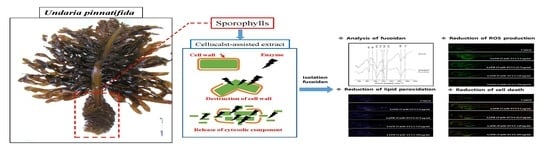
Protective Effects of Fucoidan Isolated from Celluclast-Assisted Extract of Undaria pinnatifida Sporophylls against AAPH-Induced Oxidative Stress In Vitro and In Vivo Zebrafish Model
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Composition of the FCUS
2.2. Effect of FCUS on Intracellular ROS Generation in AAPH-Stimulated Vero Cells
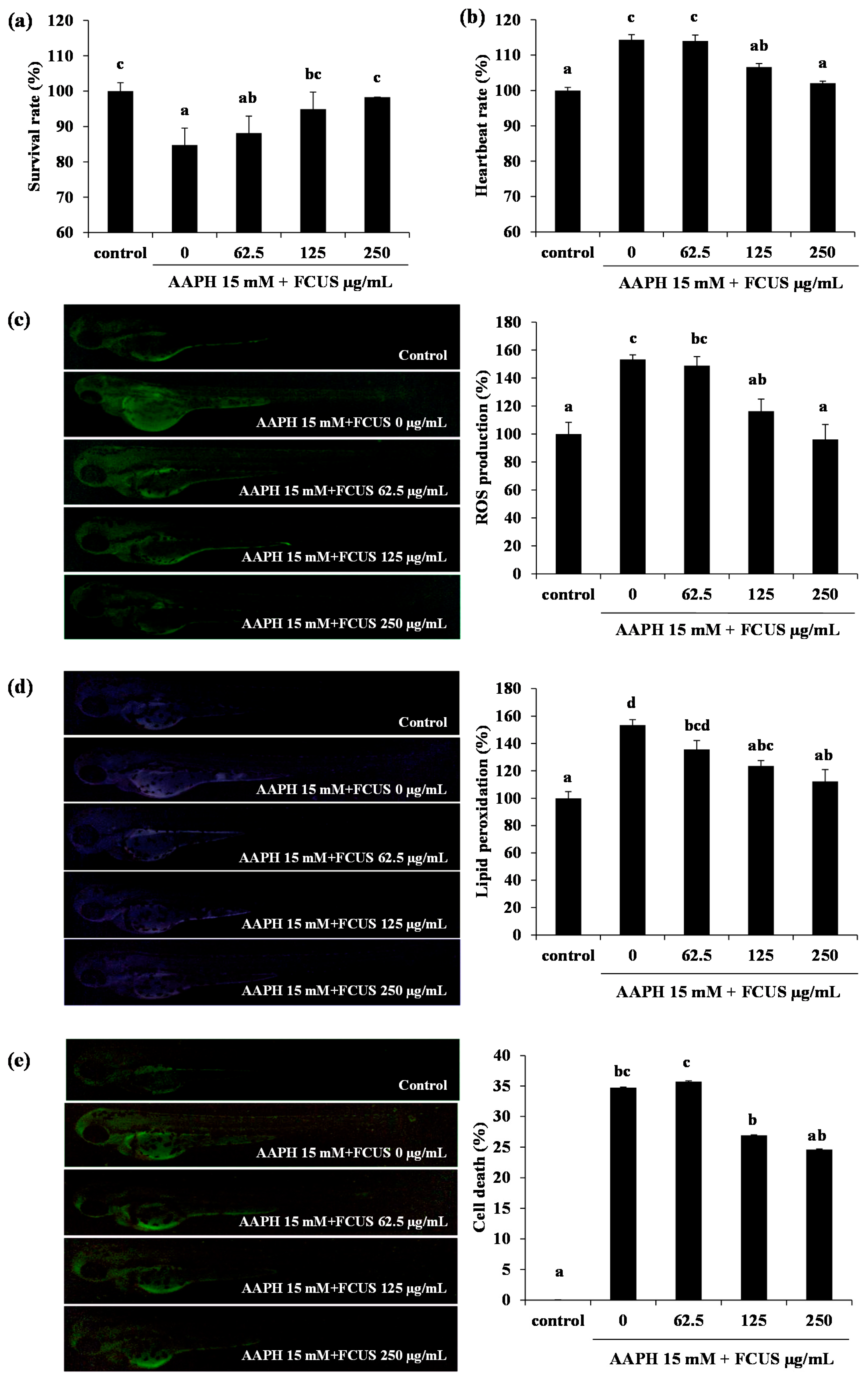
2.3. Toxicity of FCUS in Zebrafish and the Effect of FCUS on ROS and Lipid Peroxidation Production in Zebrafish
3. Materials and Methods
3.1. Material
3.2. Extraction and Purification of Fucoidan from U. pinnatifida Sporophylls
3.3. Characterization of FCUS
3.3.1. Proximate Composition and Monosaccharide Composition
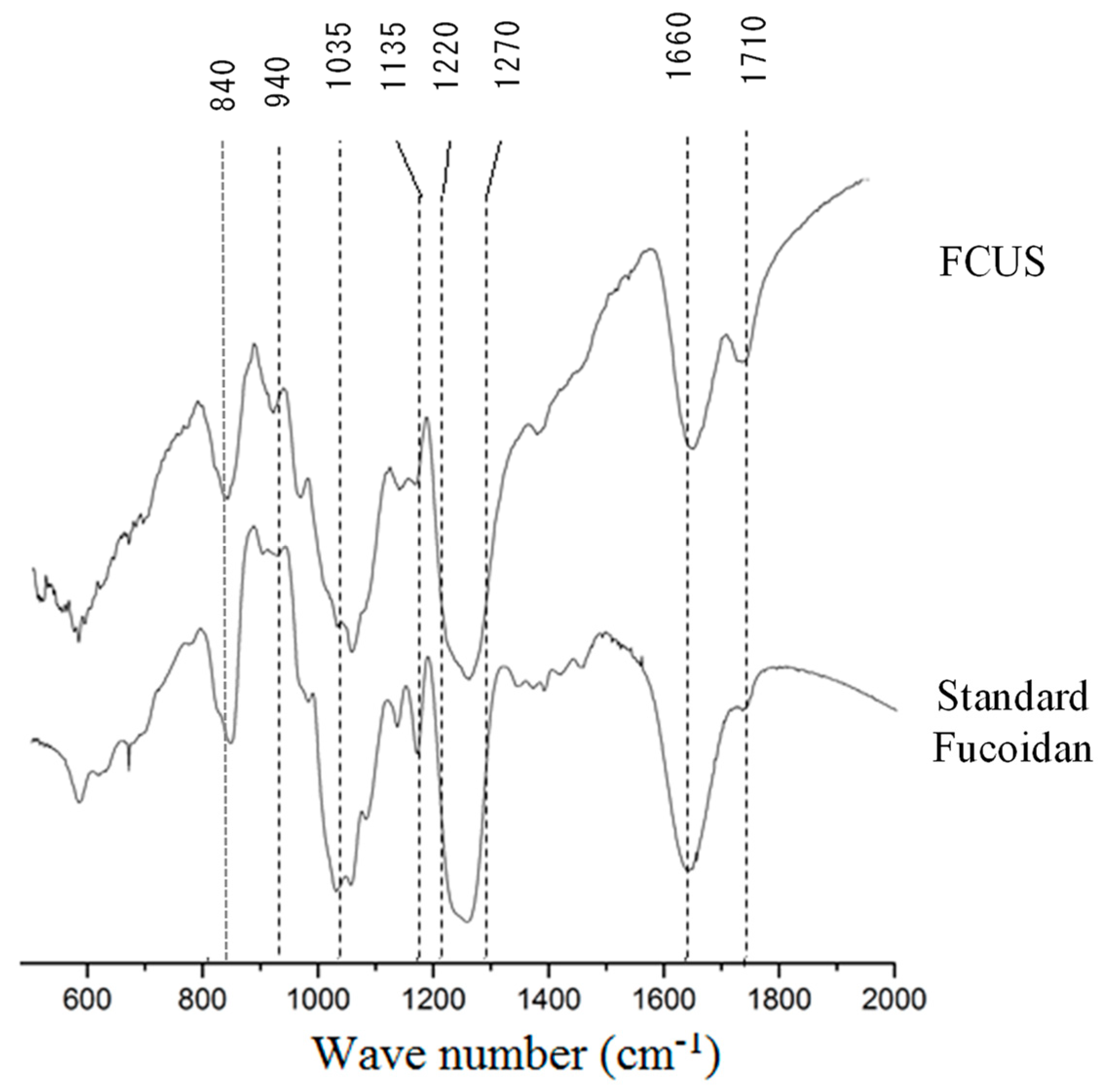
3.3.2. Fourier Transform-Infrared (FT-IR) Characterization
3.4. Cell culture and cell experiments
3.5. In Vivo Experiments
3.5.1. Maintenance of Zebrafish
3.5.2. Treatment of FCUS and AAPH to Embryos
3.5.3. Measurement of Intracellular ROS Production, Lipid Peroxidation, and Cell Death Induced by AAPH
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kishi, S.; Uchiyama, J.; Baughman, A.M.; Goto, T.; Lin, M.C.; Tsai, S.B. The zebrafish as a vertebrate model of functional aging and very gradual senescence. Exp. Gerontol. 2003, 38, 777–786. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Park, E.-J.; Lee, K.-W.; Jeon, Y.-J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresource Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.T.; Cherry, J. Progress and challenges in enzyme development for biomass utilization. In Biofuels; Springer: Berlin/Heidelberg, Germany, 2007; pp. 95–120. [Google Scholar]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnicka, F.; Copikova, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Koo, J.-G.; Choi, Y.-S.; Kwak, J.-K. Blood-anticoagulant activity of fucoidans from sporophylls of Undaria pinnatifida, Laminaria religiosa, Hizikia fusiforme and Sargassum fulvellum in Korea. Korean J. Fish. Aquat. Sci. 2001, 34, 515–520. [Google Scholar]
- Jiang, Z.D.; Okimura, T.; Yokose, T.; Yamasaki, Y.; Yamaguchi, K.; Oda, T. Effects of sulfated fucan, ascophyllan, from the brown Alga Ascophyllum nodosum on various cell lines: A comparative study on ascophyllan and fucoidan. J. Biosci. Bioeng. 2010, 110, 113–117. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Lim, S.J.; Aida, W.M.W.; Maskat, M.Y.; Mamot, S.; Ropien, J.; Mohd, D.M. Isolation and antioxidant capacity of fucoidan from selected Malaysian seaweeds. Food Hydrocolloid. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Boo, H.J.; Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, S.Y.; Cho, H.; Yoo, E.S.; Kang, H.K. Fucoidan from Undaria pinnatifida induces apoptosis in A549 human lung carcinoma cells. Phytother. Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef]
- Kim, M.J.; Jeon, J.; Lee, J.S. Fucoidan prevents high-fat diet-induced obesity in animals by suppression of fat accumulation. Phytother. Res. 2014, 28, 137–143. [Google Scholar] [CrossRef]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Lee, S.H.; Ko, C.I.; Cha, S.H.; Kang, M.C.; Kang, S.M.; Ko, S.C.; Lee, W.W.; Ko, J.Y.; Lee, J.H.; et al. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. (Tokyo) 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.C.; Tan, S.N.; Yong, J.W.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, J.S.; Kim, W.S.; Jeon, Y.J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. In Vivo 2003, 17, 245–249. [Google Scholar]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Kang, M.C.; Kim, S.Y.; Kim, Y.T.; Kim, E.A.; Lee, S.H.; Ko, S.C.; Wijesinghe, W.A.; Samarakoon, K.W.; Kim, Y.S.; Cho, J.H.; et al. In vitro and in vivo antioxidant activities of polysaccharide purified from aloe vera (Aloe barbadensis) gel. Carbohydr. Polym. 2014, 99, 365–371. [Google Scholar] [CrossRef]
- Ko, S.C.; Kang, N.; Kim, E.A.; Kang, M.C.; Lee, S.H.; Kang, S.M.; Lee, J.B.; Jeon, B.T.; Kim, S.K.; Park, S.J.; et al. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012, 47, 2005–2011. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, P.J.; Park, E.J.; Kim, S.K.; Jeon, Y.J. Antioxidant activity of enzymatic extracts from a brown seaweed Ecklonia cava by electron spin resonance spectrometry and comet assay. Eur. Food Res. Technol. 2005, 221, 41–47. [Google Scholar] [CrossRef]
- Nishino, T.; Nishioka, C.; Ura, H.; Nagumo, T. Isolation and partial characterization of a noval amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohydr. Res. 1994, 255, 213–224. [Google Scholar] [CrossRef]
- Yang, H.W.; Oh, J.Y.; Kim, E.A.; Kim, S.Y.; Kim, J.I.; Jeon, Y.J. Antioxidant Effect of Fucoidan from Miyeokgui, Marine Alga in Zebrafish Model. Journal of chitin and chitosan 2015, 20, 123–130. [Google Scholar]
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A Revised Structure for Fucoidan May Explain Some of Its Biological-Activities. J. Biol. Chem. 1993, 268, 21770–21776. [Google Scholar]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kim, E.A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; De Silva, E.D.; et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae-Seoul 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Kang, N.; Kim, S.-Y.; Rho, S.; Ko, J.-Y.; Jeon, Y.-J. Anti-fatigue activity of a mixture of seahorse (Hippocampus abdominalis) hydrolysate and red ginseng. Fish. Aquat. Sci. 2017, 20, 3. [Google Scholar] [CrossRef]
- Kim, H.-H.; Kim, H.-S.; Ko, J.-Y.; Kim, C.-Y.; Lee, J.-H.; Jeon, Y.-J. A single-step isolation of useful antioxidant compounds from Ishige okamurae by using centrifugal partition chromatography. Fish. Aquat. Sci. 2016, 19, 22. [Google Scholar] [CrossRef]
- Wang, L.; Jo, M.J.; Katagiri, R.; Harata, K.; Ohta, M.; Ogawa, A.; Kamegai, M.; Ishida, Y.; Tanoue, S.; Kimura, S.; et al. Antioxidant effects of citrus pomace extracts processed by super-heated steam. Lwt-Food Sci. Technol. 2018, 90, 331–338. [Google Scholar] [CrossRef]
- Fernando, I.P.; Kim, M.; Son, K.T.; Jeong, Y.; Jeon, Y.J. Antioxidant Activity of Marine Algal Polyphenolic Compounds: A Mechanistic Approach. J. Med. Food 2016, 19, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Hwang, J.W.; Sung, S.H.; Jeon, Y.J.; Jeong, J.H.; Jeon, B.T.; Moon, S.H.; Park, P.J. Antioxidant activity and protective effect of extract of Celosia cristata L. flower on tert-butyl hydroperoxide-induced oxidative hepatotoxicity. Food Chem. 2015, 168, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.M.; Zhao, T.T.; Zhang, Q.B.; Li, Z.; Zhao, Z.Q.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). J. Appl. Phycol. 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Camara, R.B.; Nobre, L.T.; Costa, M.S.; Almeida-Lima, J.; Farias, E.H.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Mugoni, V.; Camporeale, A.; Santoro, M.M. Analysis of oxidative stress in zebrafish embryos. J. Vis. Exp. 2014, e51328. [Google Scholar] [CrossRef]
- Kang, M.C.; Cha, S.H.; Wijesinghe, W.A.; Kang, S.M.; Lee, S.H.; Kim, E.A.; Song, C.B.; Jeon, Y.J. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013, 138, 950–955. [Google Scholar] [CrossRef]
- Cho, S.H.; Heo, S.J.; Yang, H.W.; Ko, E.Y.; Jung, M.S.; Cha, S.H.; Ahn, G.; Jeon, Y.J.; Kim, K.N. Protective Effect of 3-Bromo-4,5-Dihydroxybenzaldehyde from Polysiphonia morrowii Harvey against Hydrogen Peroxide-Induced Oxidative Stress In Vitro and In Vivo. J. Microbiol. Biotechnol. 2019, 29, 1193–1203. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Park, J.E.; Yang, J.H.; Yoon, S.J.; Lee, J.H.; Yang, E.S.; Park, J.W. Lipid peroxidation-mediated cytotoxicity and DNA damage in U937 cells. Biochimie 2002, 84, 1199–1205. [Google Scholar] [CrossRef]
- Socrier, L.; Rosselin, M.; Giraldo, A.M.G.; Chantemargue, B.; Di Meo, F.; Trouillas, P.; Durand, G.; Morandat, S. A Novel Nitrone-Trolox Conjugate Inhibits Membrane Lipid Oxidation Through Synergistic Antioxidant Effects. Biophys. J. 2019, 116, 227a. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Xue, C.H.; Xue, Y.; Li, Z.J.; Fu, X.Y. The effects of fucoidans from Laminaria japonica on AAPH mediated oxidation of human low-density lipoprotein. Acta Oceanol. Sin. 2006, 25, 124–130. [Google Scholar]
- Kuda, T.; Kunii, T.; Goto, H.; Suzuki, T.; Yano, T. Varieties of antioxidant and antibacterial properties of Ecklonia stolonifera and Ecklonia kurome products harvested and processed in the Noto Peninsula, Japan. Food Chem. 2007, 103, 900–905. [Google Scholar] [CrossRef]
- Wu, D.; Yotnda, P. Production and detection of reactive oxygen species (ROS) in cancers. J. Vis. Exp. 2011, e3357. [Google Scholar] [CrossRef]
- Papaharalambus, C.A.; Griendling, K.K. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc. Med. 2007, 17, 48–54. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Aging: ROS or TOR. Cell Cycle 2008, 7, 3344–3354. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef]
- Wang, L.; Ryu, B.; Kim, W.S.; Kim, G.H.; Jeon, Y.J. Protective effect of gallic acid derivatives from the freshwater green alga Spirogyra sp against ultraviolet B-induced apoptosis through reactive oxygen species clearance in human keratinocytes and zebrafish. Algae 2017, 32, 379–388. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| (Dry Base, %) | |
|---|---|
| Yield | 6.2 ± 1.2 |
| Carbohydrate content | 66.8 ± 0.1 |
| Protein content | 2.8 ± 0.1 |
| Sulfate content | 30.4 ± 0.1 |
| Proportion of monosaccharide | |
| Fucose | 52.3 ± 0.92 |
| Galactose | 44.5 ± 0.16 |
| Glucose | 1.0 ± 0.01 |
| Mannose | 2.2 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-Y.; Kim, E.-A.; Kang, S.I.; Yang, H.-W.; Ryu, B.; Wang, L.; Lee, J.-S.; Jeon, Y.-J. Protective Effects of Fucoidan Isolated from Celluclast-Assisted Extract of Undaria pinnatifida Sporophylls against AAPH-Induced Oxidative Stress In Vitro and In Vivo Zebrafish Model. Molecules 2020, 25, 2361. https://doi.org/10.3390/molecules25102361
Oh J-Y, Kim E-A, Kang SI, Yang H-W, Ryu B, Wang L, Lee J-S, Jeon Y-J. Protective Effects of Fucoidan Isolated from Celluclast-Assisted Extract of Undaria pinnatifida Sporophylls against AAPH-Induced Oxidative Stress In Vitro and In Vivo Zebrafish Model. Molecules. 2020; 25(10):2361. https://doi.org/10.3390/molecules25102361
Chicago/Turabian StyleOh, Jae-Young, Eun-A Kim, Sang In Kang, Hye-Won Yang, Bomi Ryu, Lei Wang, Jung-Suck Lee, and You-Jin Jeon. 2020. "Protective Effects of Fucoidan Isolated from Celluclast-Assisted Extract of Undaria pinnatifida Sporophylls against AAPH-Induced Oxidative Stress In Vitro and In Vivo Zebrafish Model" Molecules 25, no. 10: 2361. https://doi.org/10.3390/molecules25102361
APA StyleOh, J.-Y., Kim, E.-A., Kang, S. I., Yang, H.-W., Ryu, B., Wang, L., Lee, J.-S., & Jeon, Y.-J. (2020). Protective Effects of Fucoidan Isolated from Celluclast-Assisted Extract of Undaria pinnatifida Sporophylls against AAPH-Induced Oxidative Stress In Vitro and In Vivo Zebrafish Model. Molecules, 25(10), 2361. https://doi.org/10.3390/molecules25102361