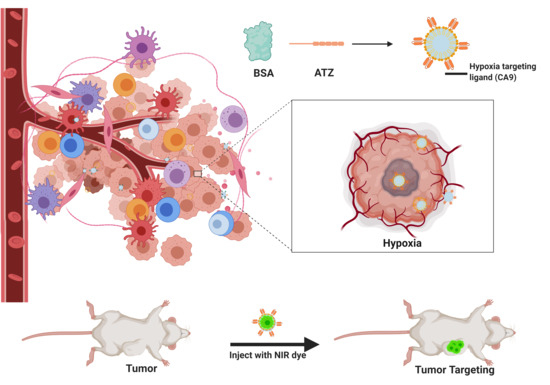
Carbonic Anhydrase-IX Guided Albumin Nanoparticles for Hypoxia-mediated Triple-Negative Breast Cancer Cell Killing and Imaging of Patient-derived Tumor
Abstract
1. Introduction
1.1. Albumin
1.2. Click Chemistry
1.3. 3,4-Difluorobenzylidene Curcumin
1.4. Carbonic Anhydrase IX Receptor
1.5. Hypoxia Targeted Nanoparticles for Better Cell Killing in TNBC
2. Results
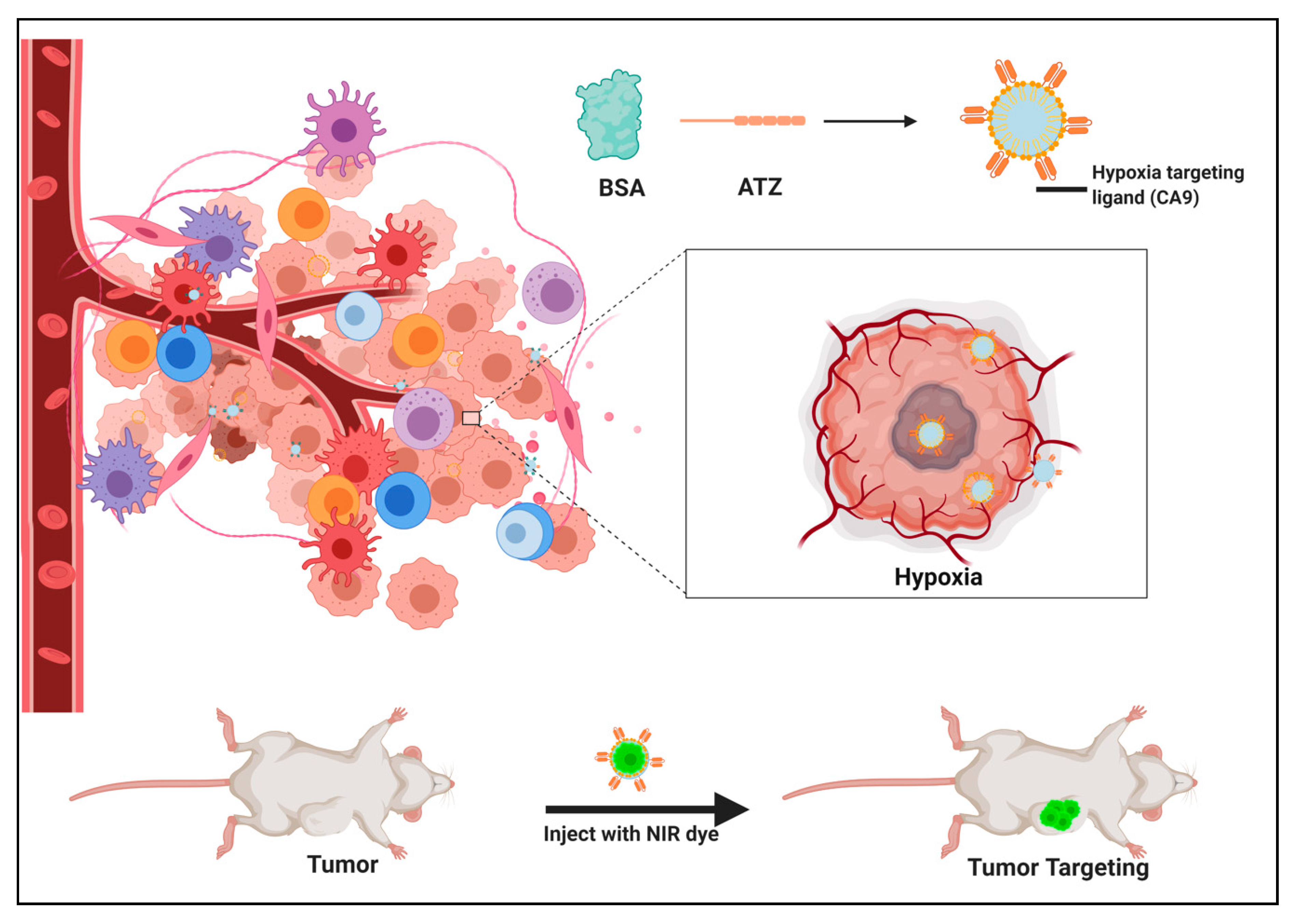
2.1. In Silico Screening for the Binding Studies
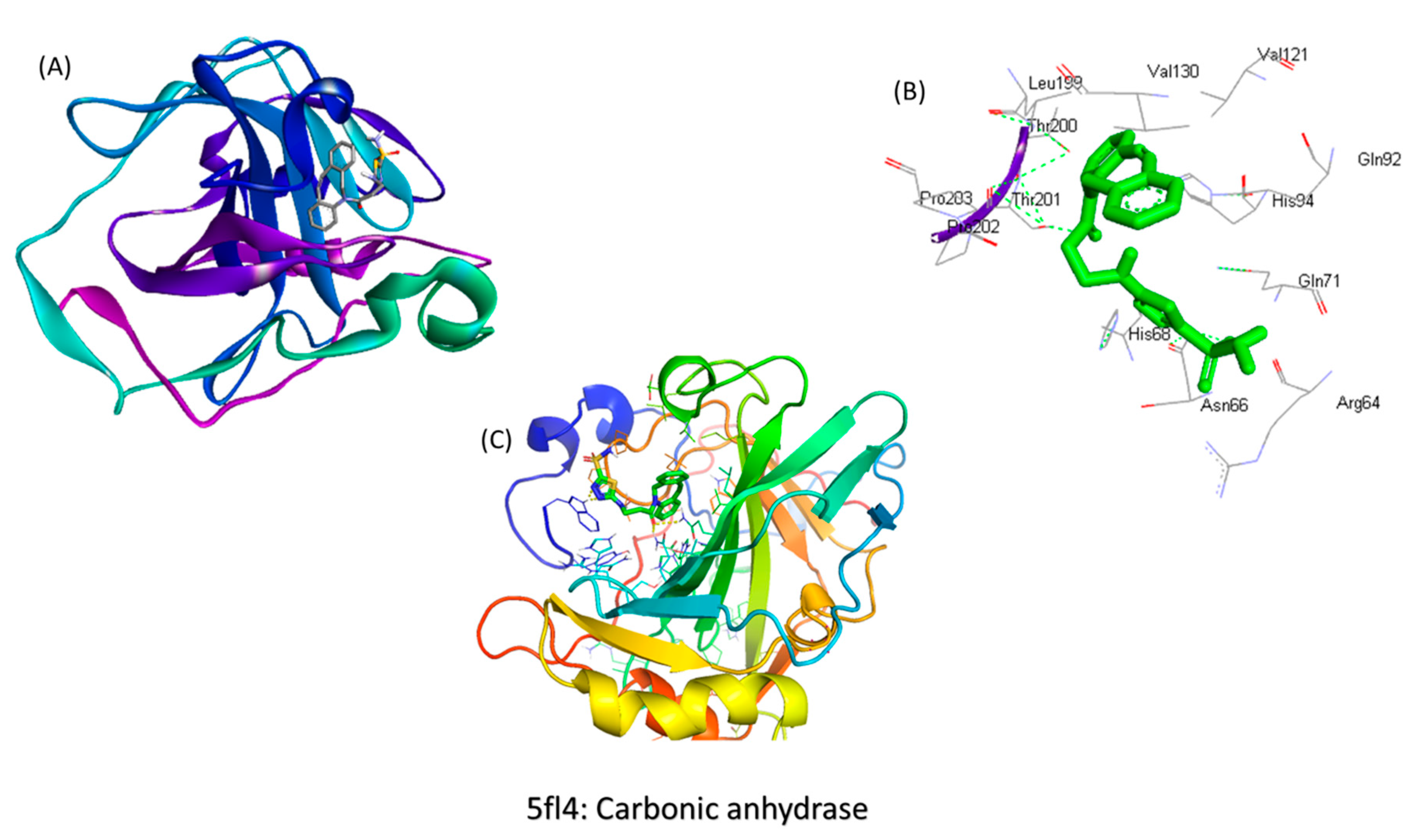
2.2. Synthesis of the Molecule
2.3. Characterization
2.3.1. Drug Loading
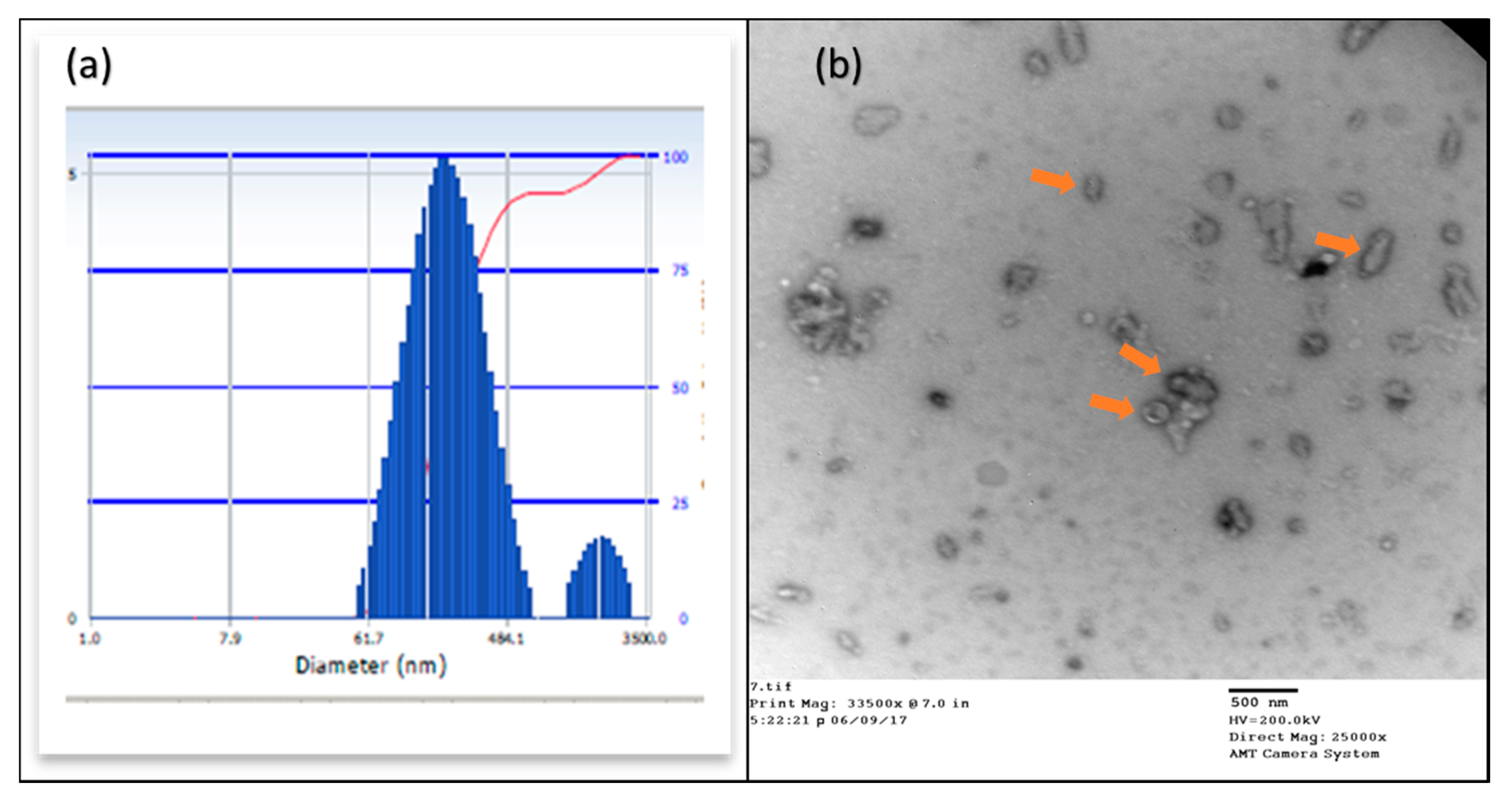
2.3.2. Particle Size Analysis
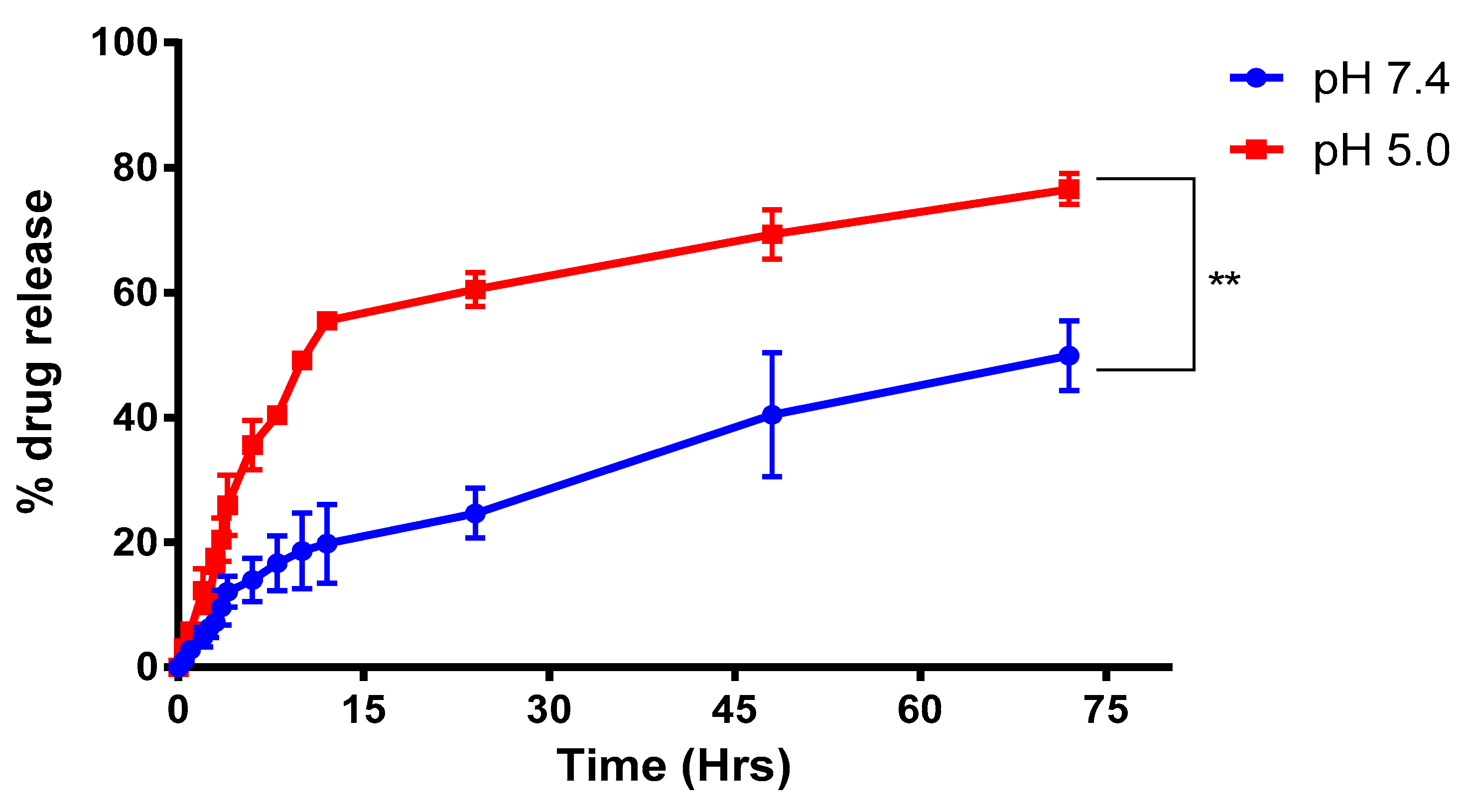
2.3.3. Drug Release Studies
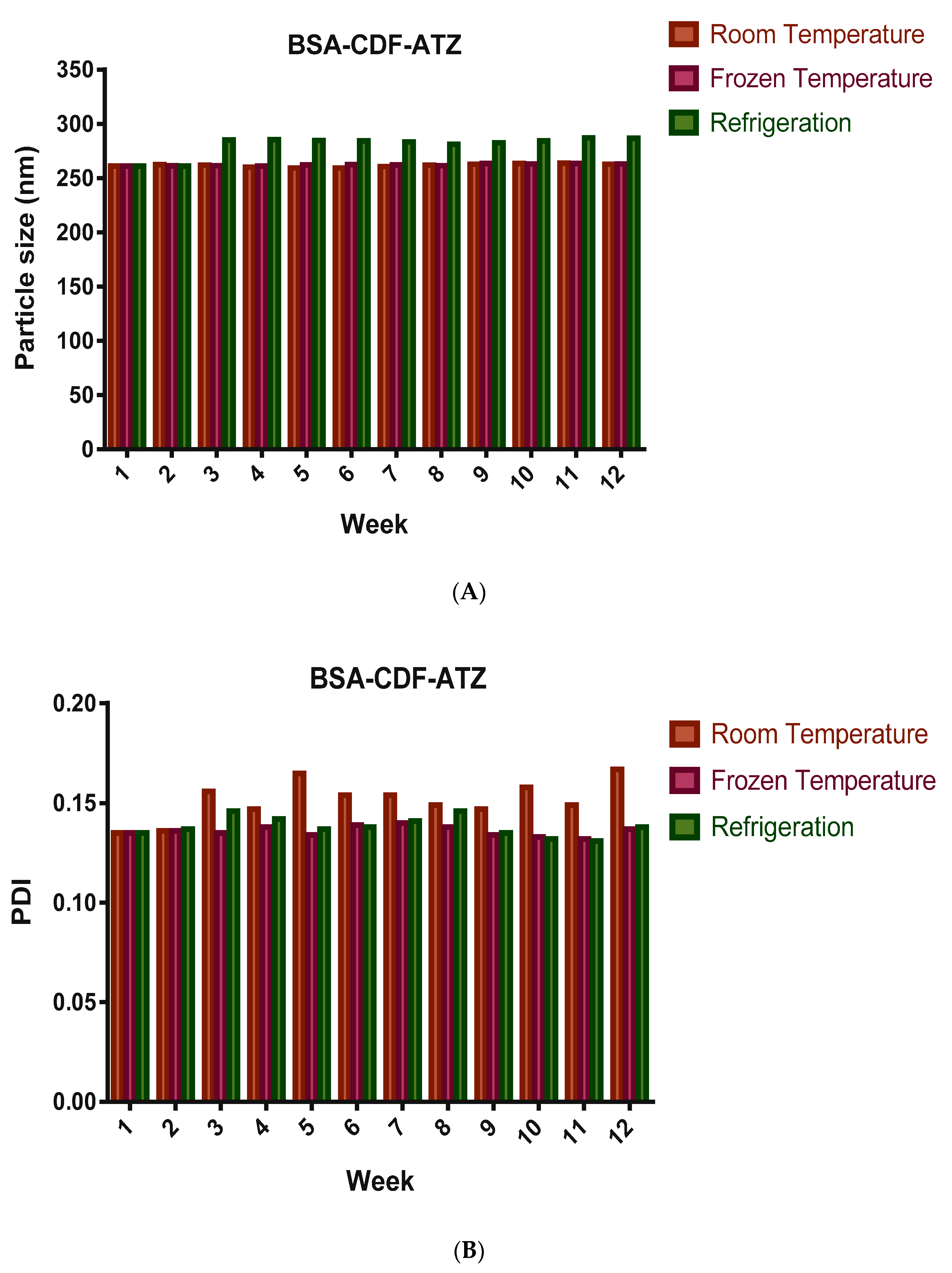
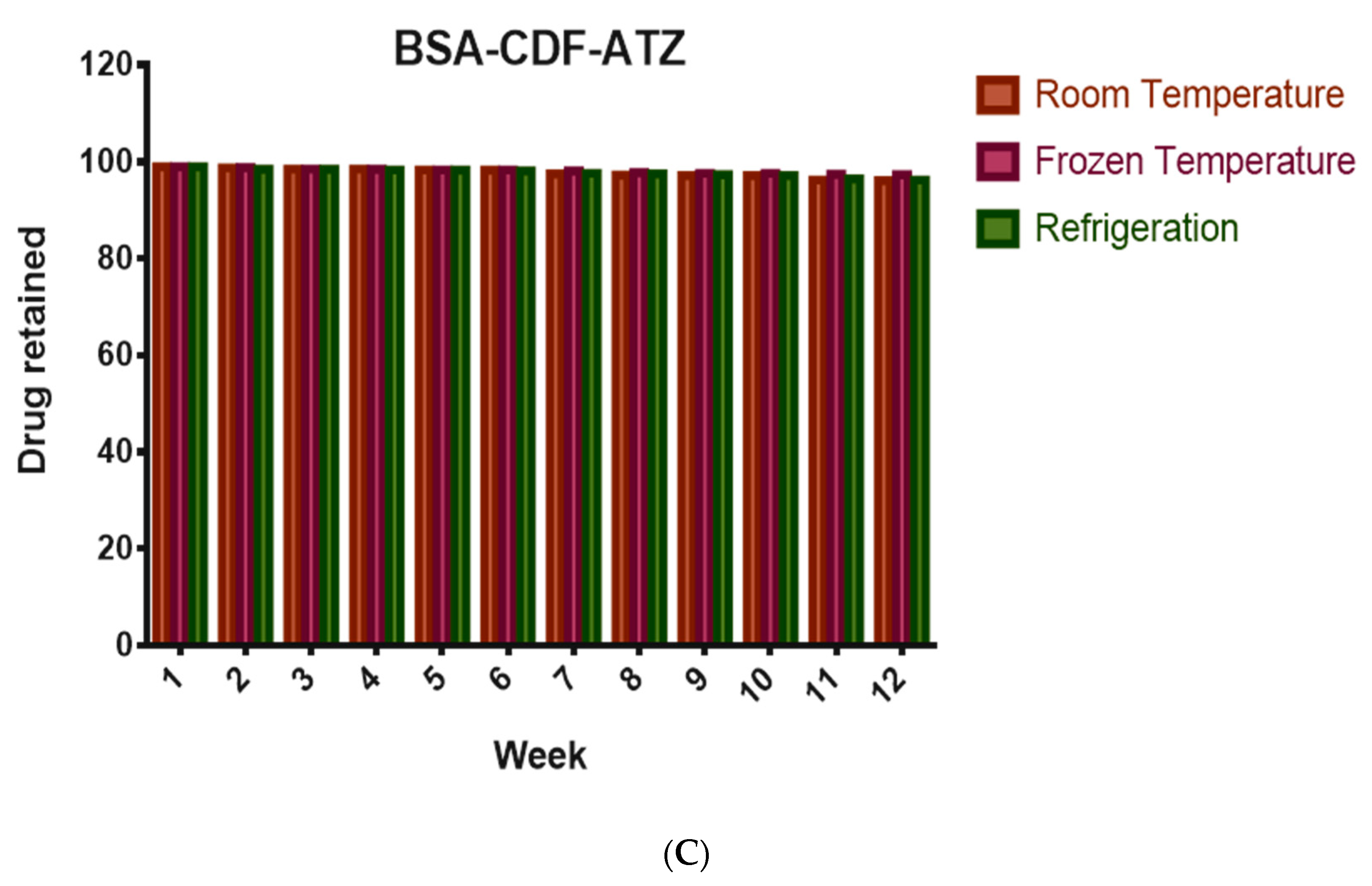
2.3.4. Shelf-Life Assessments by Stability Studies
2.3.5. In Vitro Cytotoxicity Studies for Understanding the Extent of Cell Killing
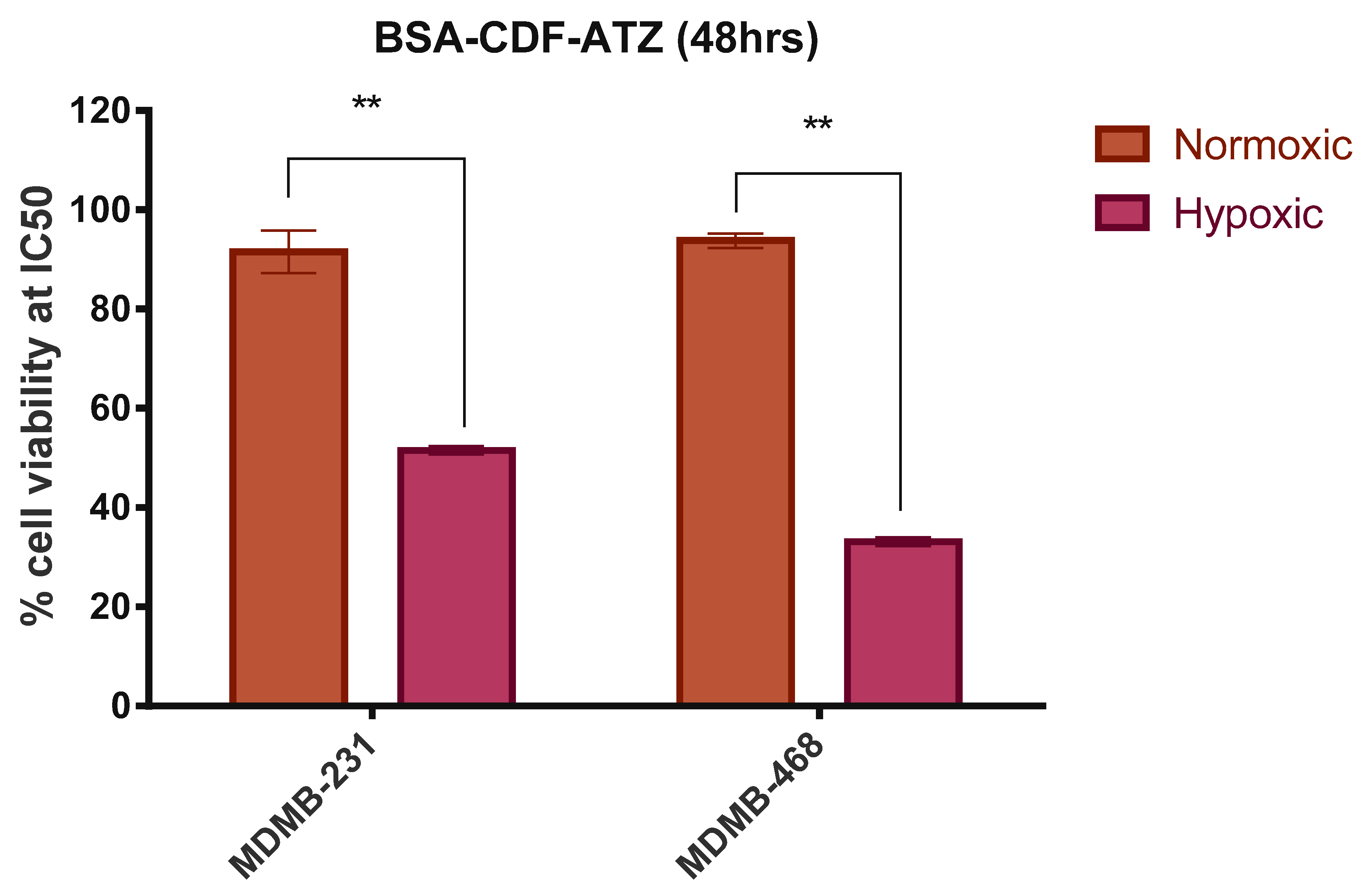
2.3.6. Comparison of in Vitro Cytotoxicity of the Drug Delivery System in Normoxic and Hypoxic Conditions
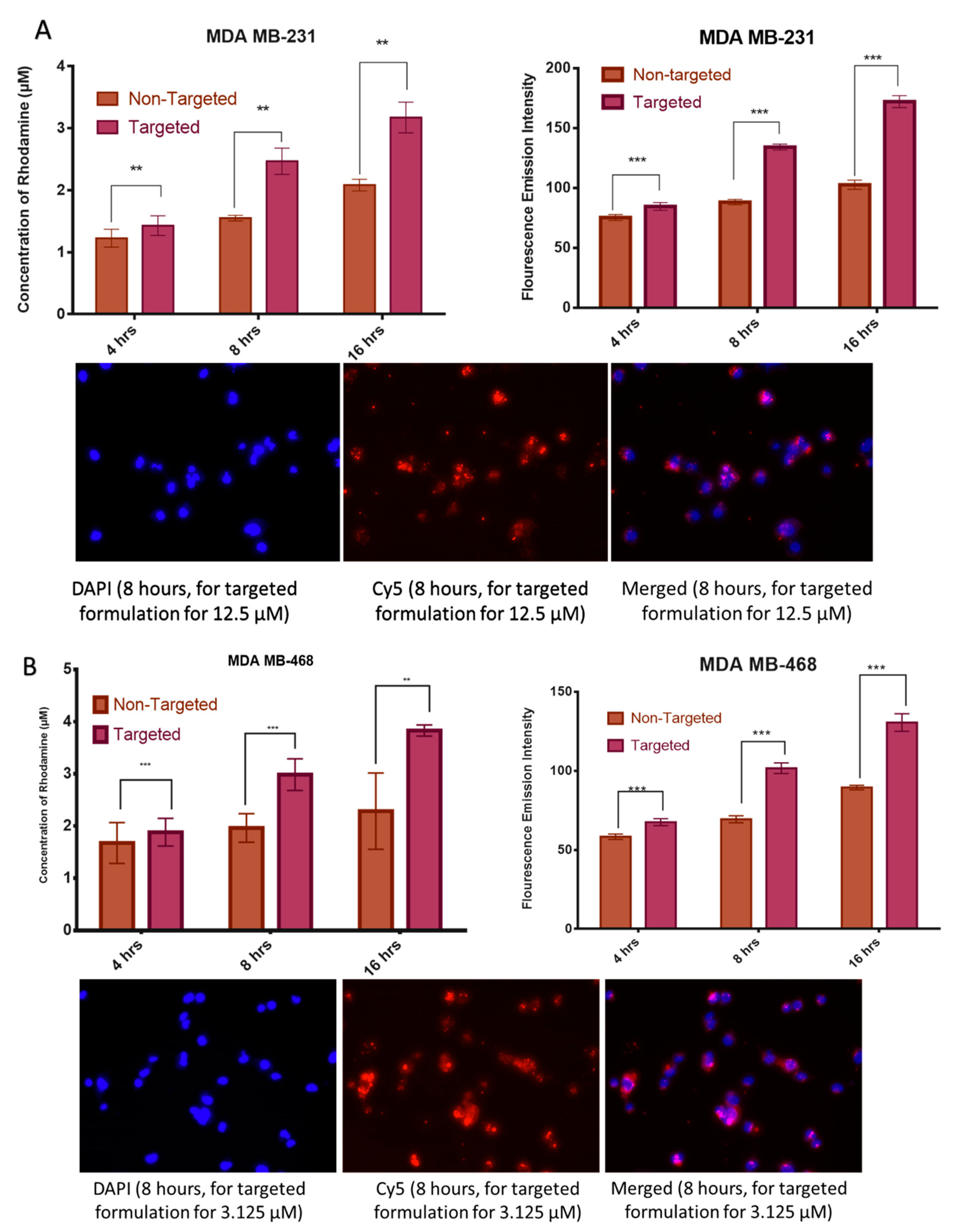
2.3.7. Cell Uptake Studies by Fluorescence Spectroscopy
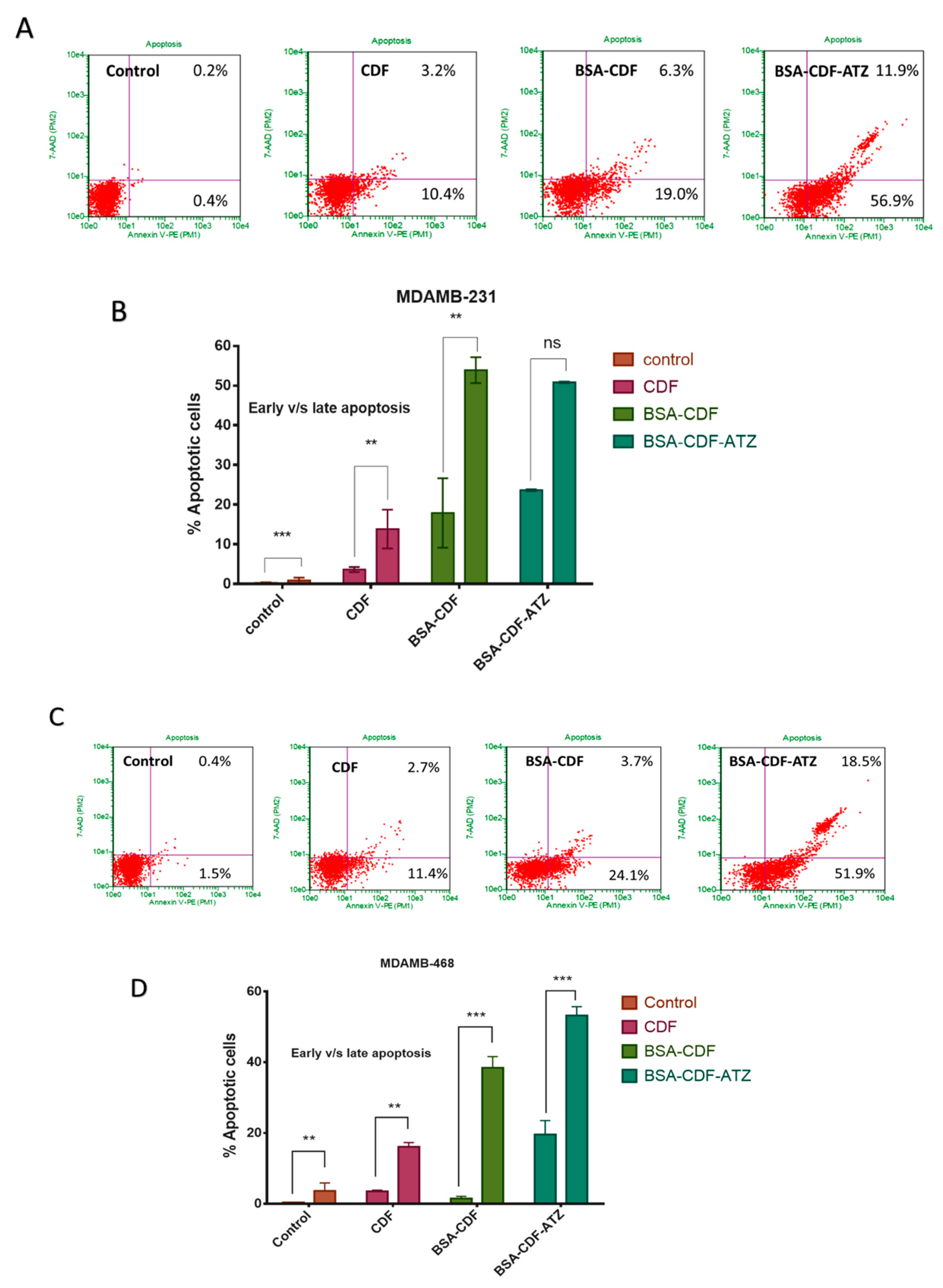
2.3.8. Extent of Apoptosis Studied by Flow Cytometry
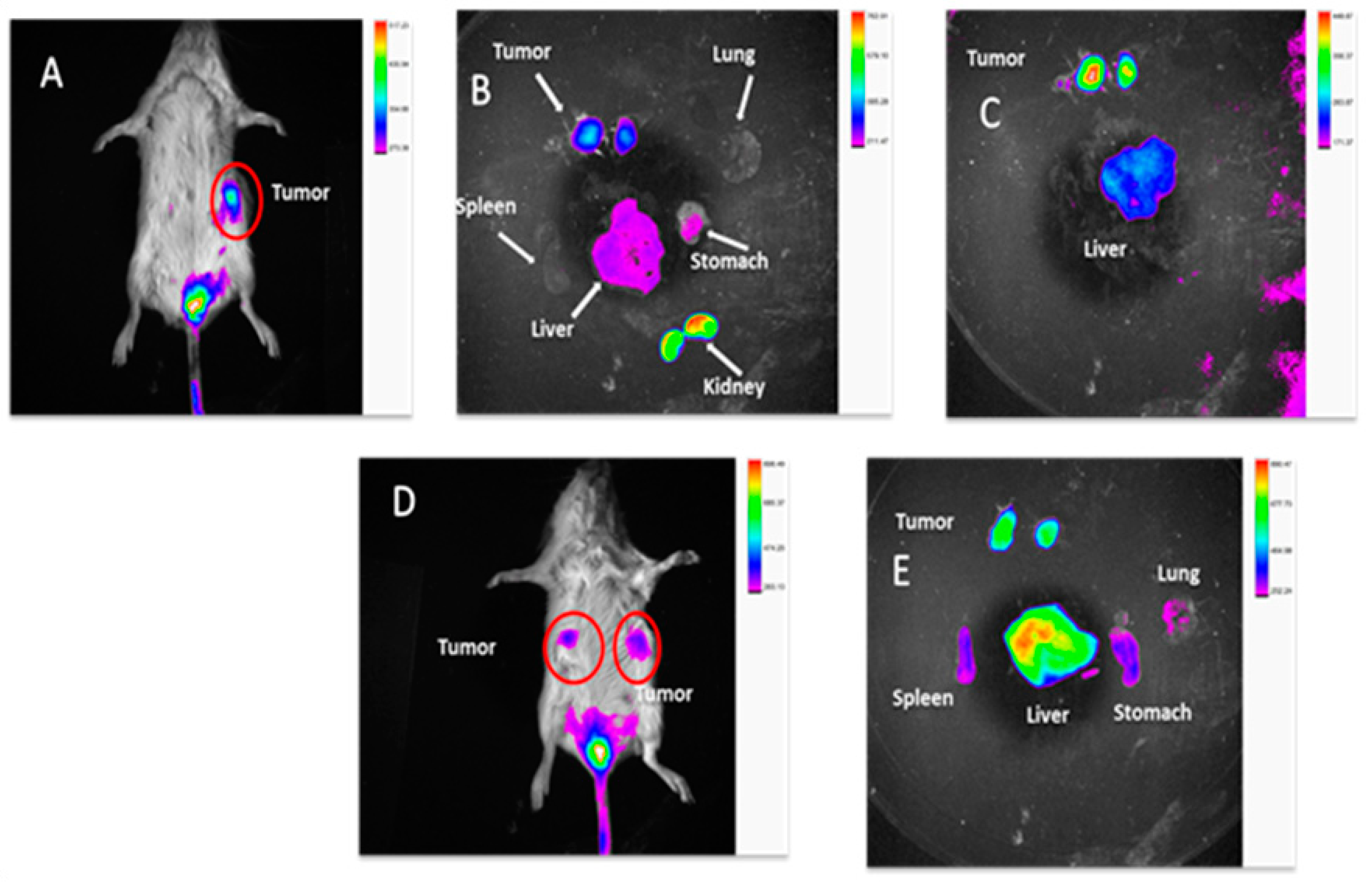
2.3.9. In Vivo TNBC PDX Tumor Targeting Efficacy in Mice
2.3.10. Statistical Analysis of the Results of Cytotoxicity Studies
3. Discussion
4. Materials and Methods
4.1. In Silico Screening for the Binding Studies
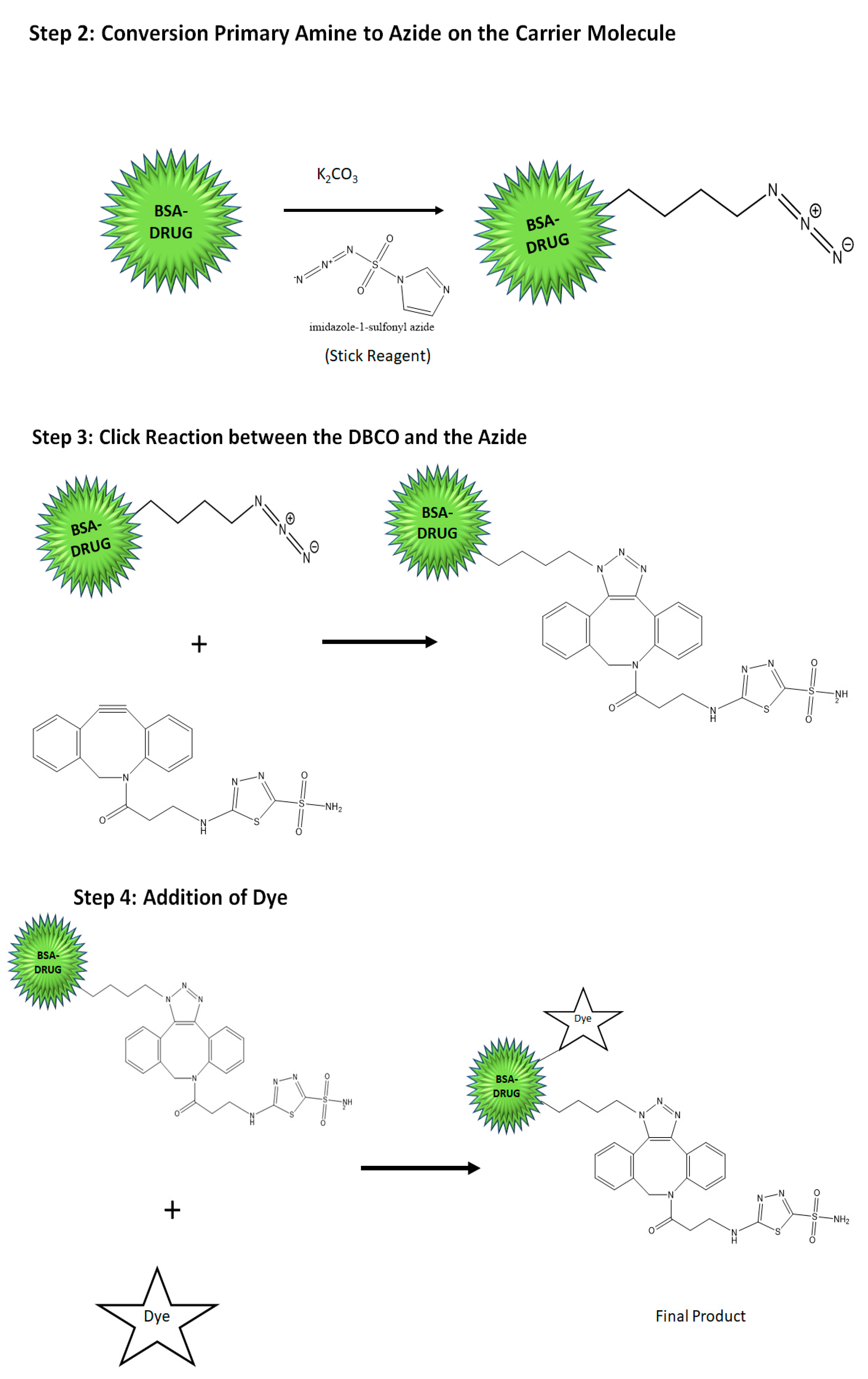
4.2. The Synthesis of the Hypoxia Targeting System
- (a)
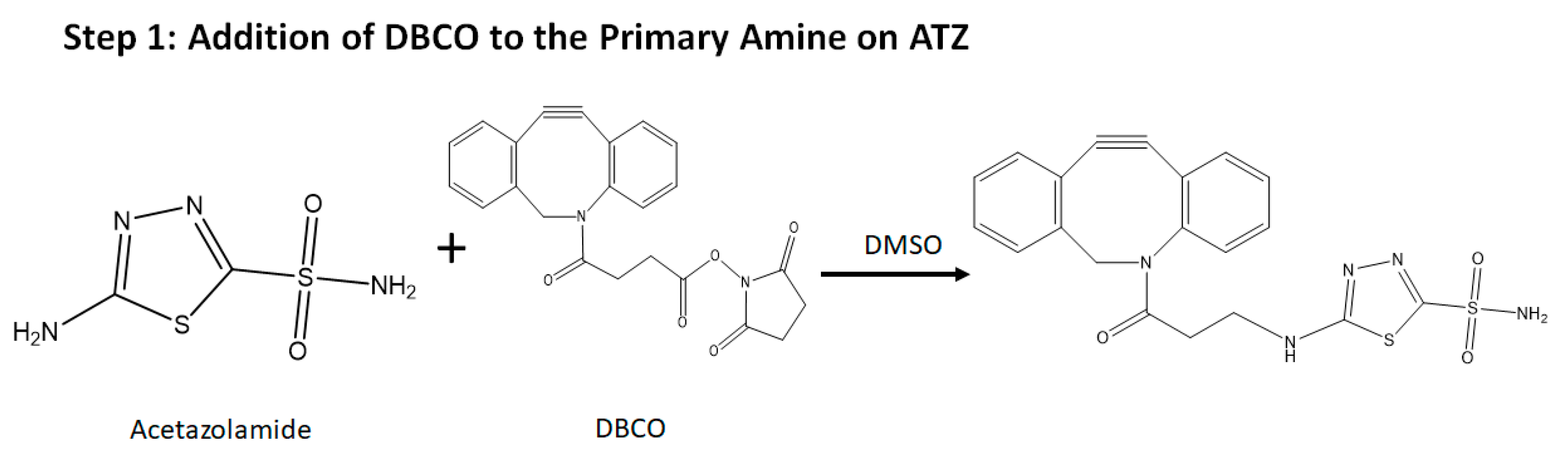
- Synthesis of the targeting ligand: Acetazolamide is the ligand bound on the BSA particle. The amide group of the acetazolamide is first activated to a primary amine group. This activated amine is conjugated to DBCO that has the functional group for click chemistry.
- (b)
- Preparation of the nanocarrier: Nanocarrier is prepared by the converting the amine group of the amino acids present on the BSA protein to azide group utilizing the Stick Reagent (imidazole-1-sulfonyl azide) and K2CO3 overnight.
- (c)
- (d)
- Conjugation of the ligand to the carrier molecule: In this step, the conjugation of the alkyne group on DBCO and the azide group on the carrier molecule with each other in a click reaction is performed at a pH of 8 and room temperature for 4–6 h. The final product is water-soluble. The product can likewise be conjugated further with an NIR dye by the click reaction or simple conjugation under stirring to result in a theranostic product.
4.3. Characterization
4.3.1. Drug Loading
4.3.2. Particle Size Analysis
4.3.3. Drug Release Studies
4.3.4. Shelf-life Assessments by Stability Studies
4.3.5. Cell Culture
4.3.6. In Vitro Cytotoxicity Studies for Understanding the Extent of Cell Killing
4.3.7. Comparative in Vitro Cytotoxicity Studies for Normoxic and Hypoxic Conditions
4.3.8. Cell Uptake Studies by Fluorescence Spectroscopy
4.3.9. Apoptosis Assay by Flow Cytometry
4.3.10. In Vivo TNBC PDX Tumor Targeting Efficacy in Mice
4.3.11. Statistical Analysis of the Results of Cytotoxicity Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- CDC. Expected New Cancer Cases and Deaths in 2020; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2016. [Google Scholar]
- Norton, K.-A.; Wallace, T.; Pandey, N.B.; Popel, A.S. An agent-based model of triple-negative breast cancer: The interplay between chemokine receptor CCR5 expression, cancer stem cells, and hypoxia. BMC Syst. Biol. 2017, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.K.; Greish, K.; Seki, T.; Okazaki, S.; Fang, J.; Takeshita, K.; Maeda, H. Polymeric micelles of zinc protoporphyrin for tumor targeted delivery based on EPR effect and singlet oxygen generation. J. Drug Target. 2007, 15, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Greish, K.; Iyer, A.K.; Fang, J.; Kawasuji, M.; Maeda, H. Enhanced permeability and retention (EPR) effect and tumor-selective delivery of anticancer drugs. Sect. Title Pharm. 2006, 10, 14. [Google Scholar]
- Daruwalla, J.; Greish, K.; Malcontenti-Wilson, C.; Muralidharan, V.; Iyer, A.; Maeda, H.; Christophi, C. Styrene maleic acid-pirarubicin disrupts tumor microcirculation and enhances the permeability of colorectal liver metastases. J. Vasc. Res. 2009, 46, 218–228. [Google Scholar] [CrossRef]
- Daruwalla, J.; Greish, K.; Nikfarjam, M.; Millar, I.; Malcontenti-Wilson, C.; Iyer, A.K.; Christophi, C. Evaluation of the effect of SMA-pirarubicin micelles on colorectal cancer liver metastases and of hyperbaric oxygen in CBA mice. J. Drug Target. 2007, 15, 487–495. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Iyer, A.K. Tumor-targeted induction of oxystress for cancer therapy. J. Drug Target. 2007, 15, 475–486. [Google Scholar] [CrossRef]
- Kesharwani, P.; Xie, L.; Mao, G.; Padhye, S.; Iyer, A.K. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surfaces B Biointerfaces 2015, 136, 413–423. [Google Scholar] [CrossRef]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Hyaluronic Acid Engineered Nanomicelles Loaded with 3,4-Difluorobenzylidene Curcumin for Targeted Killing of CD44+ Stem-Like Pancreatic Cancer Cells. Biomacromolecules 2015, 16, 3042–3053. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Alsaab, H.O.; Sau, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Folic acid conjugated polymeric micelles loaded with a curcumin difluorinated analog for targeting cervical and ovarian cancers. Colloids Surfaces B Biointerfaces 2017, 157, 490–502. [Google Scholar] [CrossRef]
- Ganesh, S.; Iyer, A.K.; Gattacceca, F.; Morrissey, D.V.; Amiji, M.M. In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J. Control. Release 2013, 172, 699–706. [Google Scholar] [CrossRef] [PubMed]
- K Iyer, A.; He, J.; M Amiji, M. Image-Guided Nanosystems for Targeted Delivery in Cancer Therapy. Curr. Med. Chem. 2012, 19, 3230–3240. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Sau, S.; Kesharwani, P.; Iyer, A.K. Polyvalent Folate-Dendrimer-Coated Iron Oxide Theranostic Nanoparticles for Simultaneous Magnetic Resonance Imaging and Precise Cancer Cell Targeting. Biomacromolecules 2017, 18, 1197–1209. [Google Scholar] [CrossRef]
- Wang, Z.; Sau, S.; Alsaab, H.O.; Iyer, A.K. CD44 directed nanomicellar payload delivery platform for selective anticancer effect and tumor specific imaging of triple negative breast cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery—New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar]
- Sau, S.; Agarwalla, P.; Mukherjee, S.; Bag, I.; Sreedhar, B.; Pal-Bhadra, M.; Patra, C.R.; Banerjee, R. Cancer cell-selective promoter recognition accompanies antitumor effect by glucocorticoid receptor-targeted gold nanoparticle. Nanoscale 2014, 6, 6745–6754. [Google Scholar] [CrossRef]
- Stockett, M.H.; Kjær, C.; Linder, M.K.; Detty, M.R.; Nielsen, S.B. Luminescence spectroscopy of chalcogen substituted rhodamine cations in vacuo. Photochem. Photobiol. Sci. 2017, 16, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.P.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin-based nanoparticles containing resveratrol: Characterization and antioxidant activity. J. Drug Deliv. Sci. Technol. 2017, 39, 147–155. [Google Scholar] [CrossRef]
- Larsen, M.T.; Kuhlmann, M.; Hvam, M.L.; Howard, K.A. Albumin-based drug delivery: Harnessing nature to cure disease. Mol. Cell. Ther. 2016, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Bhise, K.; Kashaw, S.K.; Sau, S.; Iyer, A.K. Nanostructured lipid carriers employing polyphenols as promising anticancer agents: Quality by design (QbD) approach. Int. J. Pharm. 2017, 526, 506–515. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Jain, S.; Sau, S.; Iyer, A.K. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: In vitro and ex vivo studies. J. Control. Release 2017, 253, 122–136. [Google Scholar] [CrossRef]
- Gou, Q.; Liu, L.; Wang, C.; Wu, Q.; Sun, L.; Yang, X.; Xie, Y.; Li, P.; Gong, C. Polymeric nanoassemblies entrapping curcumin overcome multidrug resistance in ovarian cancer. Colloids Surfaces B Biointerfaces 2015, 126, 26–34. [Google Scholar] [CrossRef]
- Wang, D.; Veena, M.S.; Stevenson, K.; Tang, C.; Ho, B.; Suh, J.D.; Duarte, V.M.; Faull, K.F.; Mehta, K.; Srivatsan, E.S.; et al. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor κB by an AKT-independent pathway. Clin. Cancer Res. 2008, 14, 6228–6236. [Google Scholar] [CrossRef]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-Loaded PLGA-PEG-PLGA Triblock Copolymeric Micelles: Preparation, Pharmacokinetics and Distribution In Vivo; Elsevier Inc.: Amsterdam, The Netherlands, 2011; Volume 354, ISBN 1095-7103. [Google Scholar]
- Padhye, S.; Banerjee, S.; Chavan, D.; Pandye, S.; Swamy, K.V.; Ali, S.; Li, J.; Dou, Q.P.; Sarkar, F.H. Fluorocurcumins as cyclooxygenase-2 inhibitor: Molecular docking, pharmacokinetics and tissue distribution in mice. Pharm. Res. 2009, 26, 2438–2445. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 Variant Regulates Redox Status in Cancer Cells by Stabilizing the xCT Subunit of System xc- and Thereby Promotes Tumor Growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef]
- Padhye, S.; Yang, H.; Jamadar, A.; Cui, Q.C.; Chavan, D.; Dominiak, K.; McKinney, J.; Banerjee, S.; Dou, Q.P.; Sarkar, F.H. New difluoro knoevenagel condensates of curcumin, their schiff bases and copper complexes as proteasome inhibitors and apoptosis inducers in cancer cells. Pharm. Res. 2009, 26, 1874–1880. [Google Scholar] [CrossRef]
- Dandawate, P.R.; Vyas, A.; Ahmad, A.; Banerjee, S.; Deshpande, J.; Swamy, K.V.; Jamadar, A.; Dumhe-Klaire, A.C.; Padhye, S.; Sarkar, F.H. Inclusion complex of novel curcumin analogue CDF and β-cyclodextrin (1:2) and its enhanced in vivo anticancer activity against pancreatic cancer. Pharm. Res. 2012, 29, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.K.; Zinabadi, A.; Wu, A.W.; Venkatesan, N.; Duarte, V.M.; Kang, J.J.; Dalgard, C.L.; Srivastava, M.; Sarkar, F.H.; Wang, M.B.; et al. Liposome encapsulated curcumin-difluorinated (CDF) inhibits the growth of cisplatin resistant head and neck cancer stem cells. Oncotarget 2015, 6, 18504–18517. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef]
- Kesharwani, P.; Banerjee, S.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. Parenterally administrable nano-micelles of 3,4-difluorobenzylidene curcumin for treating pancreatic cancer. Colloids Surfaces B Biointerfaces 2015, 132, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Ali, S.; Banerjee, S.; Wang, Z.; Logna, F.; Azmi, A.S.; Kong, D.; Ahmad, A.; Li, Y.; Padhye, S.; et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012, 72, 335–345. [Google Scholar] [CrossRef]
- Iyer, A.K.; Greish, K.; Fang, J.; Murakami, R.; Maeda, H. High-loading nanosized micelles of copoly(styrene-maleic acid)-zinc protoporphyrin for targeted delivery of a potent heme oxygenase inhibitor. Biomaterials 2007, 28, 1871–1881. [Google Scholar] [CrossRef]
- Svastová, E.; Hulíková, A.; Rafajová, M.; Zat’ovicová, M.; Gibadulinová, A.; Casini, A.; Cecchi, A.; Scozzafava, A.; Supuran, C.T.; Pastorek, J.; et al. Hypoxia Activates the Capacity of Tumor-Associated Carbonic Anhydrase IX to Acidify Extracellular pH. FEBS Lett 2004, 577, 439–445. [Google Scholar] [CrossRef]
- Pastorekova, S.; Pastorek, J. Carbonic Anhydrase, Its Inhibitors and Activators; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Tafreshi, N.K.; Lloyd, M.C.; Bui, M.M.; Gillies, R.J.; Morse, D.L. Carbonic Anhydrase IX as an Imaging and Therapeutic Target for Tumors and Metastases. Subcell. Biochem. 2014, 75, 221–254. [Google Scholar]
- Pastorek, J.; Pastorekova, S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin. Cancer Biol. 2015, 31, 52–64. [Google Scholar] [CrossRef]
- Sneddon, D.; Poulsen, S.A. Agents described in the Molecular Imaging and Contrast Agent Database for imaging carbonic anhydrase IX expression. J. Enzyme Inhib. Med. Chem. 2014, 29, 753–763. [Google Scholar] [CrossRef]
- Mohan, R.; Banerjee, M.; Ray, A.; Manna, T.; Wilson, L.; Owa, T.; Bhattacharyya, B.; Panda, D. Antimitotic sulfonamides inhibit microtubule assembly dynamics and cancer cell proliferation. Biochemistry 2006, 45, 5440–5449. [Google Scholar] [CrossRef]
- Kazokaitė, J.; Aspatwar, A.; Parkkila, S.; Matulis, D. An update on anticancer drug development and delivery targeting carbonic anhydrase IX. PeerJ 2017, 5, e4068. [Google Scholar] [CrossRef] [PubMed]
- Logsdon, D.P.; Grimard, M.; Luo, M.; Shahda, S.; Jiang, Y.; Tong, Y.; Yu, Z.; Zyromski, N.; Schipani, E.; Carta, F.; et al. Regulation of HIF1α under Hypoxia by APE1/Ref-1 Impacts CA9 Expression: Dual-Targeting in Patient-Derived 3D Pancreatic Cancer Models. Mol. Cancer Ther. 2016, 15, 2722–2732. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.; Peeters, S.G.J.A.; Van Kuijk, S.J.A.; Yaromina, A.; Lieuwes, N.G.; Saraya, R.; Biemans, R.; Rami, M.; Parvathaneni, N.K.; Vullo, D.; et al. Targeting carbonic anhydrase IX by nitroimidazole based sulfamides enhances the therapeutic effect of tumor irradiation: A new concept of dual targeting drugs. Radiother. Oncol. 2013, 108, 523–528. [Google Scholar] [CrossRef]
- Kanfar, N.; Tanc, M.; Dumy, P.; Supuran, C.T.; Ulrich, S.; Winum, J.Y. Effective Access to Multivalent Inhibitors of Carbonic Anhydrases Promoted by Peptide Bioconjugation. Chem. A Eur. J. 2017, 23, 6788–6794. [Google Scholar] [CrossRef] [PubMed]
- Cazzamalli, S.; Dal Corso, A.; Neri, D. Linker stability influences the anti-tumor activity of acetazolamide-drug conjugates for the therapy of renal cell carcinoma. J. Control. Release 2017, 246, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Akocak, S.; Alam, M.R.; Shabana, A.M.; Sanku, R.K.K.; Vullo, D.; Thompson, H.; Swenson, E.R.; Supuran, C.T.; Ilies, M.A. PEGylated Bis-Sulfonamide Carbonic Anhydrase Inhibitors Can Efficiently Control the Growth of Several Carbonic Anhydrase IX-Expressing Carcinomas. J. Med. Chem. 2016, 59, 5077–5088. [Google Scholar] [CrossRef]
- Supuran, C.; Briganti, F.; Tilli, S.; Chegwidden, W.; Scozzafava, A. Carbonic anhydrase inhibitors: Sulfonamides as antitumor agents? Bioorg. Med. Chem. 2001, 9, 703–714. [Google Scholar] [CrossRef]
- Maresca, A.; Temperini, C.; Pochet, L.; Masereel, B.; Scozzafava, A.; Supuran, C.T. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J. Med. Chem. 2010, 53, 335–344. [Google Scholar] [CrossRef]
- Owa, T.; Yoshino, H.; Okauchi, T.; Yoshimatsu, K.; Ozawa, Y.; Sugi, N.H.; Nagasu, T.; Koyanagi, N.; Kitoh, K. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J. Med. Chem. 1999, 42, 3789–3799. [Google Scholar] [CrossRef]
- Gabow, P.A.; Peterson, L.N. Renal and Electrolyte Disorders; Little, Brown and Company: Boston, MA, USA, 1976. [Google Scholar]
- Winum, J.Y.; Rami, M.; Scozzafava, A.; Montero, J.L.; Supuran, C. Carbonic anhydrase IX: A new druggable target for the design of antitumor agents. Med. Res. Rev. 2008, 28, 445–463. [Google Scholar] [CrossRef] [PubMed]
- Zatovicova, M.; Jelenska, L.; Hulikova, A.; Csaderova, L.; Ditte, Z.; Ditte, P.; Goliasova, T.; Pastorek, J.; Pastorekova, S. Carbonic anhydrase IX as an anticancer therapy target: Preclinical evaluation of internalizing monoclonal antibody directed to catalytic domain. Curr. Pharm. Des. 2010, 16, 3255–3263. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases as drug targets—An overview. Curr. Top. Med. Chem. 2007, 7, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention effect for selective targeting of anticancer nanomedicine: Are we there yet? Drug Discov. Today Technol. 2012, 9, e161–e166. [Google Scholar] [CrossRef]
- Tatiparti, K.; Rauf, M.A.; Sau, S. A Biomimetic Drug Delivery System Targeting Tumor Hypoxia in Triple-Negative Breast Cancers. Appl. Sci. 2020, 10, 1075. [Google Scholar] [CrossRef]
- Tatiparti, K.; Sau, S.; Gawde, K.; Iyer, A. Copper-Free ‘Click’ Chemistry-Based Synthesis and Characterization of Carbonic Anhydrase-IX Anchored Albumin-Paclitaxel Nanoparticles for Targeting Tumor Hypoxia. Int. J. Mol. Sci. 2018, 19, 838. [Google Scholar] [CrossRef]
- Gawde, K.A.; Kesharwani, P.; Sau, S.; Sarkar, F.H.; Padhye, S.; Kashaw, S.K.; Iyer, A.K. Synthesis and characterization of folate decorated albumin bio-conjugate nanoparticles loaded with a synthetic curcumin difluorinated analogue. J. Colloid Interface Sci. 2017, 496, 290–299. [Google Scholar] [CrossRef]
- Sau, S.; Alsaab, H.O.; Kashaw, S.K.; Tatiparti, K.; Iyer, A.K. Advances in antibody-drug conjugates: A new era of targeted cancer therapy. Drug Discov. Today 2017, 22, 10–1547. [Google Scholar] [CrossRef] [PubMed]
- Sau, S.; Alsaab, H.O.; Bhise, K.; Alzhrani, R.; Nabil, G.; Iyer, A.K. Multifunctional nanoparticles for cancer immunotherapy: A groundbreaking approach for reprogramming malfunctioned tumor environment. J. Control. Release 2018, 274, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Lutz, J.F.; Zarafshani, Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne “click” chemistry. Adv. Drug Deliv. Rev. 2008, 60, 958–970. [Google Scholar] [CrossRef]
- Kolb, H.C.; Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Best, M.D. Click chemistry and bioorthogonal reactions: Unprecedented selectivity in the labeling of biological molecules. Biochemistry 2009, 48, 6571–6584. [Google Scholar] [CrossRef]
- Totobenazara, J.; Burke, A.J. New click-chemistry methods for 1,2,3-triazoles synthesis: Recent advances and applications. Tetrahedron Lett. 2015, 56, 2853–2859. [Google Scholar] [CrossRef]
- Huang, B.; Kukowska-Latallo, J.F.; Tang, S.; Zong, H.; Johnson, K.B.; Desai, A.; Gordon, C.L.; Leroueil, P.R.; Baker, J.R. The facile synthesis of multifunctional PAMAM dendrimer conjugates through copper-free click chemistry. Bioorg. Med. Chem. Lett. 2012, 22, 3152–3156. [Google Scholar] [CrossRef]
- Ziperstein, M.J.; Guzman, A.; Kaufman, L.J. Breast cancer cell line aggregate morphology does not predict invasive capacity. PLoS ONE 2015, 10, 9–e0139523. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Zatovicova, M.; Pastorek, J. Cancer-Associated Carbonic Anhydrases and Their Inhibition. Curr. Pharm. Des. 2008, 14, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Helena Ng, H.L.; Lu, A.; Lin, G.; Qin, L.; Yang, Z. The potential of liposomes with carbonic anhydrase IX to deliver anticancer ingredients to cancer cells in Vivo. Int. J. Mol. Sci. 2014, 16, 230–255. [Google Scholar]
- Supuran, C.T. Carbonic anhydrase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 3467–3474. [Google Scholar] [CrossRef]
- Monti, S.M.; Supuran, C.T.; De Simone, G. Carbonic Anhydrase IX as a Target for Designing Novel Anticancer Drugs. Curr. Med. Chem. 2012, 19, 821–830. [Google Scholar] [CrossRef]
- Winum, J.-Y.; Scozzafava, A.; Montero, J.-L.; Supuran, C.T. Inhibition of carbonic anhydrase IX: A new strategy against cancer. Anticancer. Agents Med. Chem. 2009, 9, 693–702. [Google Scholar] [CrossRef]
- Almansour, A.I.; Arumugam, N.; Suresh Kumar, R.; Mahalingam, S.M.; Sau, S.; Bianchini, G.; Menéndez, J.C.; Altaf, M.; Ghabbour, H.A. Design, synthesis and antiproliferative activity of decarbonyl luotonin analogues. Eur. J. Med. Chem. 2017, 138, 932–941. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Iyer, A.K. Stumuli-Responsive Bio-Hybrid Nanogels: An Emerging Platform in Medicinal Arena. Glob. J. Nanomed. 2017, 1, 6–8. [Google Scholar]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Aquino-Parsons, C.; MacPhail, S.H.; Liao, S.Y.; Stanbridge, E.J.; Raleigh, J.A.; Lerman, M.I. Carbonic anhydrase 9 as an endogenous marker for hypoxic cells in cervical cancer. Cancer Res. 2001, 61, 8924–8929. [Google Scholar] [PubMed]
- Supuran, C.T.; Scientifico, P.; Bioinorganica, C. Carbonic Anhydrase IX: A New Drug Target for Designing Diagnostic Tools and Antitumor Agents. 2009, 37, 259–270. Hacettepe J. Biol. Chem. 2009, 37, 259–270. [Google Scholar]
- Cianchi, F.; Vinci, M.M.C.; Supuran, C.T.C.; Peruzzi, B.; De Giuli, P.; Fasolis, G.; Perigli, G.; Pastorekova, S.; Papucci, L.; Pini, A.; et al. Selective inhibition of carbonic anhydrase IX decreases cell proliferation and induces ceramide-mediated apoptosis in human cancer cells. J. Pharmacol. Exp. Ther. 2010, 334, 710–719. [Google Scholar] [CrossRef]
- Tan, E.Y.; Yan, M.; Campo, L.; Han, C.; Takano, E.; Turley, H.; Candiloro, I.; Pezzella, F.; Gatter, K.C.; Millar, E.K.A.; et al. The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br. J. Cancer 2009, 100, 405–411. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds …… are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatiparti, K.; Rauf, M.A.; Sau, S.; Iyer, A.K. Carbonic Anhydrase-IX Guided Albumin Nanoparticles for Hypoxia-mediated Triple-Negative Breast Cancer Cell Killing and Imaging of Patient-derived Tumor. Molecules 2020, 25, 2362. https://doi.org/10.3390/molecules25102362
Tatiparti K, Rauf MA, Sau S, Iyer AK. Carbonic Anhydrase-IX Guided Albumin Nanoparticles for Hypoxia-mediated Triple-Negative Breast Cancer Cell Killing and Imaging of Patient-derived Tumor. Molecules. 2020; 25(10):2362. https://doi.org/10.3390/molecules25102362
Chicago/Turabian StyleTatiparti, Katyayani, Mohd Ahmar Rauf, Samaresh Sau, and Arun K. Iyer. 2020. "Carbonic Anhydrase-IX Guided Albumin Nanoparticles for Hypoxia-mediated Triple-Negative Breast Cancer Cell Killing and Imaging of Patient-derived Tumor" Molecules 25, no. 10: 2362. https://doi.org/10.3390/molecules25102362
APA StyleTatiparti, K., Rauf, M. A., Sau, S., & Iyer, A. K. (2020). Carbonic Anhydrase-IX Guided Albumin Nanoparticles for Hypoxia-mediated Triple-Negative Breast Cancer Cell Killing and Imaging of Patient-derived Tumor. Molecules, 25(10), 2362. https://doi.org/10.3390/molecules25102362