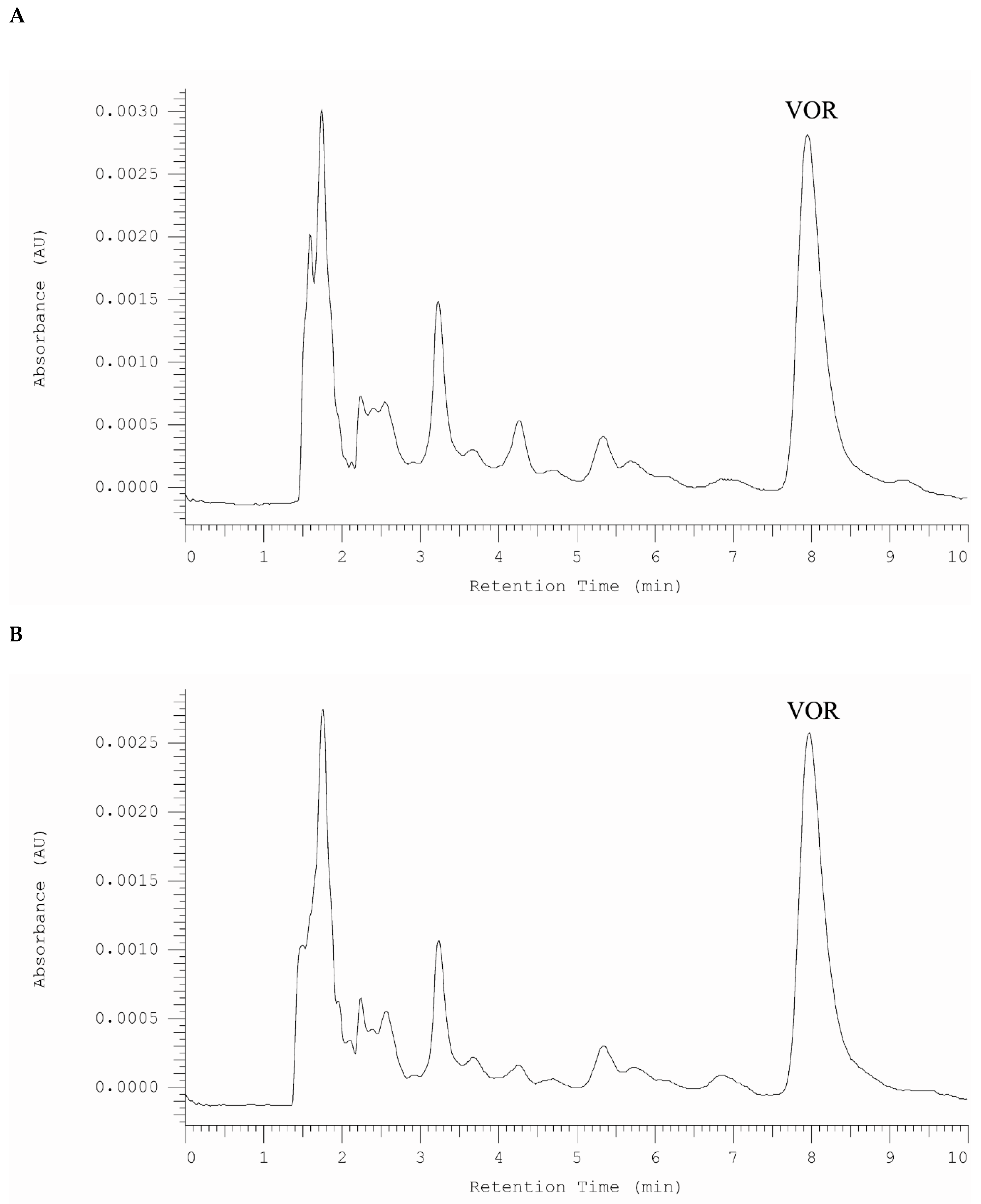
2.4.1. Analysis of Vortioxetine in Serum, Saliva, and Urine Samples by HPLC-DAD
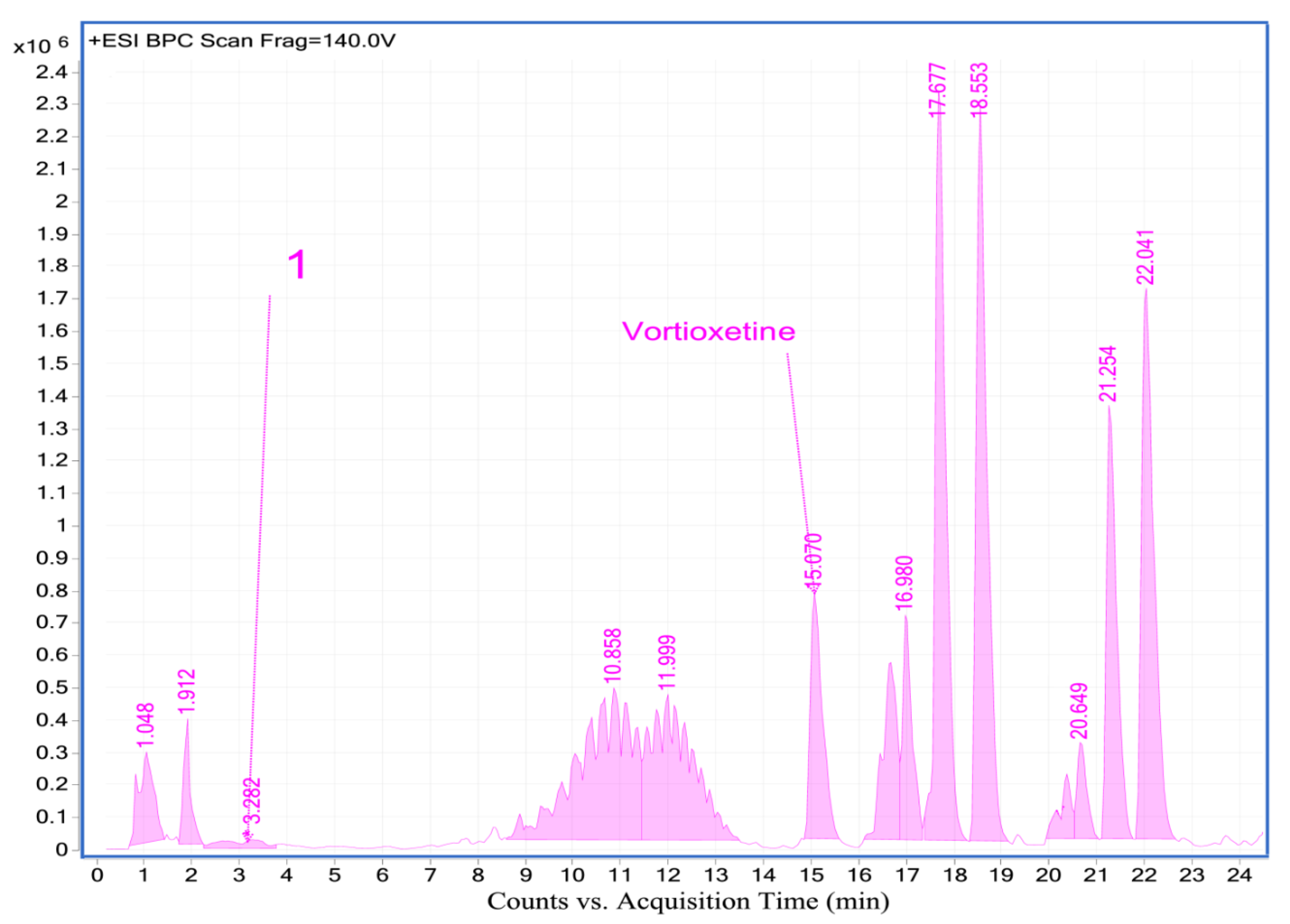
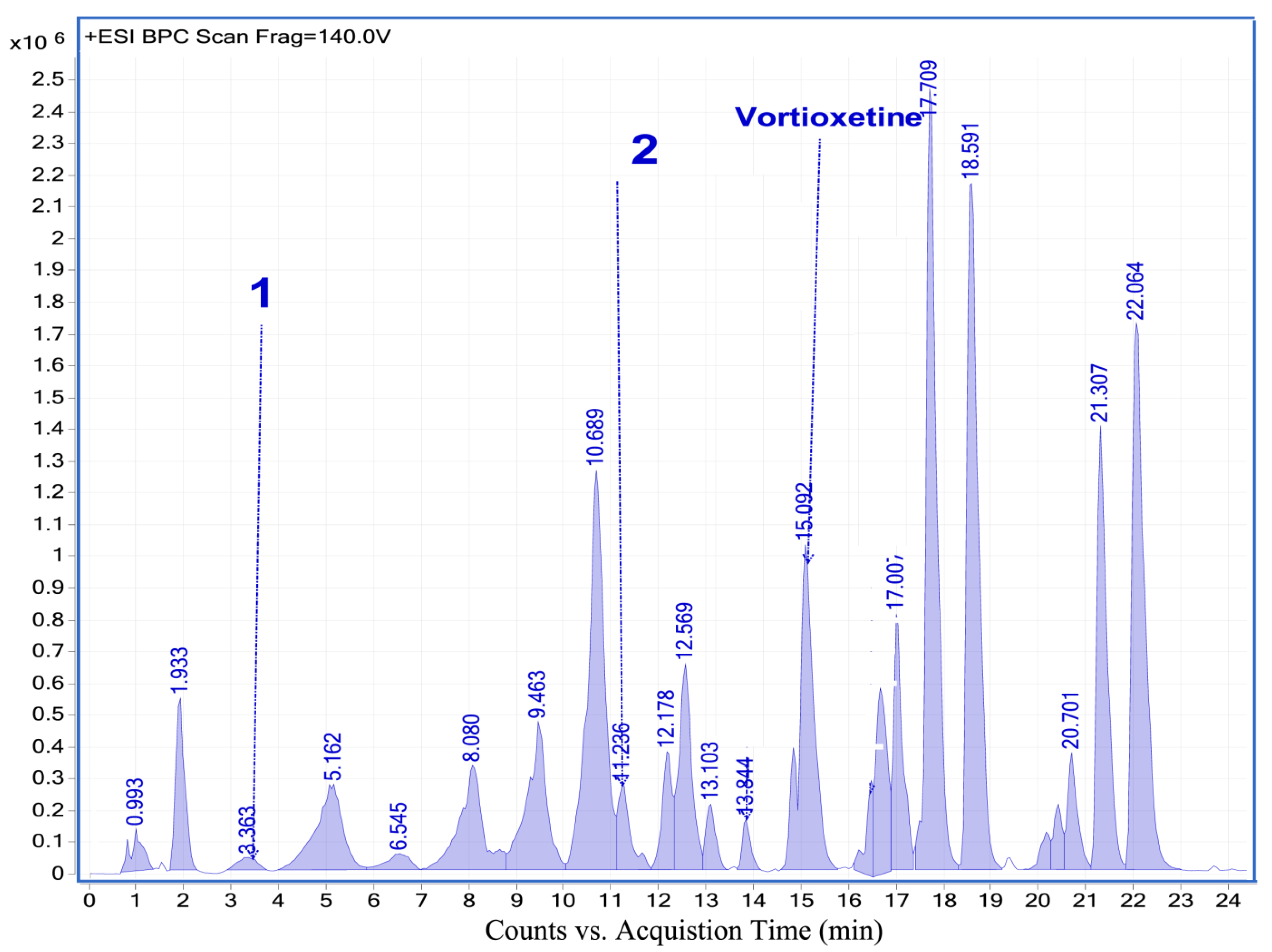
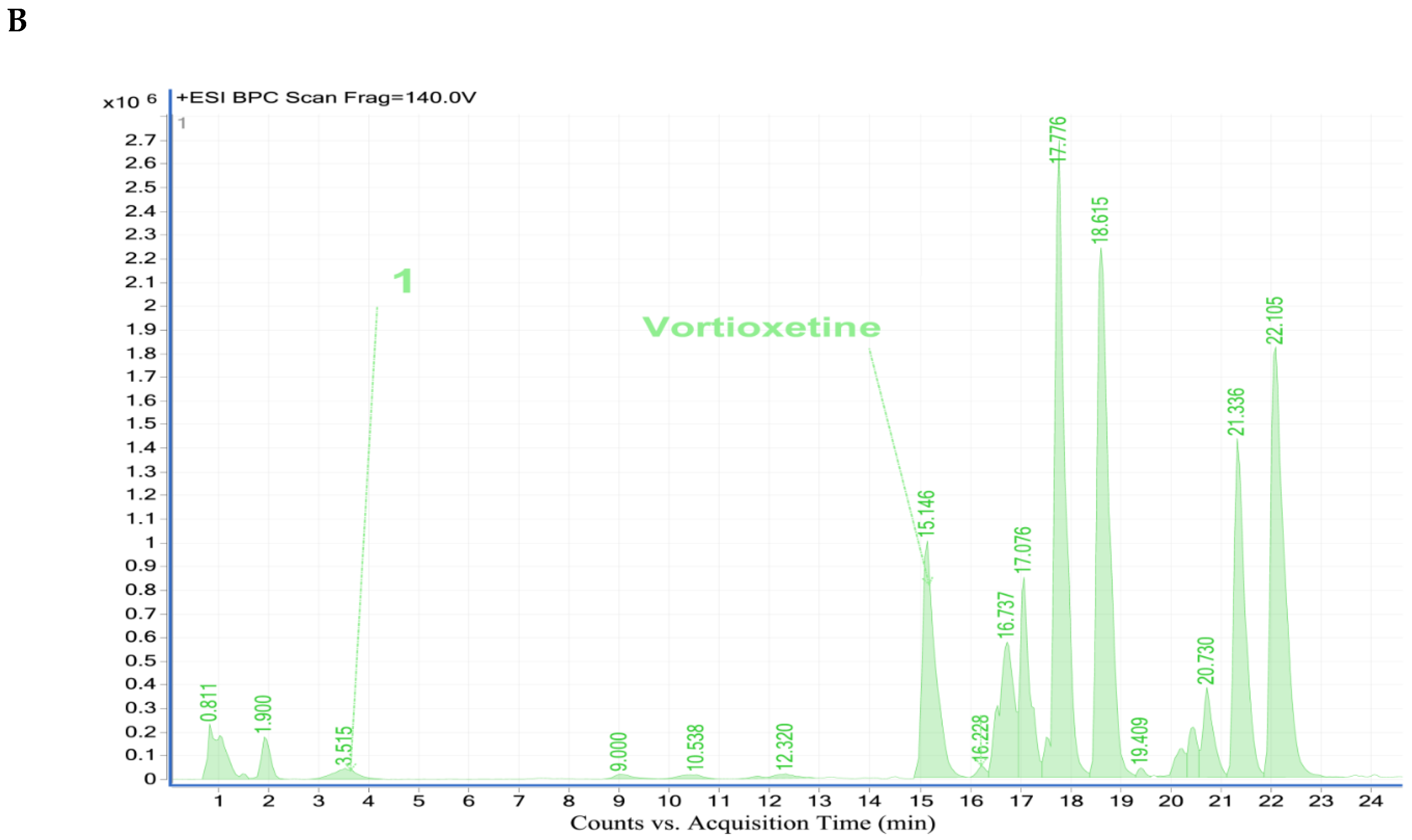
2.4.2. Analysis of Vortioxetine in Serum, Saliva and Urine Samples by LC-QTOF-MS
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Apparatus and LC Conditions
3.2.1. LC-DAD Conditions
3.2.2. LC-QTOF-MS Conditions
3.3. Serum, Saliva, and Urine Samples Collection
3.3.1. Serum
3.3.2. Saliva
3.3.3. Urine
3.4. SPE Procedure for Isolation of Vortioxetine from Serum, Saliva, and Urine
3.4.1. Preparation of Serum Samples
3.4.2. Preparation of Saliva Samples
3.4.3. Preparation of Urine Samples
3.5. Matrix Effects, Process Efficiency, and Extraction Recovery
3.6. Preparation of Stock Solution and Working Solutions
3.7. Log P Calculation
3.7.1. Calculation by Computer Programs
3.7.2. Determination by LC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mørk, A.; Montezinho, L.P.; Miller, S.; Trippodi-Murphy, C.; Plath, N.; Li, Y.; Gulinello, M.; Sanchez, C. Vortioxetine (Lu AA21004), a novel multimodal antidepressant, enhances memory in rats. Pharmacol. Biochem. Behav. 2013, 105, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.S.; Sanchez, C.; Celada, P.; Ar, F. Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: Focus on glutamatergic and GABAergic neurotransmission. Neuropharmacology 2016, 108, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Riga, M.S.; Teruel-Martí, V.; Sanchez, C.; Celada, P.; Artigas, F. Subchronic vortioxetine treatment ebut not escitaloprame enhances pyramidal neuron activity in the rat prefrontal cortex. Neuropharmacology 2017, 113, 148–155. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, V.; Fekete, S.; Clarke, A.; Veuthey, J.-L.; Guillarme, D. Recent advances in chromatography for pharmaceutical analysis. Anal. Chem. 2019, 91, 210–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, S.; Panc, Y.; Li, T.; Xu, Z.-S.; Shao, M.-M. Simultaneous quantification of vortioxetine, carvedilol and its active metabolite 4-hydroxyphenyl carvedilol in rat plasma byUPLC–MS/MS: Application to their pharmacokinetic interaction study. J. Pharm. Biomed. Anal. 2016, 128, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Gu, E.-M.; Huang, C.; Liang, B.; Yuan, L.; Lana, T.; Hu, G.; Zhou, H. An UPLC–MS/MS method for the quantitation of vortioxetine in rat plasma: Application to a pharmacokinetic study. J. Chromatogr. B 2015, 997, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Zou, Y.; Jia, B.; Wu, L.; Yang, Z.; Yuan, F.; Zhang, L. Pharmacokinetic and metabolic studies of Vortioxetine in rats using ultra high performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2018, 41, 4469–4479. [Google Scholar] [CrossRef] [PubMed]
- De Diego, M.; Correa, D.; Mennickent, S.; Godoy, R.; Vergara, C. Determination of vortioxetine and its degradation product in bulk and tablets, by LC-DAD and MS/MS methods. Biomed. Chromatogr. 2018, 32, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, N.; Ma, X.; Xiong, K.; Sun, L.; Zou, Q.; Yao, L. Stability-indicating reversed-phase HPLC method development and characterization of impurities in vortioxetine utilizing LC–MS, IR and NMR. J. Pharm. Biomed. Anal. 2016, 117, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Petruczynik, A.; Buszewski, B.; Szultka-Młyńska, M.; Karakuła-Juchnowicz, H.; Waksmundzka-Hajnos, M. Determination of vortioxetine in human serum and saliva samples by HPLC-DAD and HPLC-MS. Acta Chromatogr. 2017, 29, 325–344. [Google Scholar] [CrossRef]
- Waller, J.A.; Tamm, J.A.; Abdourahman, A.; Pehrson, A.L.; Li, Y.; Cajina, M.; Sánchez, C. Chronic vortioxetine treatment in rodents Modulates gene expression of neurodevelopmental and plasticity markers. Eur. Neuropsychopharm. 2017, 27, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Yan, Z.; Yang, H.A. Sensitive Precolumn Derivatization Method for Determination of Piperazine in Vortioxetine Hydrobromide Using a C8 Column and High-Performance Liquid Chromatography–Mass Spectrometry. Anal. Sci. 2016, 32, 1333–1338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, G.; Lee, R.; Højer, A.-M.; Buchbjerg, J.K.; Serenko, M.; Zhao, Z. Pharmacokinetic Drug Interactions Involving Vortioxetine (Lu AA21004), a Multimodal Antidepressant. Clin. Drug Investig. 2013, 33, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Spieler, D.; Namendorf, C.; Namendorf, T.; Uhr, M. abcb1ab p-glycoprotein is involved in the uptake of the novel antidepressant vortioxetine into the brain of mice. J. Psychiatr. Res. 2019, 109, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Sztanke, K. Retention Data for Some Carbonyl Derivatives of Imidazo[2,1-c][1,2,4]triazine in Reversed-Phase Systems in TLC and HPLC and their Use for Determination of Lipophilicity. Part 1. Lipophilicity of 8-Aryl-3-phenyl-6,7-dihydro-4H-imidazo[2,1-c][1,2,4]triazin-4-ones. JPC-J. Planar Chromatogr. 2005, 18, 274–281. [Google Scholar] [CrossRef]
Sample Availability: Sample of vortioxetine and its metabolites are not available from the authors. |



| Stationary Phase | Mobile Phase | tR | As | N/m |
|---|---|---|---|---|
| HILIC A | 90% ACN and formate buffer pH 3.8 | 4.89 | 1.01 | 394,510 |
| HILIC A | 90% ACN 0.1 M HCOONH4 | 6.26 | 0.91 | 10,290 |
| HILIC B | 90% ACN and formate buffer pH 3.8 | 3.06 | 1.17 | 183,320 |
| HILIC B | 90% ACN 0.1 M HCOONH4 | 3.10 | 1.01 | 88,800 |
| HILIC N | 90% ACN and formate buffer pH 3.8 | 3.49 | 1.08 | 136,170 |
| HILIC N | 90% ACN 0.1 M HCOONH4 | 3.45 | 1.01 | 153,230 |
| SCX | 25% ACN, and formate buffer 2.5 | 3.15 | 1.15 | 15,810 |
| SCX | 25% ACN and formate buffer pH 3.8 | 6.93 | 1.02 | 32,260 |
| Hydro RP | 80% MeOH, acetate buffer at pH 3.6 0.01 M OSNa | 4.74 | 0.77 | 13,170 |
| Hydro RP | 75% MeOH acetate buffer at pH 3.6 0.01 M OSNa | 8.72 | 0.80 | 19,370 |
| Hydro RP | 70% MeOH acetate buffer at H 3.6 0.01 M OSNa | 17.41 | 0.80 | 27,540 |
| Hydro RP | 65% MeOH acetate buffer at pH 3.6 0.01 M OSNa | 39.40 | 0.89 | 36,800 |
| Hydro RP | 60% MeOH acetate buffer at pH 3.6 0.01 M OSNa | 90.92 | 0.95 | 51,910 |
| Polar RP | 80% MeOH acetate buffer at pH 3.6 0.01 M OSNa | 3.66 | 1.12 | 18,890 |
| Polar RP | 75% MeOH acetate buffer at pH 3.6 0.01 M OSNa | 5.00 | 0.96 | 18,060 |
| Polar RP | 70% MeOH acetate buffer at pH 3.6 0.01 M OSNa | 7.34 | 0.92 | 19,190 |
| Polar RP | 65% MeOH acetate buffer at pH 3.6 0.01 M OSNa | 12.59 | 0.91 | 22,290 |
| Polar RP | 60% MeOH acetate buffer at pH 3.6 0.01 M OSNa | 25.80 | 0.94 | 27,890 |
| Polar RP | 55% MeOH acetate buffer at pH 3.6 0.01 M OSNa | 59.32 | 1.01 | 36,550 |
| Chromatographic System | Log kw | |
|---|---|---|
| Column | Mobile Phase | |
| Hydro RP | MeCN + 0.1% HCOOH | 4.06 |
| MeOH + 0.1% HCOOH | 4.73 | |
| MeOH, acetic buffer at pH 3.5 + 0.025 M DEA | 4.00 | |
| MeCN, acetic buffer at pH 3.5 + 0.025 M DEA | 6.52 | |
| MeOH, acetic buffer at pH 3.6 + 0.01 M OSA-Na | 6.10 | |
| Polar RP | MeOH, acetic buffer at pH 3.5 + 0.025 M DEA | 4.25 |
| MeCN, acetic buffer at pH 3.5 + 0.025 M DEA | 4.23 | |
| MeOH b.oct. pH 3.6 0.01 M OSA-Na | 4.65 | |
| Average | 4.82 | |
| Program | Log P |
|---|---|
| miLogP | 4.08 |
| ALOGPS | 4.51 |
| ChemAxon | 4.76 |
| ALogP | 3.86 |
| CLOGP | 4.92 |
| XLogP3-AA | 4.20 |
| cLogP | 4.76 |
| Average | 4.44 |
| Sample | Concentration Added (ng/mL) | Recovery (%) | Extraction Efficiency (%) | Matrix Effect (%) |
|---|---|---|---|---|
| Serum | 40 | 105.26 | 111.05 | 105.51 |
| Urine | 40 | 76.11 | 81.41 | 106.96 |
| Saliva | 40 | 101.93 | 75.62 | 74.19 |
| Compound | Structure |
|---|---|
| Vortioxetine (Lu AA21004) | 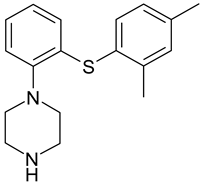 |
| Lu AA34442 (M0) | 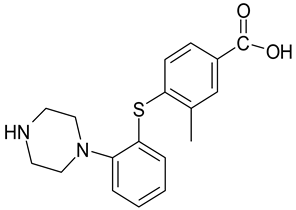 |
| Lu AE22404 | 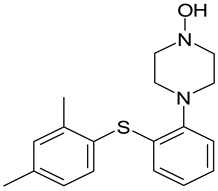 |
| No | Tentatively Assignment | Retention Time [min] | Formula | Molecular Ion [M+H]+ | Fragment Ions | Biological Samples Where Vortioxetine/ Metabolite was Detected | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum | Saliva | Urine | |||||||||||
| 1 h | 2 h | 4 h | 8 h | 10 h | 24 h | ||||||||
| Vortioxetine | 15.09 | C18H23N2S+ | 299.1557 | 150.0341; 109.0068 | + | + | + | + | + | + | + | + | |
| 1 | LU AE22404 | 3.36 | C18H23N2OS+ | 315.1539 | 191.0625; 120.0780 | + | + | + | + | + | + | + | + |
| 2 | LU AA34443 (M0) | 11.24 | C18H23N2O2S+ | 329.1366 | 286.0877; 150.0367; 109.0088 | - | - | - | - | - | - | - | + |
| Column | Functional Group | Lenght (mm) | I.D. (mm) | Endcaped | Particle Size (μm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) |
|---|---|---|---|---|---|---|---|---|
| Synergi Polar RP | Ether-linked phenyl | 150 | 4.6 | Proprietary (polar group) | 4 | 80 | 475 | 11 |
| Synergi Hydro-RP | Octadecyl (C18) | 150 | 4.6 | Proprietary (polar group) | 4 | 80 | 475 | 19 |
| ACE HILIC-A | Proprietary SIL | 150 | 4.6 | NO | 5 | 100 | 300 | - |
| ACE HILIC-B | Proprietary Aminopropyl | 150 | 4.6 | NO | 5 | 100 | 300 | 4 |
| ACE HILIC-C | Proprietary Polyhydroxy | 150 | 4.6 | NO | 5 | 100 | 300 | 7 |
| Luna SCX | Benzene Sulfonic Acid | 150 | 4.6 | NO | 5 | 100 | 400 | 0.55 Sulfur Load |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruczynik, A.; Wróblewski, K.; Wojtanowski, K.; Mroczek, T.; Juchnowicz, D.; Karakuła-Juchnowicz, H.; Tuzimski, T. Comparison of Various Chromatographic Systems for Identification of Vortioxetine in Bulk Drug Substance, Human Serum, Saliva, and Urine Samples by HPLC-DAD and LC-QTOF-MS. Molecules 2020, 25, 2483. https://doi.org/10.3390/molecules25112483
Petruczynik A, Wróblewski K, Wojtanowski K, Mroczek T, Juchnowicz D, Karakuła-Juchnowicz H, Tuzimski T. Comparison of Various Chromatographic Systems for Identification of Vortioxetine in Bulk Drug Substance, Human Serum, Saliva, and Urine Samples by HPLC-DAD and LC-QTOF-MS. Molecules. 2020; 25(11):2483. https://doi.org/10.3390/molecules25112483
Chicago/Turabian StylePetruczynik, Anna, Karol Wróblewski, Krzysztof Wojtanowski, Tomasz Mroczek, Dariusz Juchnowicz, Hanna Karakuła-Juchnowicz, and Tomasz Tuzimski. 2020. "Comparison of Various Chromatographic Systems for Identification of Vortioxetine in Bulk Drug Substance, Human Serum, Saliva, and Urine Samples by HPLC-DAD and LC-QTOF-MS" Molecules 25, no. 11: 2483. https://doi.org/10.3390/molecules25112483
APA StylePetruczynik, A., Wróblewski, K., Wojtanowski, K., Mroczek, T., Juchnowicz, D., Karakuła-Juchnowicz, H., & Tuzimski, T. (2020). Comparison of Various Chromatographic Systems for Identification of Vortioxetine in Bulk Drug Substance, Human Serum, Saliva, and Urine Samples by HPLC-DAD and LC-QTOF-MS. Molecules, 25(11), 2483. https://doi.org/10.3390/molecules25112483










