NMR-Based Chemical Profiling, Isolation and Evaluation of the Cytotoxic Potential of the Diterpenoid Siderol from Cultivated Sideritis euboea Heldr.
Abstract
1. Introduction
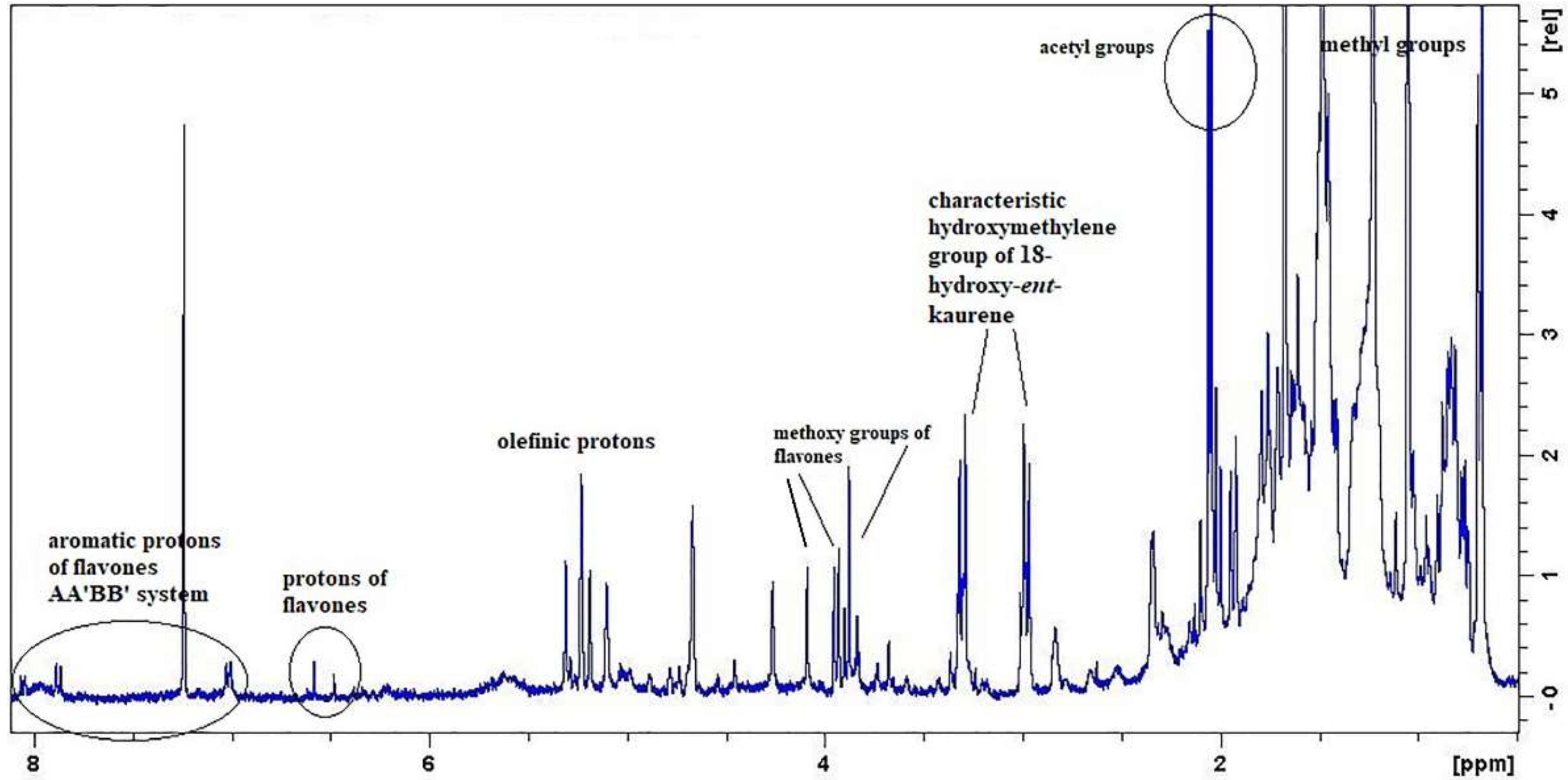
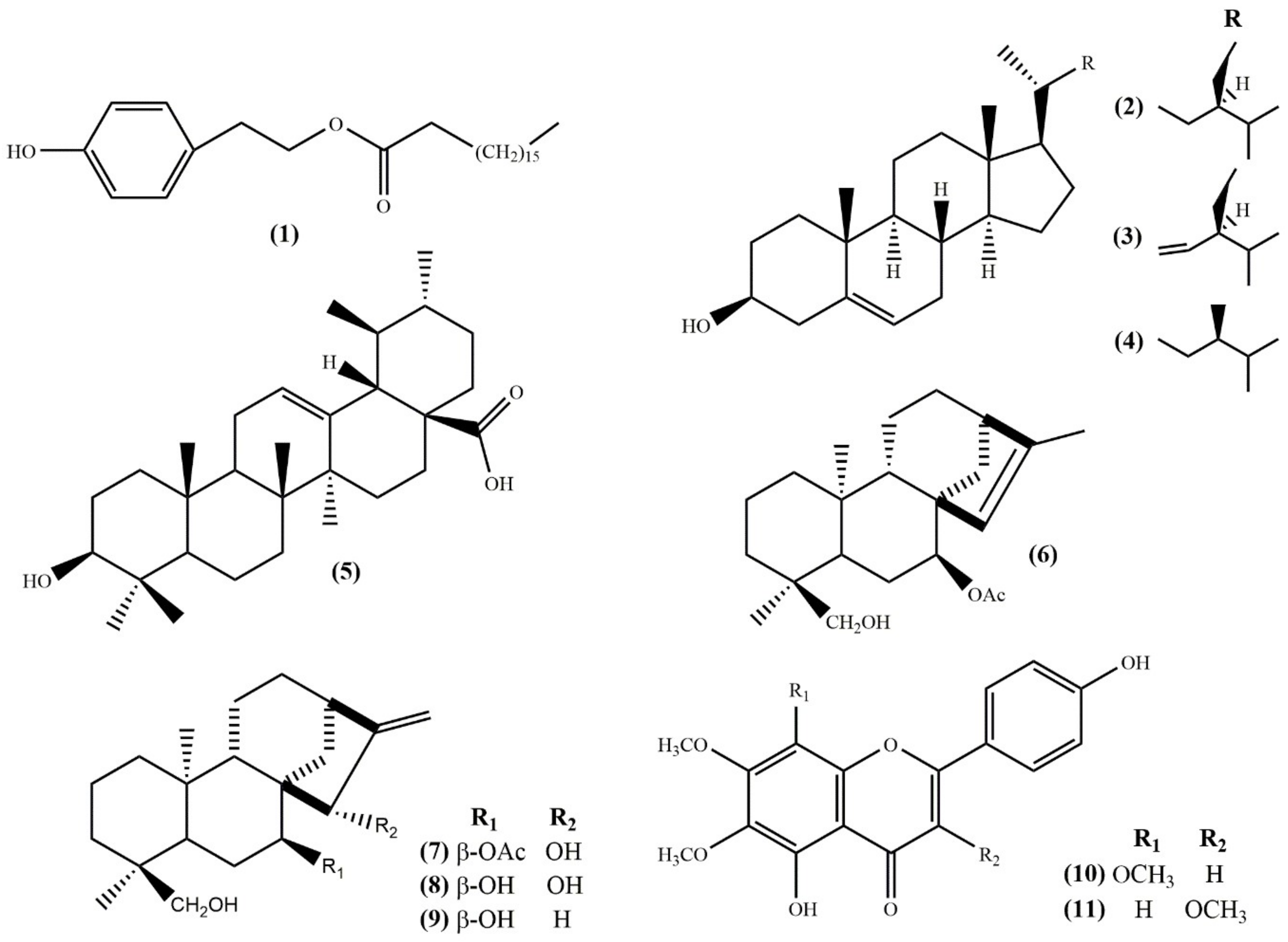
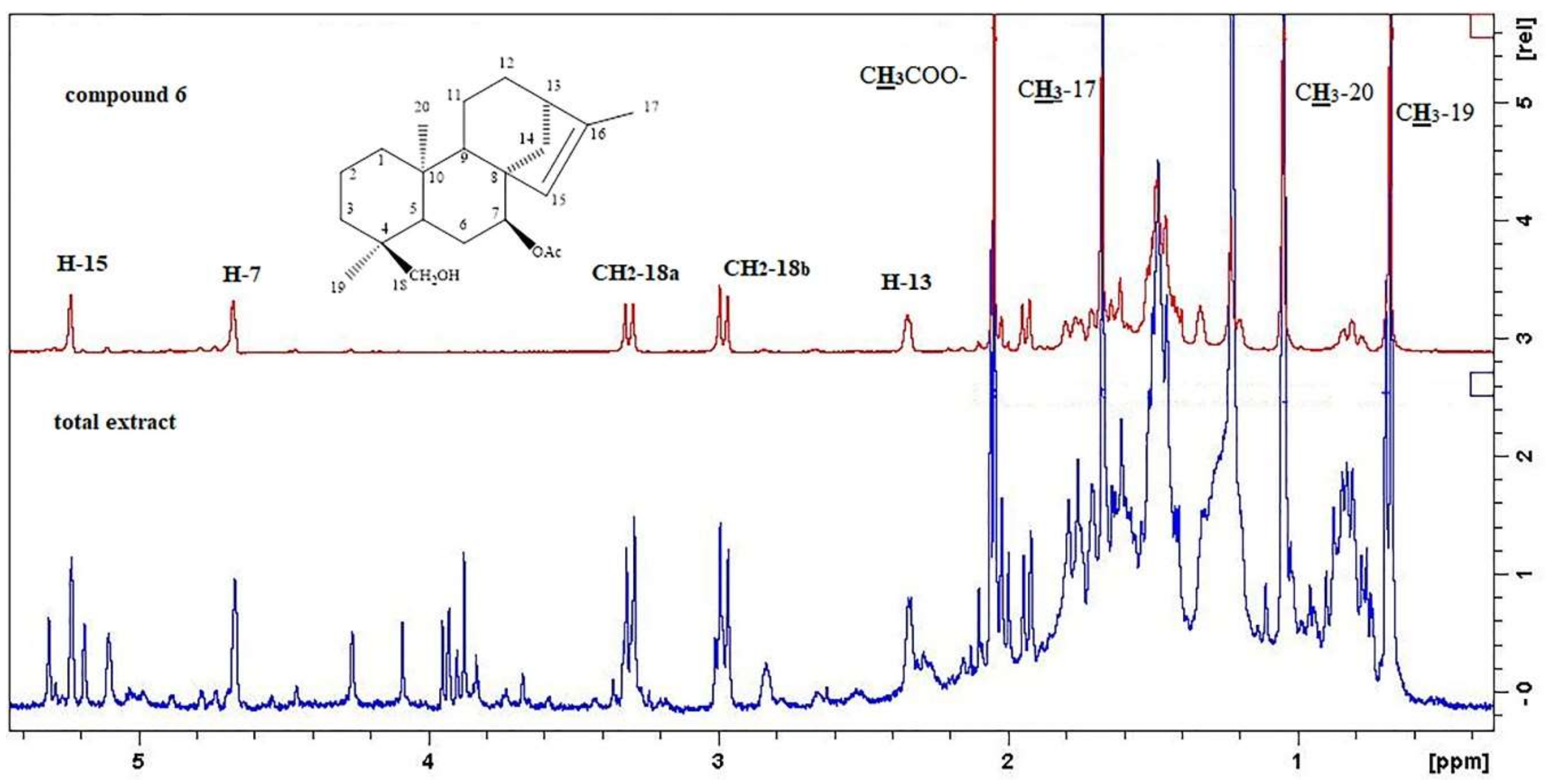
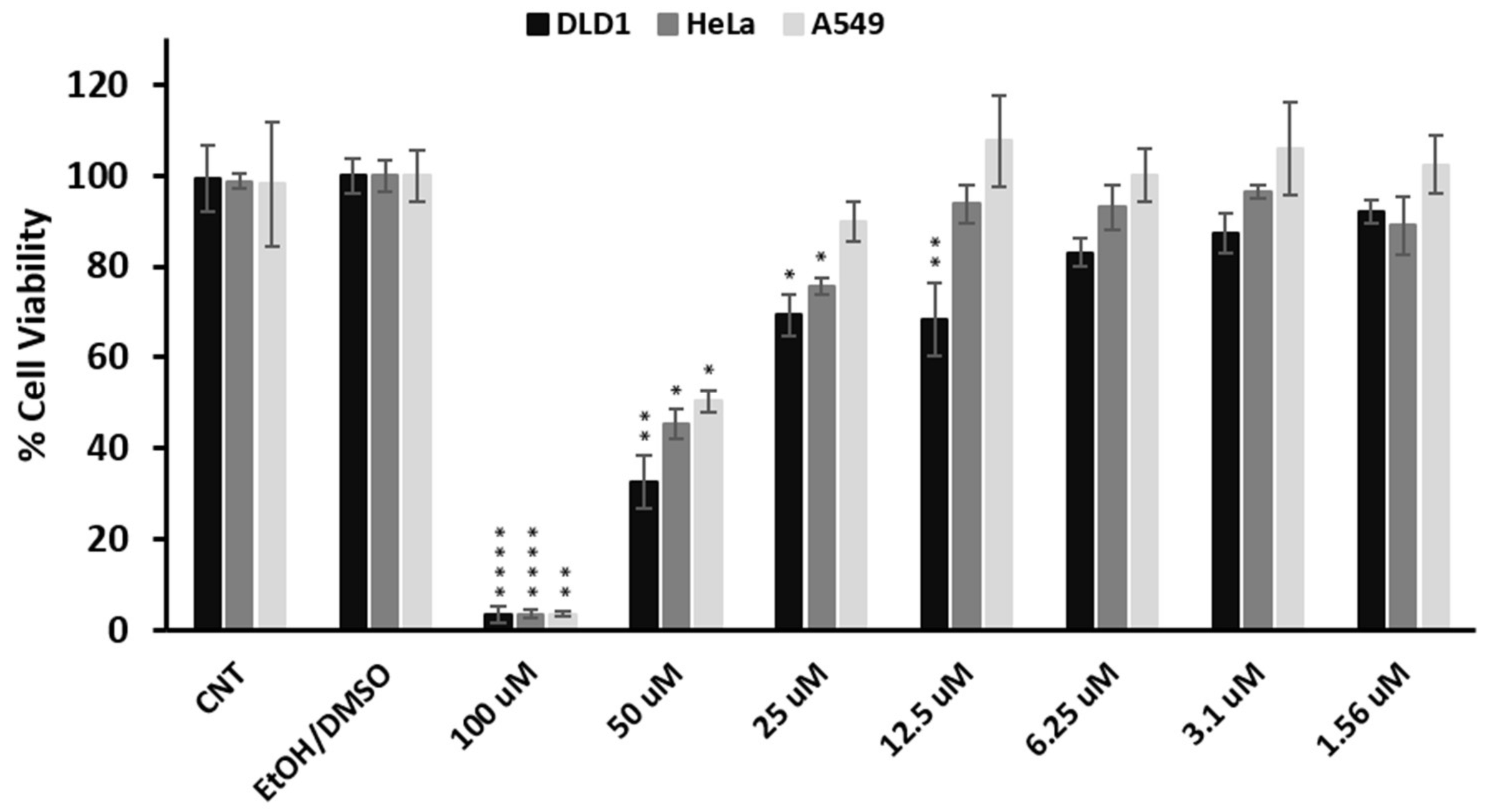
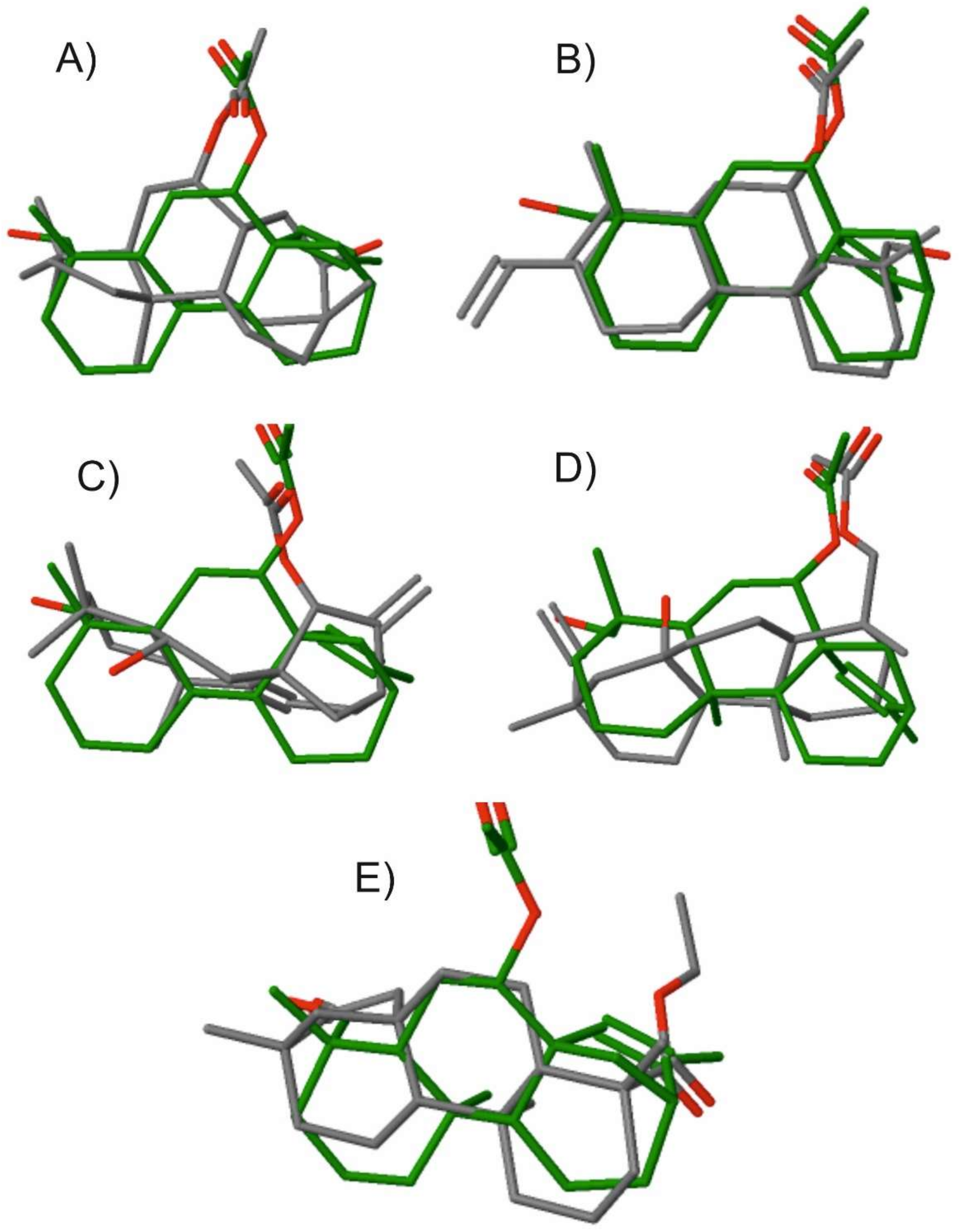
2. Results and Discussion
3. Materials and Methods
3.1. General Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cell Cultures
3.5. Viability Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aneva, I.; Zhelev, P.; Kozuharova, E.; Danova, K.; Nabavi, S.F.; Behzad, S. Genus Sideritis, section Empedoclia in southeastern Europe and Turkey–studies in ethnopharmacology and recent progress of biological activities. DARU J. Pharm. Sci. 2019, 27, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Rosselli, S.; Pibiri, I.; Kilgore, N.; Lee, K.-H. Anti-HIV Agents Derived from the ent -Kaurane Diterpenoid Linearol. J. Nat. Prod. 2002, 65, 1594–1597. [Google Scholar] [CrossRef] [PubMed]
- Ertaş, A.; Öztürk, M.; Boğa, M.; Topçu, G. Antioxidant and Anticholinesterase Activity Evaluation of ent -kaurane Diterpenoids from Sideritis arguta. J. Nat. Prod. 2009, 72, 500–502. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Duarte, A.I.; Carretero, M.E.; Moreira, P.I.; Gómez-Serranillos, M.P. Kaurane diterpenes as mitochondrial alterations preventive agents under experimental oxidative stress conditions. Pharm. Biol. 2016, 54, 705–711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hanson, J.R.; Nichols, T.; Mukhrish, Y.; Bagley, M.C. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2019, 36, 1499–1512. [Google Scholar] [CrossRef]
- Liu, W.; Feng, X.; Zheng, Y.; Huang, C.-H.; Nakano, C.; Hoshino, T.; Bogue, S.; Ko, T.-P.; Chen, C.-C.; Cui, Y.; et al. Structure, function and inhibition of ent-kaurene synthase from Bradyrhizobium japonicum. Sci. Rep. 2015, 4, 6214. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC). Assessment report on Sideritis scardica Griseb.; Sideritis clandestina (Bory &Chaub.) Hayek; Sideritis raeseri Boiss. & Heldr.; Sideritis syriaca L., herba. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-sideritis-scardica-griseb-sideritis-clandestina-bory-chaub-hayek-sideritis_en.pdf (accessed on 19 May 2020).
- Wightman, E.L.; Jackson, P.A.; Khan, J.; Forster, J.; Heiner, F.; Feistel, B.; Suarez, C.G.; Pischel, I.; Kennedy, D.O. The acute and chronic cognitive and cerebral blood flow effects of a Sideritis scardica (Greek mountain tea) extract: A double blind, randomized, placebo controlled, parallel groups study in healthy humans. Nutrients 2018, 10, 955. [Google Scholar] [CrossRef]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef]
- Fraga, B.M. Phytochemistry and chemotaxonomy of Sideritis species from the Mediterranean region. Phytochemistry 2012, 76, 7–24. [Google Scholar] [CrossRef]
- Piozzi, F.; Bruno, M.; Rosselli, S.; Maggio, A. The Diterpenoids from the Genus Sideritis. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2006; Volume 33, pp. 493–540. ISBN 978-0-444-52717-2. [Google Scholar]
- Kilic, T.; Yildiz, Y.K.; Goren, A.C.; Tumen, G.; Topcu, G. Phytochemical Analysis of Some Sideritis Species of Turkey. Chem. Nat. Compd. 2003, 39, 453–456. [Google Scholar] [CrossRef]
- Loğoğlu, E.; Arslan, S.; Öktemer, A.; Şakõyan, İ. Biological activities of some natural compounds from Sideritis sipylea Boiss. Phytother. Res. 2006, 20, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Topçu, G.; Gören, A.C. Biological Activity of Diterpenoids Isolated from Anatolian Lamiaceae Plants. Rec. Nat. Prod. 2007, 1–16. [Google Scholar]
- Topçu, G.; Ertaş, A.; Öztürk, M.; Dinçel, D.; Kılıç, T.; Halfon, B. Ent-kaurane diterpenoids isolated from Sideritis congesta. Phytochem. Lett. 2011, 4, 436–439. [Google Scholar] [CrossRef]
- Tomou, E.; Chatzopoulou, P.; Skaltsa, H. NMR analysis of cultivated Sideritis euboea Heldr. Phytochem. Anal. 2019, 31, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Öksüz, S.; Topçu, G. A eudesmanolide and other constituents from Inula graveolens. Phytochemistry 1992, 31, 195–197. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Isolation of Stigmasterol and β-Sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239–242. [Google Scholar] [CrossRef]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of Phytosterols and Triterpenoids with Potential Anti-Cancer Activity in Bran of Black Non-Glutinous Rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Yen, G.-C. Extraction and Identification of Antioxidative Components of Hsian-tsao (Mesona procumbens Hemsl.). LWT Food Sci. Technol. 2001, 34, 306–311. [Google Scholar] [CrossRef]
- Piozzi, F.; Venturella, P.; Bellino, A.; Mondelli, R. Diterpenes from Sideritis sicula Ucria. Tetrahedron 1968, 24, 4073–4081. [Google Scholar] [CrossRef]
- Venturella, P.; Bellino, A. Eubotriol and eubol, new diterpenes from Sideritis euboea. Experientia 1977, 92, 1270–1271. [Google Scholar] [CrossRef]
- Çarikçi, S.; Kiliç, T.; Azizoğlu, A.; Topçu, G. Chemical Constituents of Two Endemic Sideritis Species from Turkey with Antioxidant Activity. Rec. Nat. Prod. 2012, 6, 101–109. [Google Scholar]
- Harborn, J.B. The Flavonoids: Advances in Research since 1986. Journal of Chemical Education 1995, 72, 73. [Google Scholar]
- Wollenweber, E.; Hradetzky, D.; Mann, K.; Roitman, J.N.; Yatskievych, G.; Proksch, M.; Proksch, P. Exudate Flavonoids from Aerial Parts of Five Ambrosia Species. J. Plant Physiol. 1987, 131, 37–43. [Google Scholar] [CrossRef]
- Venturella, P.; Bellino, A. Diterpenes from Greek Sideritis species. Fitoterapia 1977, 48, 3–4. [Google Scholar]
- Halfon, B.; Çiftçi, E.; Topçu, G. Flavonoid constituents of Sideritis caesarea. Turk. J. Chem. 2013, 9, 464–472. [Google Scholar] [CrossRef]
- Faiella, L.; Piaz, F.D.; Bader, A.; Braca, A. Diterpenes and phenolic compounds from Sideritis pullulans. Phytochemistry 2014, 106, 164–170. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Xia, Y.-X.; Liang, Z.-M.; Tsang, S.W.; Zhang, H.-J. Mechanistic Pathways and Molecular Targets of Plant-Derived Anticancer ent-Kaurane Diterpenes. Biomolecules 2020, 10, 144. [Google Scholar] [CrossRef]
- Takahashi, J.; Gomes, D.; Lyra, F.; dos Santos, G.; Martins, L. The Remarkable Structural Diversity Achieved in ent-Kaurane Diterpenes by Fungal Biotransformations. Molecules 2014, 19, 1856–1886. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. α-Pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma (HepG2) cells. Biosci. Rep. 2018, BSR20180980. [Google Scholar] [CrossRef]
- Hashemzaei, M.; Far, A.D.; Yari, A.; Heravi, R.E.; Tabrizian, K.; Taghdisi, S.M.; Sadegh, S.E.; Tsarouhas, K.; Kouretas, D.; Tzanakakis, G.; et al. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol. Rep. 2017, 38, 819–828. [Google Scholar] [CrossRef]
- Choi, M.-S.; Kim, S.-H.; Kuh, H.-J. Penetration of paclitaxel and 5-fluorouracil in multicellular layers of human colorectal cancer cells. Oncol. Rep. 2011, 25. [Google Scholar] [CrossRef]
- Li, B.-L.; Li, B.; Zhang, R.-L.; Zhao, J.-J.; Wang, X.-F.; Liu, Y.-M.; Shi, Y.-P.; Liu, J.-B.; Chen, B.-Q. Synthesis and antiproliferative evaluation of novel 1,2,4-triazole derivatives incorporating benzisoselenazolone scaffold. Bioorg. Med. Chem. Lett. 2016, 26, 1279–1281. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, J.; Cook, J.; Lipschultz, C.; Teague, D.; Fisher, J.; Mitchell, J. Cytotoxic studies of paclitaxel (Taxol®) in human tumour cell lines. Br. J. Cancer 1993, 68, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Banegas-Luna, A.J.; Cerón-Carrasco, J.P.; Puertas-Martín, S.; Pérez-Sánchez, H. BRUSELAS: HPC generic and customizable software architecture for 3D ligand-based virtual screening of large molecular databases. J. Chem. Inf. Model. 2019, 59, 2805–2817. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomou, E.-M.; Chatziathanasiadou, M.V.; Chatzopoulou, P.; Tzakos, A.G.; Skaltsa, H. NMR-Based Chemical Profiling, Isolation and Evaluation of the Cytotoxic Potential of the Diterpenoid Siderol from Cultivated Sideritis euboea Heldr. Molecules 2020, 25, 2382. https://doi.org/10.3390/molecules25102382
Tomou E-M, Chatziathanasiadou MV, Chatzopoulou P, Tzakos AG, Skaltsa H. NMR-Based Chemical Profiling, Isolation and Evaluation of the Cytotoxic Potential of the Diterpenoid Siderol from Cultivated Sideritis euboea Heldr. Molecules. 2020; 25(10):2382. https://doi.org/10.3390/molecules25102382
Chicago/Turabian StyleTomou, Ekaterina-Michaela, Maria V. Chatziathanasiadou, Paschalina Chatzopoulou, Andreas G. Tzakos, and Helen Skaltsa. 2020. "NMR-Based Chemical Profiling, Isolation and Evaluation of the Cytotoxic Potential of the Diterpenoid Siderol from Cultivated Sideritis euboea Heldr." Molecules 25, no. 10: 2382. https://doi.org/10.3390/molecules25102382
APA StyleTomou, E.-M., Chatziathanasiadou, M. V., Chatzopoulou, P., Tzakos, A. G., & Skaltsa, H. (2020). NMR-Based Chemical Profiling, Isolation and Evaluation of the Cytotoxic Potential of the Diterpenoid Siderol from Cultivated Sideritis euboea Heldr. Molecules, 25(10), 2382. https://doi.org/10.3390/molecules25102382







