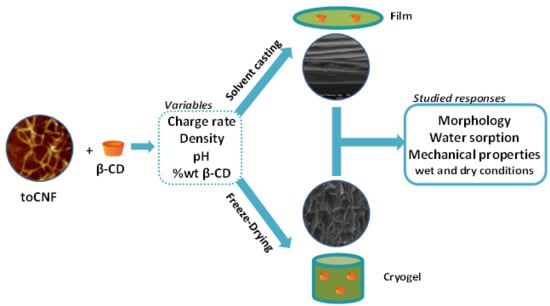
Production and Mechanical Characterisation of TEMPO-Oxidised Cellulose Nanofibrils/β-Cyclodextrin Films and Cryogels
Abstract
1. Introduction
2. Results and Discussion
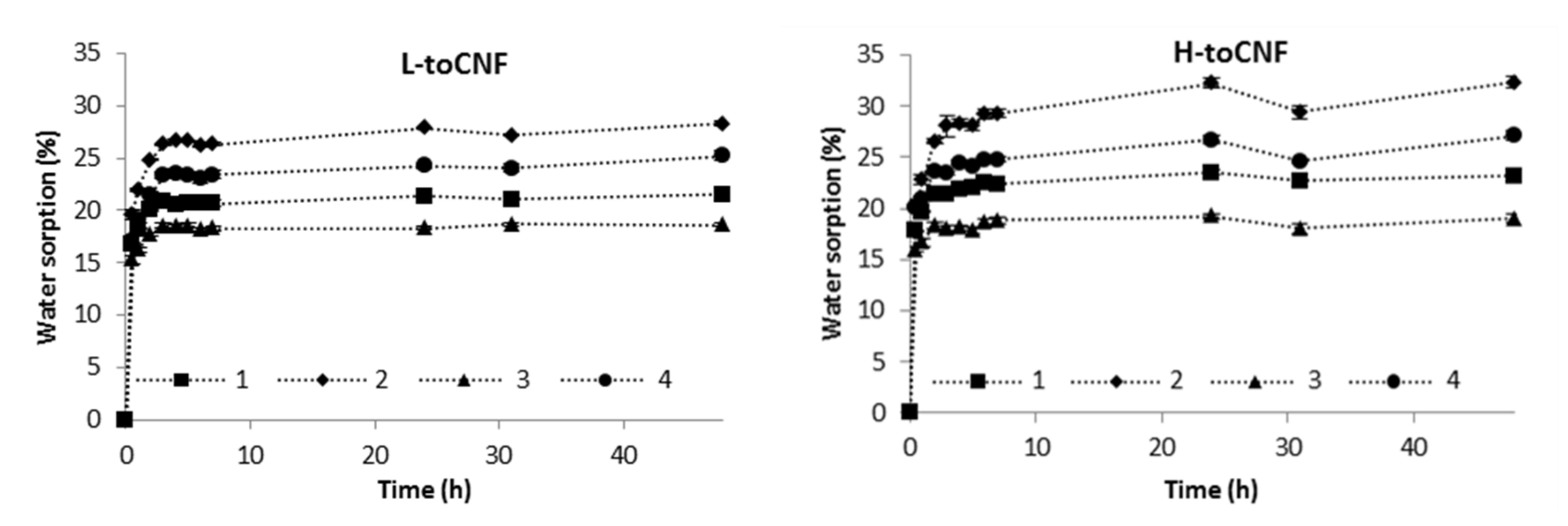
2.1. Water Sorption Analysis
2.1.1. Films
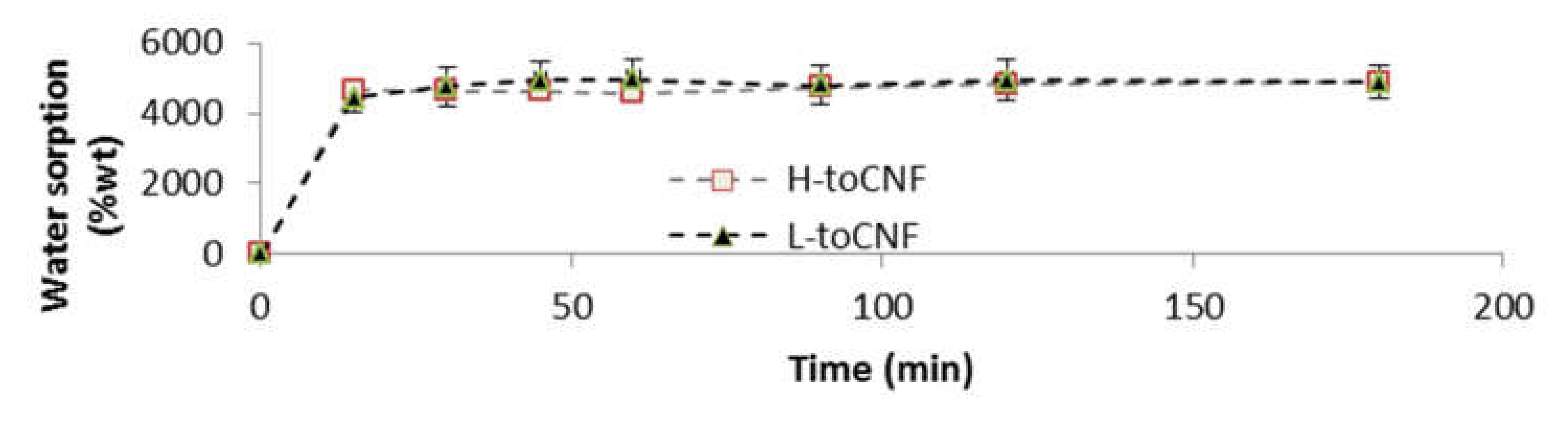
2.1.2. Cryogels
2.2. Mechanical Characterisation
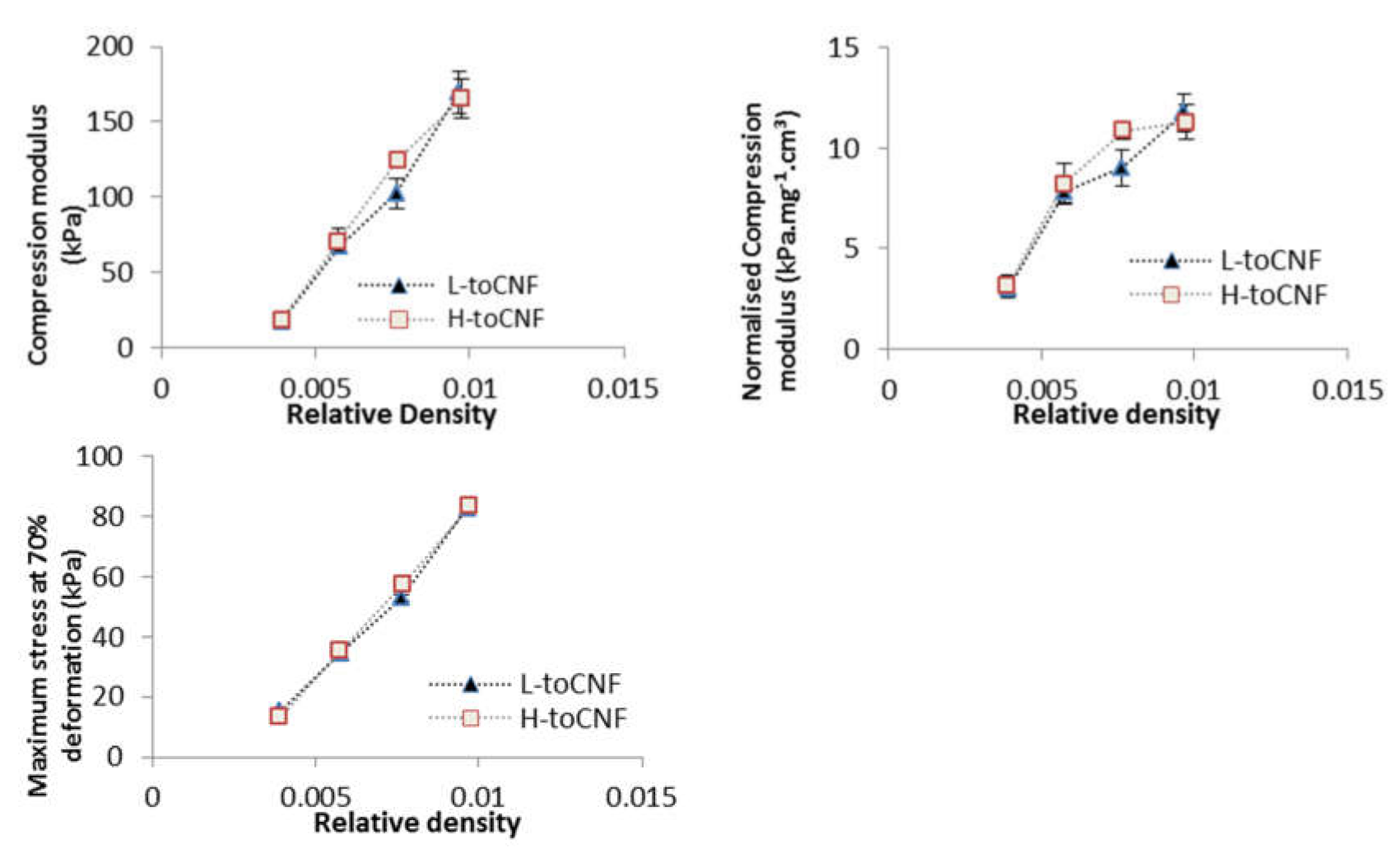
2.2.1. Impact of Density
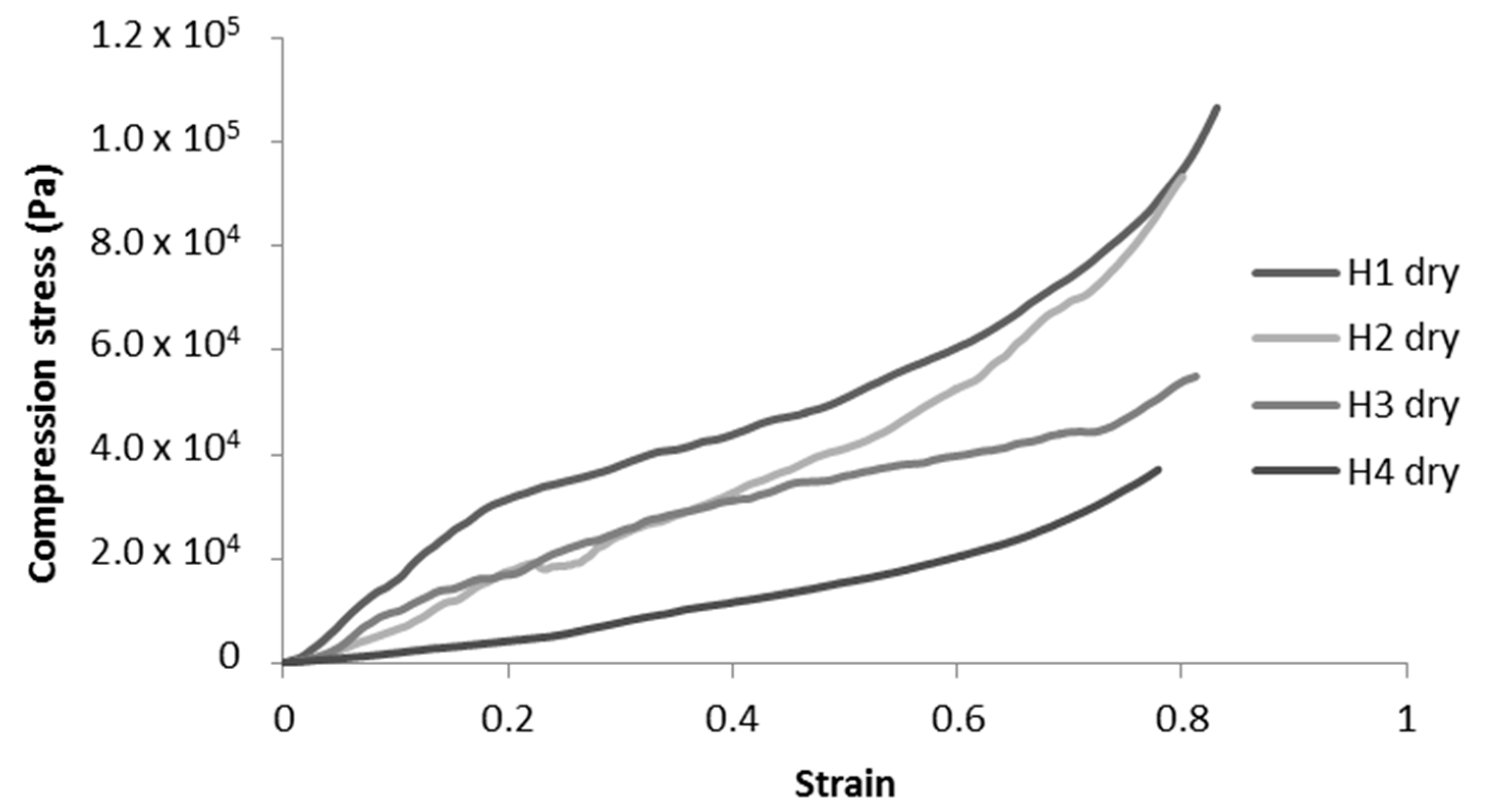
2.2.2. Impact of pH and Cyclodextrins on Dry Cryogels
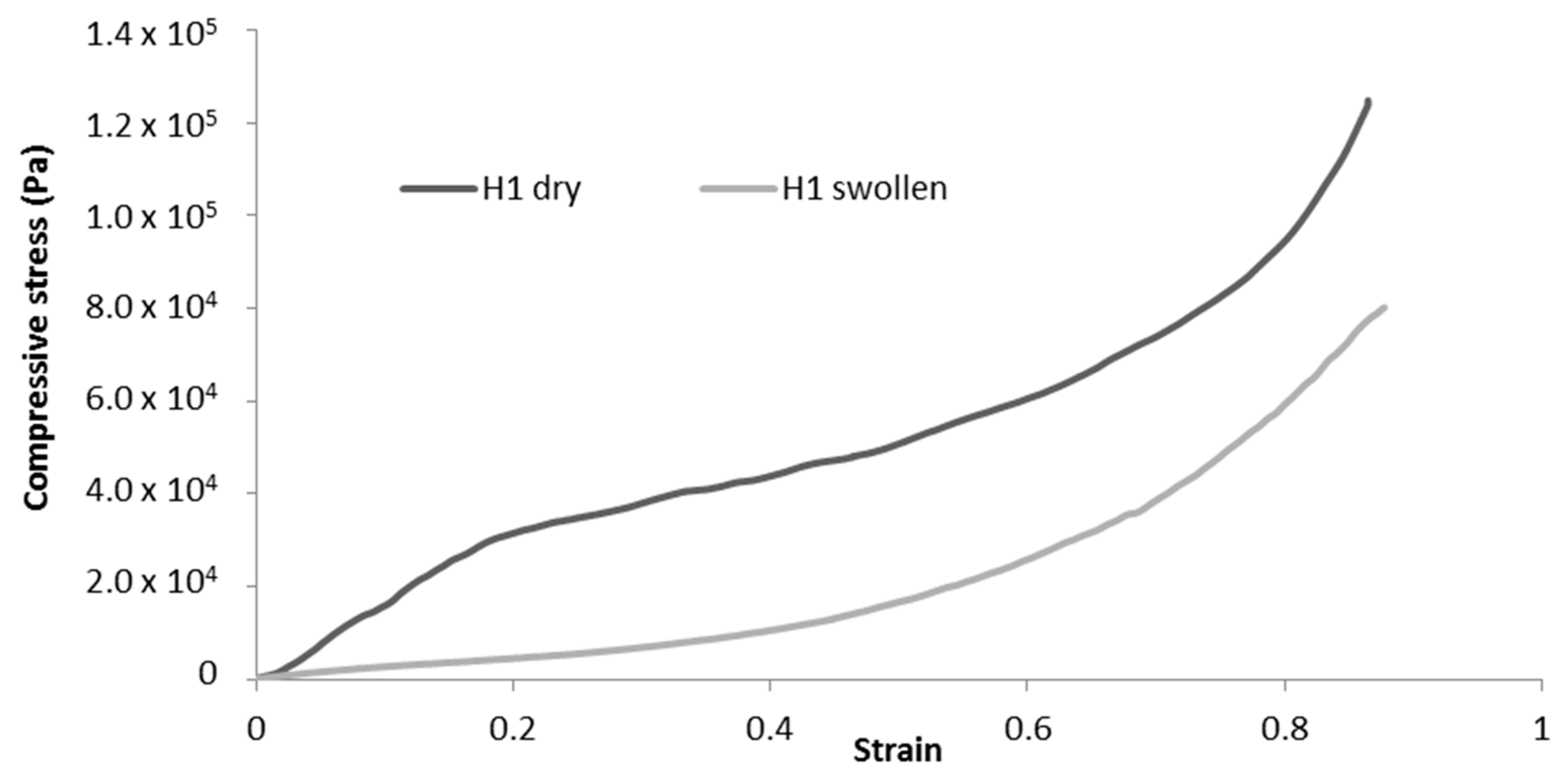
2.2.3. Difference Between Dry and Swollen Cryogels
3. Materials and Methods
3.1. Materials
3.2. Preparation of toCNF With Two Different Charge Contents
3.3. Determination of the Charge Content
3.4. Material Processing
3.4.1. Film Processing
3.4.2. Cryogel Processing
3.5. Water Sorption Analysis
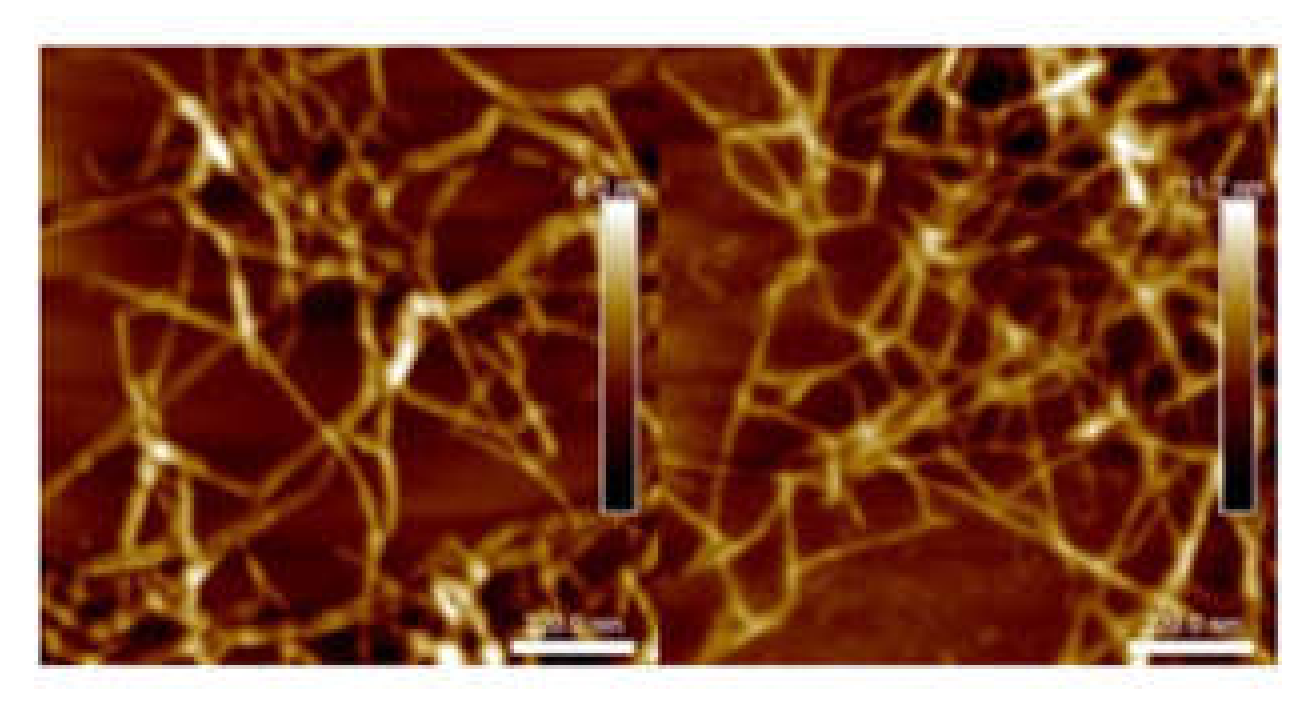
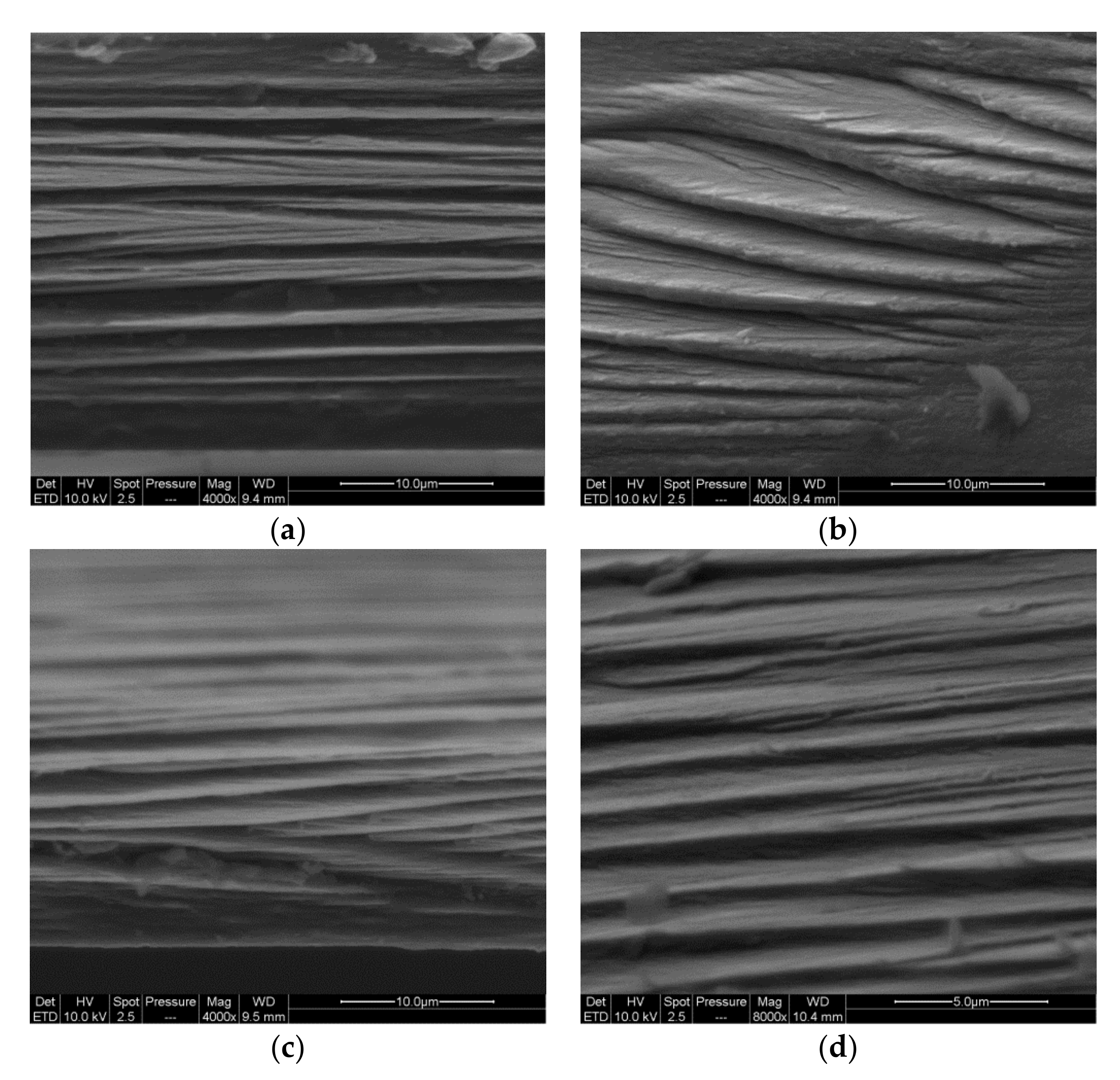
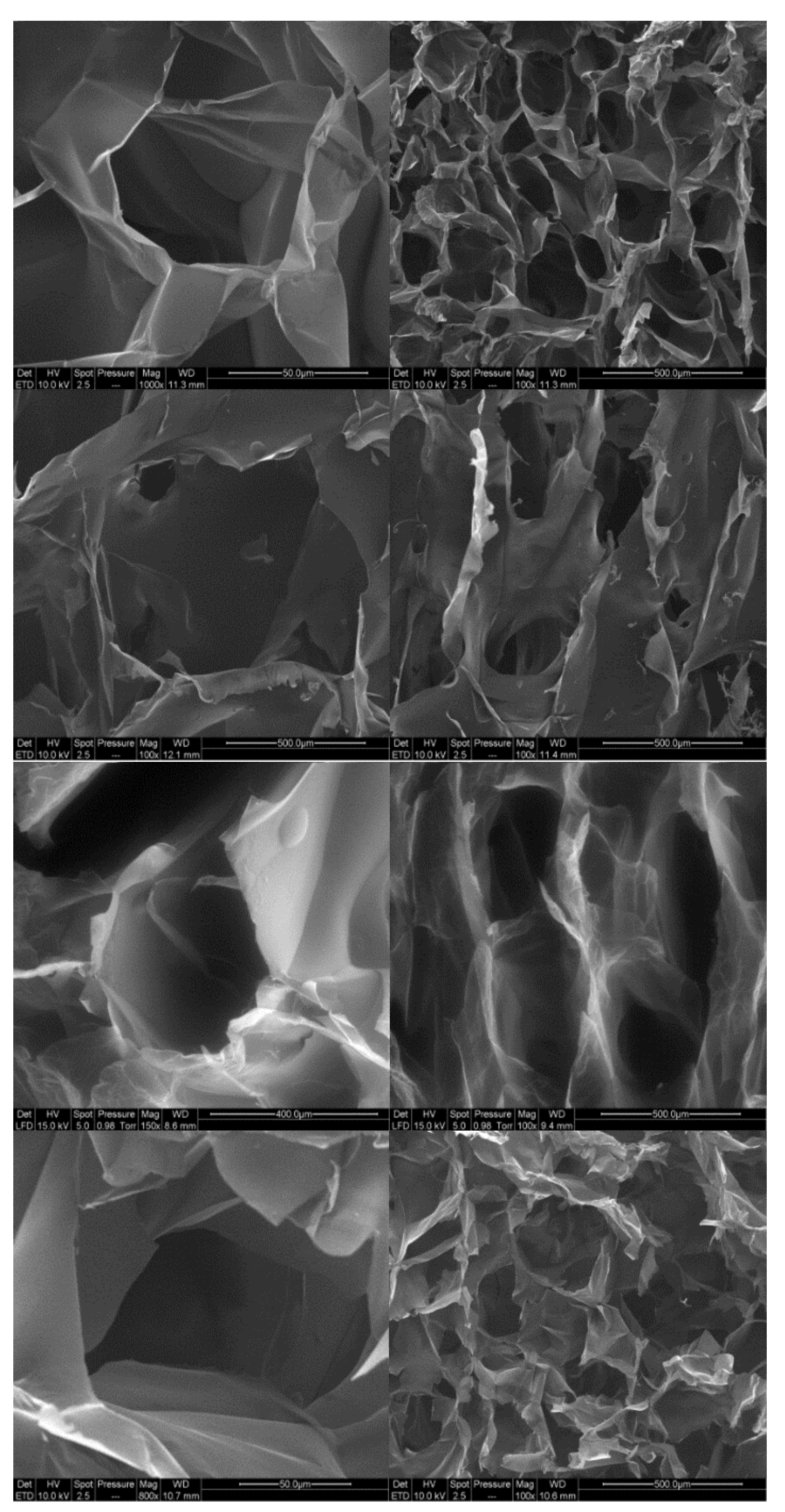
3.6. Microscopy
3.7. Mechanical Characterisation
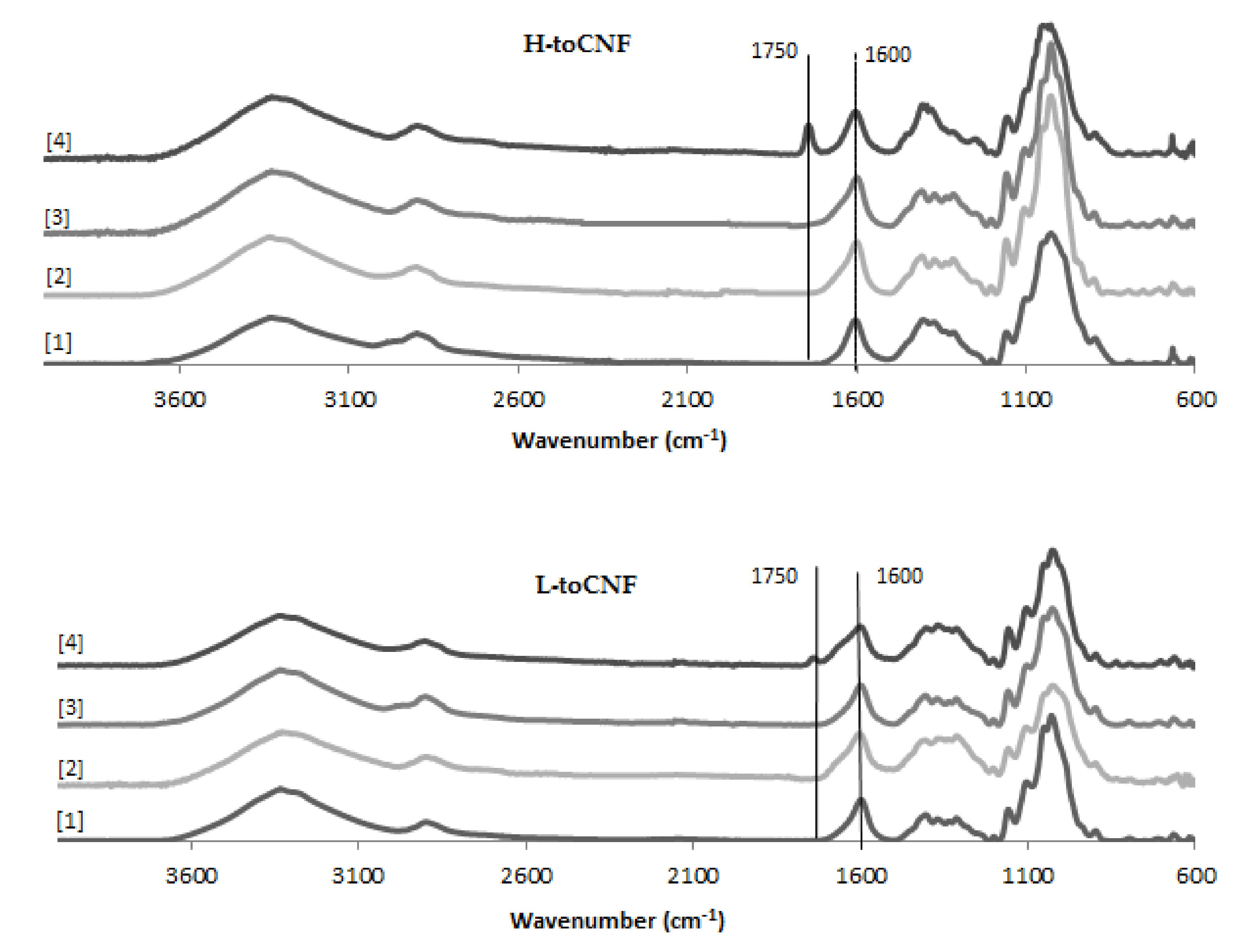
3.8. Fourier Transform Infrared Spectroscopy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turbak, A.F.; Snyder, F.W. Microfibrillated Cellulose; Patent and Trademark Office: Washington, DC, USA, 1983. [Google Scholar]
- Taipale, T.; Österberg, A.; Nykänen, M.; Ruokolainen, J.; Laine, J. Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength. Cellulose 2010, 17, 1005–1020. [Google Scholar] [CrossRef]
- Josset, S.; Orsolini, P.; Siqueira, G.; Tejado, A.; Tingaut, P.; Zimmermann, T. Energy consumption of the nanofibrillation of bleached pulp, wheat straw and recycled newspaper through a grinding process. Nord. Pulp Pap. Res. J. 2014, 29, 167–175. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2018. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, C.; Pal, L.; Rojas, O.J. Nanocellulose in thin films, coatings, and plies for packaging applications: A Review. BioResources 2017, 12, 2143–2233. [Google Scholar] [CrossRef]
- Kolakovic, R.; Peltonen, L.; Laukkanen, A.; Hirvonen, J.; Laaksonen, T. Nanofibrillar cellulose films for controlled drug delivery. Eur. J. Pharm. Biopharm. 2012, 82, 308–315. [Google Scholar] [CrossRef]
- Torstensen, J.Ø.; Liu, M.; Jin, S.-A.; Deng, L.; Hawari, A.I.; Syverud, K.; Spontak, R.J.; Gregersen, Ø.W. Swelling and free-volume characteristics of TEMPO-oxidized cellulose nanofibril films. Biomacromolecules 2018, 19, 1016–1025. [Google Scholar] [CrossRef]
- Kontturi, K.S.; Biegaj, K.; Mautner, A.; Woodward, R.T.; Wilson, B.P.; Johansson, L.-S.; Lee, K.-Y.; Heng-Orcid, J.Y.Y.; Bismarck, A.; Kontturi, E. Noncovalent surface modification of cellulose nanopapers by adsorption of polymers from aprotic solvents. Langmuir 2017, 33, 5707–5712. [Google Scholar] [CrossRef]
- Orsolini, P.; Michen, B.; Huch, A.; Tingaut, P.; Caseri, W.R.; Zimmermann, T. Characterization of pores in dense nanopapers and nanofibrillated cellulose membranes: A critical assessment of established methods. ACS Appl. Mater. Interfaces 2015, 7, 25884–25897. [Google Scholar] [CrossRef] [PubMed]
- Darpentigny, C.; Nonglaton, G.; Bras, J.; Jean, B. Highly absorbent cellulose nanofibrils aerogels prepared by supercritical drying. Carbohydr. Polym. 2020, 229, 115560. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Buchtová, N.; Pradille, C.; Budtova, J.-L. Mechanical properties of cellulose aerogels and cryogels. Soft Matter 2019, 15, 7901–7908. [Google Scholar] [CrossRef] [PubMed]
- Darpentigny, C.; Molina-Boisseau, S.; Nonglaton, G.; Bras, J.; Jean, B. Ice-templated freeze-dried cryogels from tunicate cellulose nanocrystals with high specific surface area and anisotropic morphological and mechanical properties. Cellulose 2020, 27, 233–247. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergström, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- Gupta, S.; Martoïa, F.; Orgéas, L.; Dumont, P. Ice-templated porous nanocellulose-based materials: Current progress and opportunities for materials engineering. Appl. Sci. 2018, 8, 2463. [Google Scholar] [CrossRef]
- Martoïa, F.; Cochereau, T.; Dumont, P.J.J.; Orgéas, L.; Terrien, M.; Belgacem, M.N. Cellulose nanofibril foams: Links between ice-templating conditions, microstructures and mechanical properties. Mater. Des. 2016, 104, 376–391. [Google Scholar] [CrossRef]
- Rees, A.; Powell, L.C.; Chinga-Carrasco, G.; Gethin, D.T.; Syverud, K.; Hill, K.E.; Thomas, D.W. 3D Bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. Biomed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Hakkarainen, T.; Koivuniemi, R.; Kosonen, M.; Escobedo-Lucea, C.; Sanz-Garcia, A.; Vuola, J.; Valtonen, J.; Tammela, P.; Mäkitie, A.; Luukko, K.; et al. Nanofibrillar cellulose wound dressing in skin graft donor site treatment. J. Control. Release 2016, 244, 292–301. [Google Scholar] [CrossRef]
- Campodoni, E.; Heggset, E.B.; Rashad, A.; Ramírez-Rodríguez, G.B.; Mustafa, K.; Syverud, K.; Tampieri, A.; Sandri, M. Polymeric 3D scaffolds for tissue regeneration: Evaluation of biopolymer nanocomposite reinforced with cellulose nanofibrils. Mater. Sci. Eng. C 2019, 94, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Basu, A.; Celma, G.; Strømme, M.; Ferraz, N. In vitro and in vivo evaluation of the wound healing properties of nanofibrillated cellulose hydrogels. ACS Appl. Bio Mater. 2018, 1, 1853–1863. [Google Scholar] [CrossRef]
- Fiorati, A.; Contessi-Negrini, N.; Baschenis, E.; Altomare, L.; Faré, S.; Giacometti-Schieroni, A.; Piovani, D.; Mendichi, R.; Ferro, M.; Castiglione, F.; et al. TEMPO-nanocellulose/Ca2+ hydrogels: Ibuprofen drug diffusion and in vitro cytocompatibility. Materials 2020, 13, 183. [Google Scholar] [CrossRef]
- Rashad, A.; Mustafa, K.; Heggset, E.B.; Syverud, K. Cytocompatibility of wood-derived cellulose nanofibril hydrogels with different surface chemistry. Biomacromolecules 2017, 18, 1238–1248. [Google Scholar] [CrossRef]
- Syverud, K.; Kirsebom, H.; Hajizadeh, S.; Chinga-Carrasco, G. Cross-linking cellulose nanofibrils for potential elastic cryo-structured gels. Nanoscale Res. Lett. 2011, 6, 626. [Google Scholar] [CrossRef]
- Syverud, K.; Pettersen, S.R.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels—Scaffolds with potential in tissue engineering. Cellulose 2015, 22, 473–481. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins: Basic science and product development: Pharmaceutical applications of cyclodextrins. J. Pharm. Pharmacol. 2010, 62, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fourmentin, M.; Morin-Crini, N. Principales Applications des Complexes d’Inclusion Cyclodextrine/Substrat; French Society of Chemistry: Paris, France, 2019; p. 21. [Google Scholar]
- Morin-Crini, N.; Winterton, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, É.; Crini, G. Water-insoluble β-cyclodextrin–epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polym. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]
- Otero-Espinar, F.J.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C.; Blanco-Méndez, J. Cyclodextrins in drug delivery systems. J. Drug Deliv. Sci. Technol. 2010, 20, 289–301. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef]
- Cusola, O.; Tabary, N.; Belgacem, M.N.; Bras, J. Cyclodextrin functionalization of several cellulosic substrates for prolonged release of antibacterial agents. J. Appl. Polym. Sci. 2013, 129, 604–613. [Google Scholar] [CrossRef]
- Lukášek, J.; Hauzerová, Š.; Havlíčková, K.; Strnadová, K.; Mašek, K.; Stuchlík, M.; Stibor, I.; Jenčová, V.; Řezanka, M. Cyclodextrin-polypyrrole coatings of scaffolds for tissue engineering. Polymers 2019, 11, 459. [Google Scholar] [CrossRef]
- Venuti, V.; Venuti, V.; Rossi, B.; Mele, A.; Melone, L.; Punta, C.; Majolino, D.; Masciovecchio, C.; Caldera, F.; Trotta, F. Tuning structural parameters for the optimization of drug delivery performance of cyclodextrin-based nanosponges. Expert Opin. Drug Deliv. 2017, 14, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; García-González, C.A.; Concheiro, A. Cyclodextrins as versatile building blocks for regenerative medicine. J. Control. Release 2017, 268, 269–281. [Google Scholar] [CrossRef]
- Grier, W.K.; Tiffany, A.S.; Ramsey, M.D.; Harley, B.A.C. Incorporating β-cyclodextrin into collagen scaffolds to sequester growth factors and modulate mesenchymal stem cell activity. Acta Biomaterialia 2018, 76, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Nada, A.A.; Abdellatif, F.H.H.; Ali, E.A.; Abdelazeem, R.A.; Soliman, A.A.S.; Abou-Zeid, N.Y. Cellulose-based click-scaffolds: Synthesis, characterization and biofabrications. Carbohydr. Polym. 2018, 199, 610–618. [Google Scholar] [CrossRef]
- Kim, K.O.; Kim, G.J.; Kim, J.H. A cellulose/β-cyclodextrin nanofiber patch as a wearable epidermal glucose sensor. RSC Adv. 2019, 9, 22790–22794. [Google Scholar] [CrossRef]
- Saini, S.; Quinot, D.; Lavoine, N.; Belgacem, M.N.; Bras, J. β-Cyclodextrin-grafted TEMPO-oxidized cellulose nanofibers for sustained release of essential oil. J. Mater. Sci. 2017, 52, 3849–3861. [Google Scholar] [CrossRef]
- Yuan, G.; Prabakaranb, M.; Sunc, Q.; Jung, L.; Chung, S.; Mayakrishnan, I.-M.; Song, G.; Kim, K.-H.; Soo, I. Cyclodextrin functionalized cellulose nanofiber composites for the faster adsorption of toluene from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 70, 352–358. [Google Scholar] [CrossRef]
- Ruiz-Palomero, C.; Soriano, M.L.; Valcárcel, M. β-Cyclodextrin decorated nanocellulose: A smart approach towards the selective fluorimetric determination of danofloxacin in milk samples. Analyst 2015, 140, 3431–3438. [Google Scholar] [CrossRef]
- Aytac, Z.; Sen, H.S.; Durgun, E.; Uyar, T. Sulfisoxazole/cyclodextrin inclusion complex incorporated in electrospun hydroxypropyl cellulose nanofibers as drug delivery system. Colloids Surf. B Biointerfaces 2015, 128, 331–338. [Google Scholar] [CrossRef]
- Lavoine, N.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Controlled release of chlorhexidine digluconate using β-cyclodextrin and microfibrillated cellulose. Colloids Surf. B Biointerfaces 2014, 121, 196–205. [Google Scholar] [CrossRef]
- Lavoine, N.; Givord, C.; Tabary, N.; Desloges, I.; Martel, B.; Bras, J. Elaboration of a new antibacterial bio-nano-material for food-packaging by synergistic action of cyclodextrin and microfibrillated cellulose. Innov. Food Sci. Emerg. Technol. 2014, 26, 330–340. [Google Scholar] [CrossRef]
- De Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Controlled release of carvacrol and curcumin: Bio-based food packaging by synergism action of TEMPO-oxidized cellulose nanocrystals and cyclodextrin. Cellulose 2018, 25, 1249–1263. [Google Scholar] [CrossRef]
- Jimenez, A.; Jaramillo, F.; Hemraz, U.; Boluk, Y.; Ckless, K.; Sunasee, R. Effect of surface organic coatings of cellulose nanocrystals on the viability of mammalian cell line. Nanotechnol. Sci. Appl. 2017, 10, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.O.; Tabary, N.; Martel, B.; Gandini, A.; Belgacem, N.; Bras, J. Effect of different carboxylic acids in cyclodextrin functionalization of cellulose nanocrystals for prolonged release of carvacrol. Mater. Sci. Eng. C 2016, 69, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Ndong-Ntoutoume, G.M.A.; Graneta, R.; Pierre, J.; Frédérique, M.; Légera, D.Y.; Fidanzi-Dugasa, C.; Lequartb, V.; Jolyb, N.; Liagrea, V.; Chaleixa, B.; et al. Development of curcumin–cyclodextrin/cellulose nanocrystals complexes: New anticancer drug delivery systems. Bioorganic Med. Chem. Lett. 2016, 26, 941–945. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Supramolecular hydrogels from in situ host–guest inclusion between chemically modified cellulose nanocrystals and cyclodextrin. Biomacromolecules 2013, 14, 871–880. [Google Scholar] [CrossRef]
- Okubayashi, S.; Griesser, U.J.; Bechtold, T. A kinetic study of moisture sorption and desorption on lyocell fibers. Carbohydr. Polym. 2004, 58, 293–299. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: From Nature to High Performance Tailored Materials; De Gruyter: Berlin, Germany, 2012. [Google Scholar]
- Belbekhouche, S.; Bras, J.; Siqueira, G.; Chappey, C.; Lebrun, L.; Khelifi, B.; Marais, S.; Dufresne, A. Water sorption behavior and gas barrier properties of cellulose whiskers and microfibrils films. Carbohydr. Polym. 2011, 83, 1740–1748. [Google Scholar] [CrossRef]
- Gibson, L.A.; Ashby, M. Cellular Solids, Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Mills, N. Polymer Foams Handbook: Engineering and Biomechanics Applications and Design Guide; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Goel, A.; Nene, S.N. Modifications in the phenolphthalein method for spectrophotometric estimation of beta cyclodextrin. Starch Stärke 1995, 47, 399–400. [Google Scholar] [CrossRef]
- Orelma, H.; Filpponen, I.; Johansson, L.-S.; Österberg, M.; Rojas, O.J.; Laine, J. Surface functionalized nanofibrillar cellulose (NFC) film as a platform for immunoassays and diagnostics. Biointerphases 2012, 7, 61. [Google Scholar] [CrossRef]
- Heggset, E.B.; Strand, B.L.; Sundby, K.W.; Simon, S.; Chinga-Carrasco, G.; Syverud, K. Viscoelastic properties of nanocellulose based inks for 3D printing and mechanical properties of CNF/alginate biocomposite gels. Cellulose 2018. [Google Scholar] [CrossRef]
- Weishaupt, R.; Siqueira, G.; Schubert, M.; Tingaut, P.; Maniura-Weber, K.; Zimmermann, T.; Thöny-Meyer, L.; Faccio, G.; Ihssen, J. TEMPO-oxidized nanofibrillated cellulose as a high density carrier for bioactive molecules. Biomacromolecules 2015, 16, 3640–3650. [Google Scholar] [CrossRef] [PubMed]

| Nanocellulose | CD | Functionalisation Strategy | Application | Source |
|---|---|---|---|---|
| toCNFs | βCD | Direct grafting | Release of essential oil | [48] |
| CNFs | βCD | Cross-linking with citric acid | Depollution | [49] |
| toCNFs | CMβCD | Amidation via EDC/NHS | Depollution | [50] |
| HP-CNFs | HPβCD | Electrospinning | Drug release | [51] |
| CNFs | βCD | Coating/Adsorption | Drug release | [52] |
| CNFs | βCD | Cross-linking with citric acid | Antibacterial packaging | [53] |
| toCNF | βCD | Noncovalent interaction | Drug Delivery/ Tissue Engineering | [29] |
| toCNCs | βCD/HPβCD | Direct grafting | Release of essential oil | [54] |
| CNCs | βCD | Grafting with epichlorohydrin | Tissue engineering | [55] |
| CNCs | βCD | Crosslinking with fumaric and succinic acid | Release of essential oil | [56] |
| CNCs | βCD | Ionic interaction | Drug delivery | [57] |
| CNCs | βCD | Grafting with epichlorohydrin | Supramolecular hydrogels | [58] |
| Sample Code | βCD | pH |
|---|---|---|
| 1 | 0 | Neutral |
| 2 | Acidic | |
| 3 | 10 wt% | Neutral |
| 4 | Acidic |
| Sample | Water Sorption Equilibrium after 48 h (wt%) | % of Sorption after 30 min | ||
|---|---|---|---|---|
| L-toCNF | H-toCNF | L-toCNF | H-toCNF | |
| 1 | 21.5 ± 0.2 | 23.2 ± 0.2 | 77.6 ± 0.2 | 76.7 ± 1.2 |
| 2 | 28.3 ± 0.3 | 32.3 ± 0.6 | 69.3 ± 2.0 | 62.9 ± 2.1 |
| 3 | 18.6 ± 0.1 | 19.0 ± 0.4 | 82.0 ± 1.5 | 83.9 ± 0.9 |
| 4 | 25.2 ± 0.5 | 27.1 ± 0.5 | 67.9 ± 2.0 | 74.1 ± 0.5 |
| Initial Dry Content | Porosity (%) | Compression Modulus (kPa) | Normalised Compression Modulus (kPa·mg−1·cm3) | Maximum Stress at 70% Deformation (kPa) | ||||
|---|---|---|---|---|---|---|---|---|
| L-toCNF | H-toCNF | L-toCNF | H-toCNF | L-toCNF | H-toCNF | L-toCNF | H-toCNF | |
| 0.4 wt% | 99.6 | 99.6 | 17 ± 2 | 18 ± 3 | 3.0 ± 0.3 | 3.1 ± 0.6 | 15 ± 1 | 13 ± 1 |
| 0.6 wt% | 99.4 | 99.4 | 68 ± 5 | 71 ± 9 | 7.8 ± 0.6 | 8.2 ± 1.0 | 35 ± 1 | 35 ± 1 |
| 0.8 wt% | 99.2 | 99.2 | 102 ± 10 | 125 ± 4 | 9.0 ± 0.9 | 10.9 ± 0.4 | 53 ± 1 | 57 ± 2 |
| 1 wt% | 99.0 | 99.0 | 170 ± 14 | 165 ± 13 | 11.8 ± 0.9 | 11.3 ± 0.9 | 83 ± 1 | 84 ± 1 |
| Cryogel Density (mg/cm3) | Compression Modulus (kPa) | Normalised Compression Modulus (kPa·mg−1·cm3) | Maximum Stress at 70% Deformation (kPa) | |||||
|---|---|---|---|---|---|---|---|---|
| L-toCNF | H-toCNF | L-toCNF | H-toCNF | L-toCNF | H-toCNF | L-toCNF | H-toCNF | |
| 1 | 14.89 ± 0.47 | 16.07 ± 0.57 | 104 ± 13 | 150 ± 10 | 7.0 ± 0.8 | 9.4 ± 0.8 | 64 ± 3 | 65 ± 3 |
| 2 | 25.32 ± 0.84 | 20.82 ± 1.08 | 34 ± 6 | 76 ± 6 | 1.4 ± 0.2 | 3.7 ± 0.3 | 58 ± 7 | 63 ± 3 |
| 3 | 17.91 ± 0.60 | 18.45 ± 1.66 | 59 ± 10 | 84 ± 13 | 3.3 ± 0.5 | 4.7 ± 0.3 | 42 ± 2 | 43 ± 2 |
| 4 | 20.85 ± 0.96 | 26.41 ± 2.19 | 37 ± 3 | 17 ± 3 | 1.8 ± 0.2 | 0.6 ± 0.1 | 59 ± 5 | 24 ± 4 |
| Compression Modulus (kPa) | Diminution of Compression Modulus (%) | Diminution of Maximum Stress at 70% Deformation (%) | ||||
|---|---|---|---|---|---|---|
| Casting Conditions | L-toCNF | H-toCNF | L-toCNF | H-toCNF | L-toCNF | H-toCNF |
| 1 | 45 ± 4 | 33 ± 4 | −57 % | −78% | −4% | −54% |
| 2 | 9 ± 1 | 8 ± 1 | −75 % | −90% | −82% | −90% |
| 3 | 6 ± 1 | 7 ± 1 | −89% | −92% | −90% | −89% |
| 4 | 9 ± 1 | 9 ± 4 | −77% | −49% | −82% | −73% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michel, B.; Bras, J.; Dufresne, A.; Heggset, E.B.; Syverud, K. Production and Mechanical Characterisation of TEMPO-Oxidised Cellulose Nanofibrils/β-Cyclodextrin Films and Cryogels. Molecules 2020, 25, 2381. https://doi.org/10.3390/molecules25102381
Michel B, Bras J, Dufresne A, Heggset EB, Syverud K. Production and Mechanical Characterisation of TEMPO-Oxidised Cellulose Nanofibrils/β-Cyclodextrin Films and Cryogels. Molecules. 2020; 25(10):2381. https://doi.org/10.3390/molecules25102381
Chicago/Turabian StyleMichel, Bastien, Julien Bras, Alain Dufresne, Ellinor B. Heggset, and Kristin Syverud. 2020. "Production and Mechanical Characterisation of TEMPO-Oxidised Cellulose Nanofibrils/β-Cyclodextrin Films and Cryogels" Molecules 25, no. 10: 2381. https://doi.org/10.3390/molecules25102381
APA StyleMichel, B., Bras, J., Dufresne, A., Heggset, E. B., & Syverud, K. (2020). Production and Mechanical Characterisation of TEMPO-Oxidised Cellulose Nanofibrils/β-Cyclodextrin Films and Cryogels. Molecules, 25(10), 2381. https://doi.org/10.3390/molecules25102381