Self-Healing Properties of Bioinspired Amorphous CaCO3/Polyphosphate-Supplemented Cement
Abstract
1. Introduction
2. Results
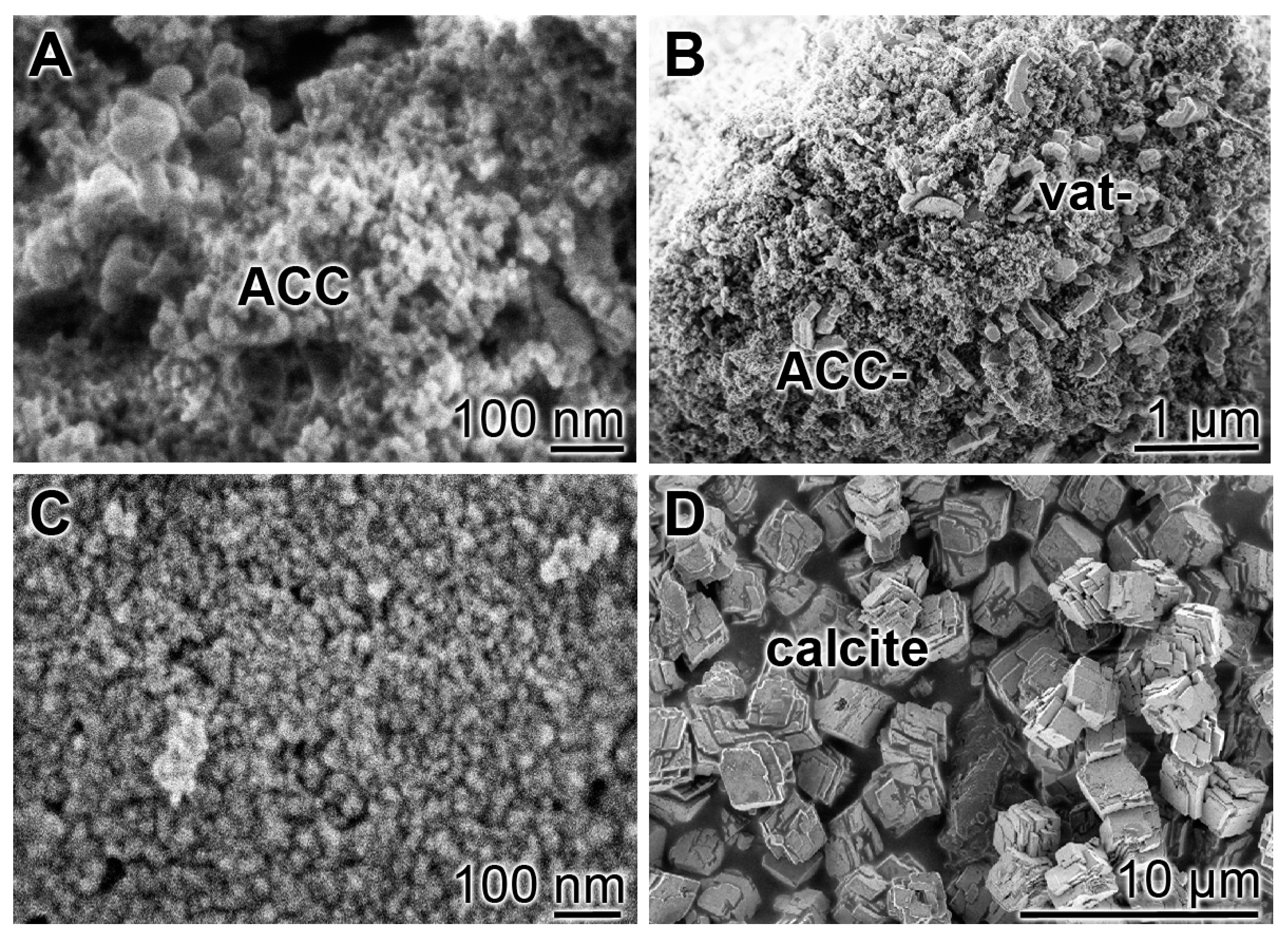
2.1. Morphology of Amorphous Calcium Carbonate Nanoparticles
2.2. XRD Analyses of ACC-NP
2.3. Morphology of Cement Supplemented with “ACCP10”; “hCEM•ACCP10”
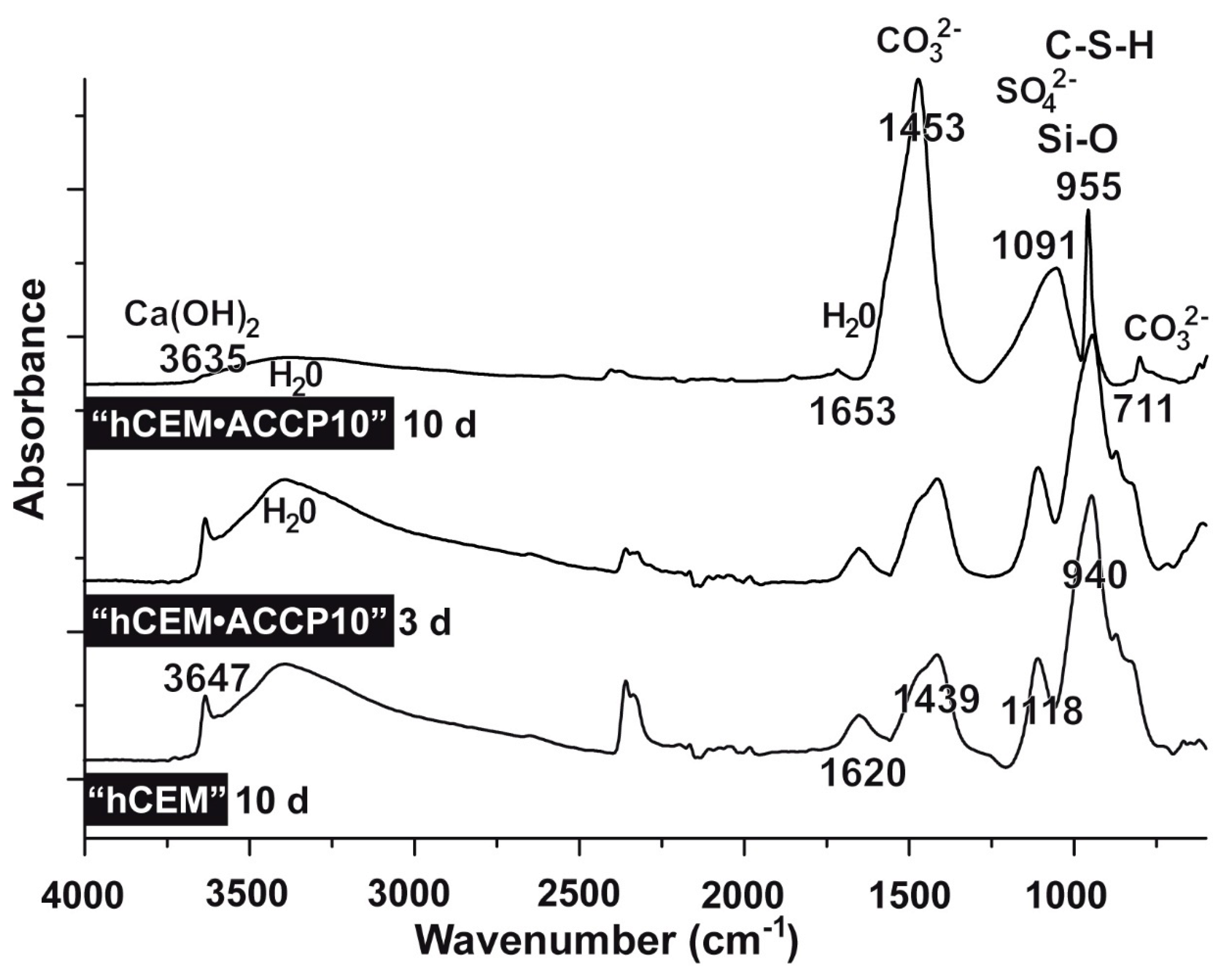
2.4. FTIR Analyses
2.5. XRD Analyses of Cement Samples
2.6. Mechanical Properties–Compression Testing
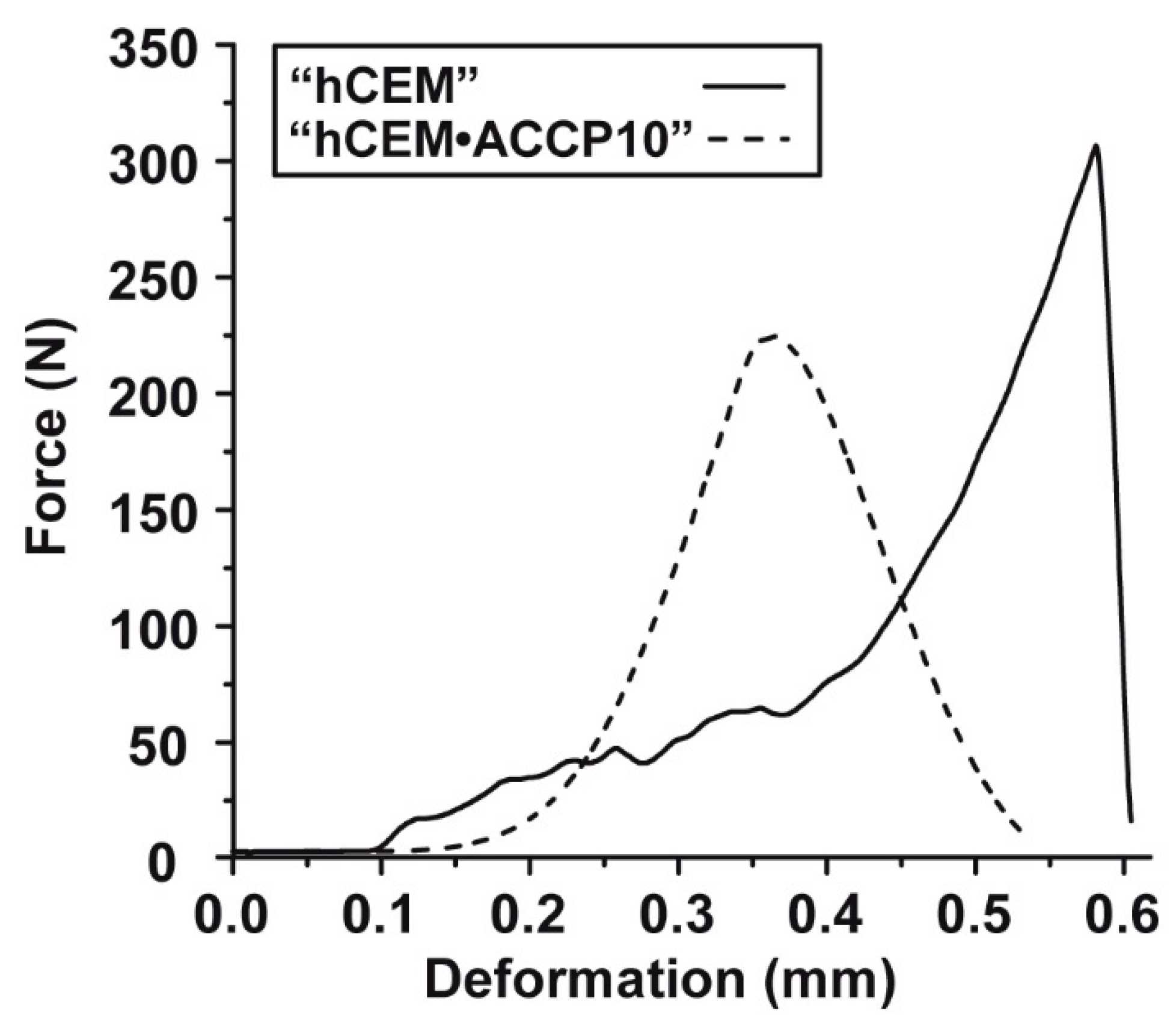
2.7. Mechanical Testing: 3-Point Bending Studies
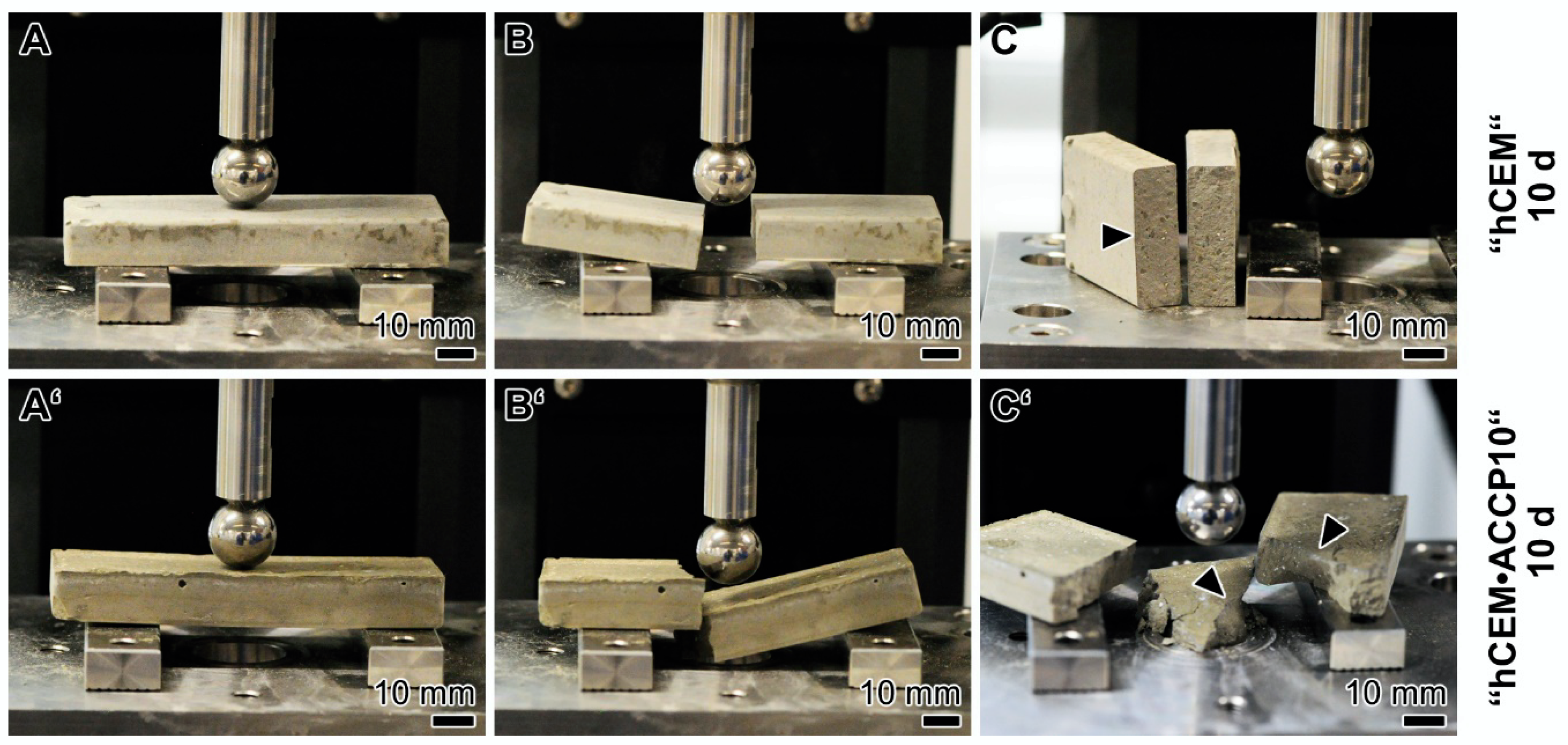
2.8. Self-healing Potential
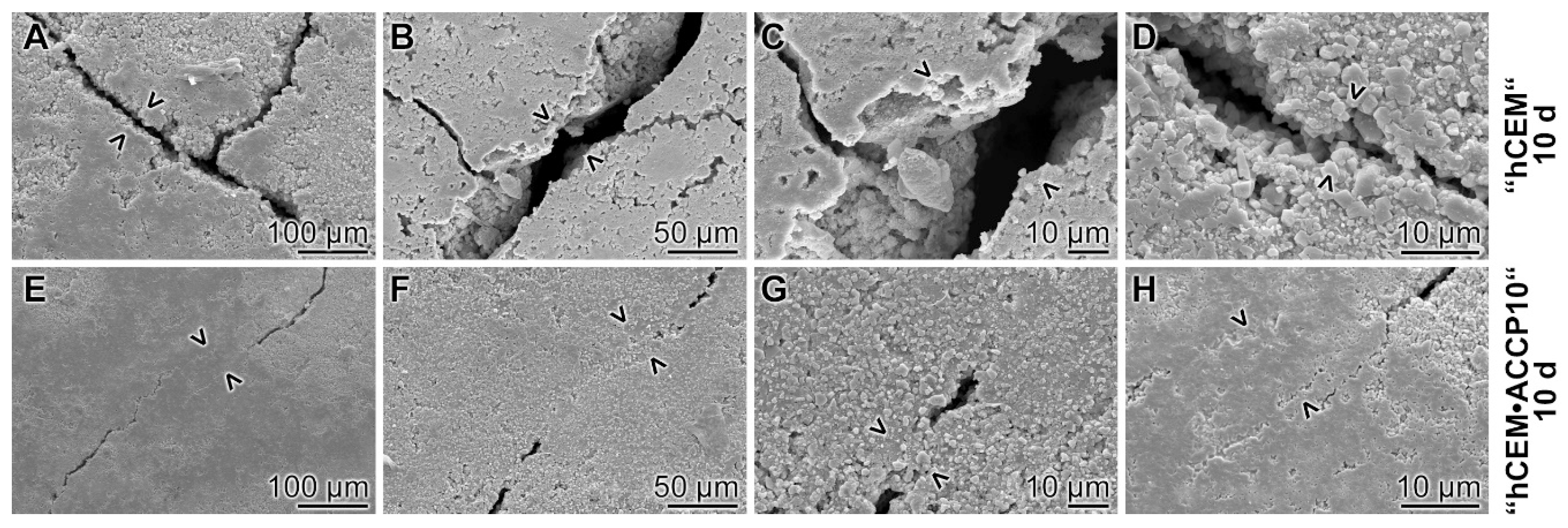
2.9. Surface Texture
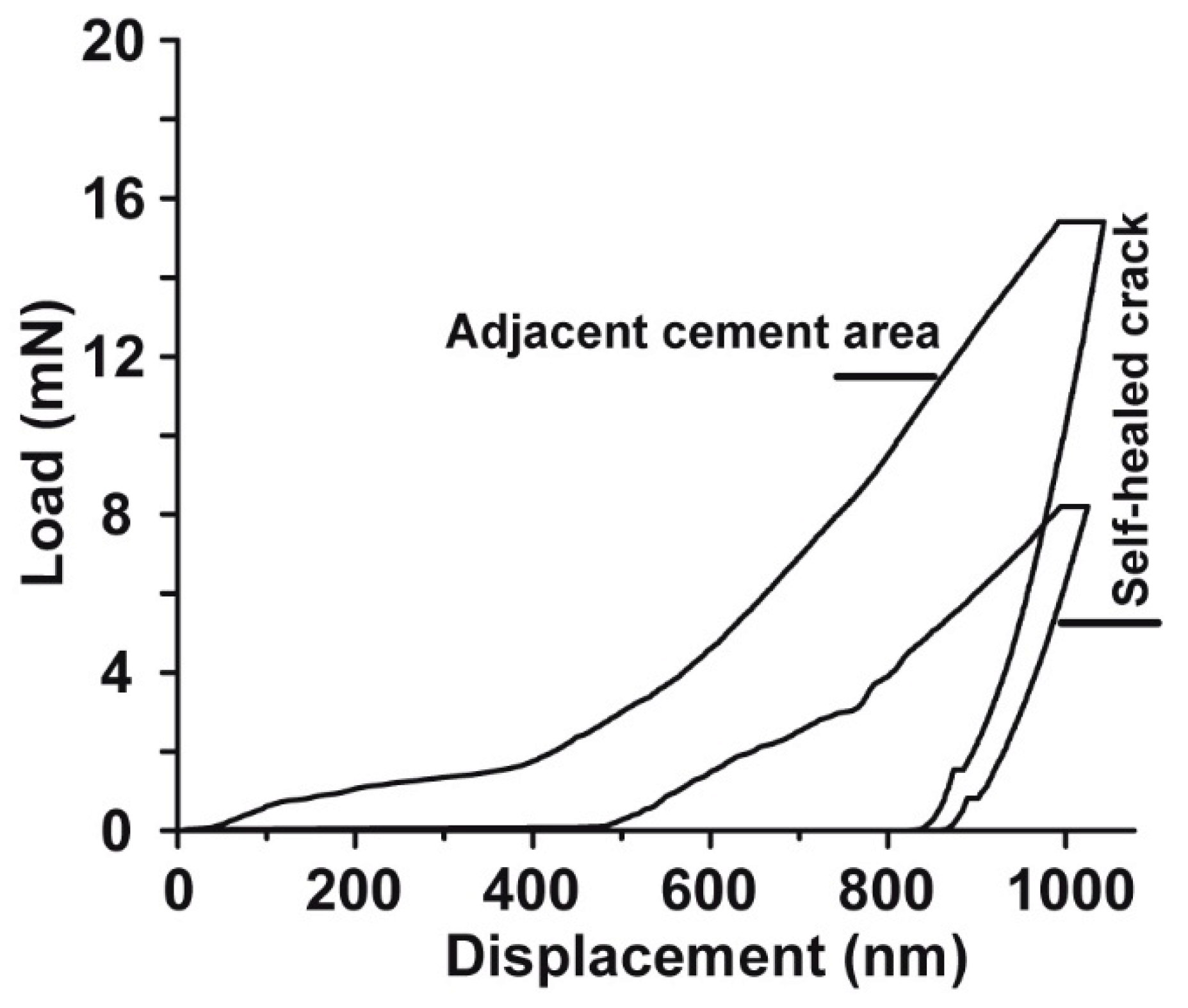
2.10. Mechanical Properties within Resealed Microcracks
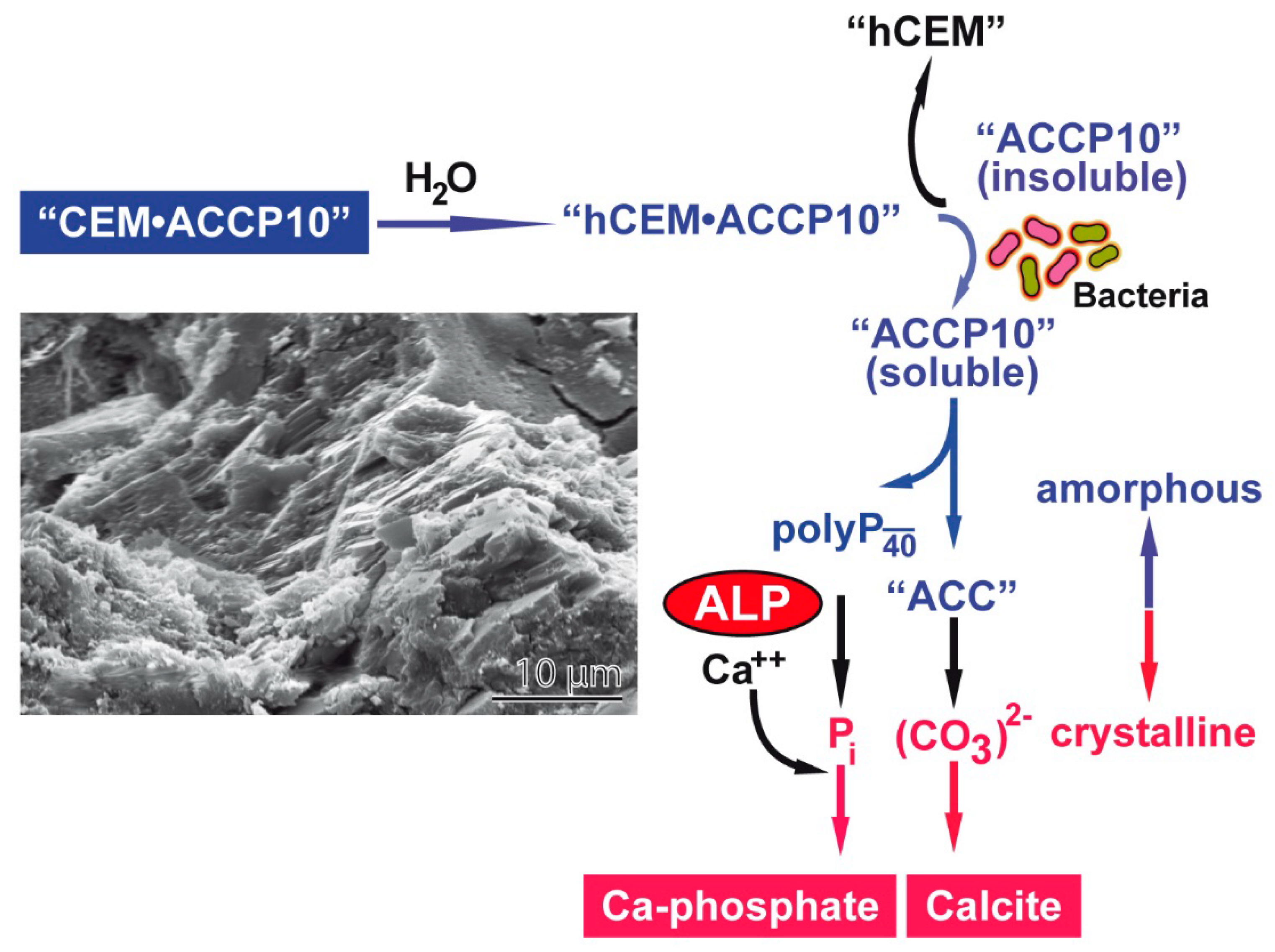
2.11. Analysis of Material Oozed into Cracks
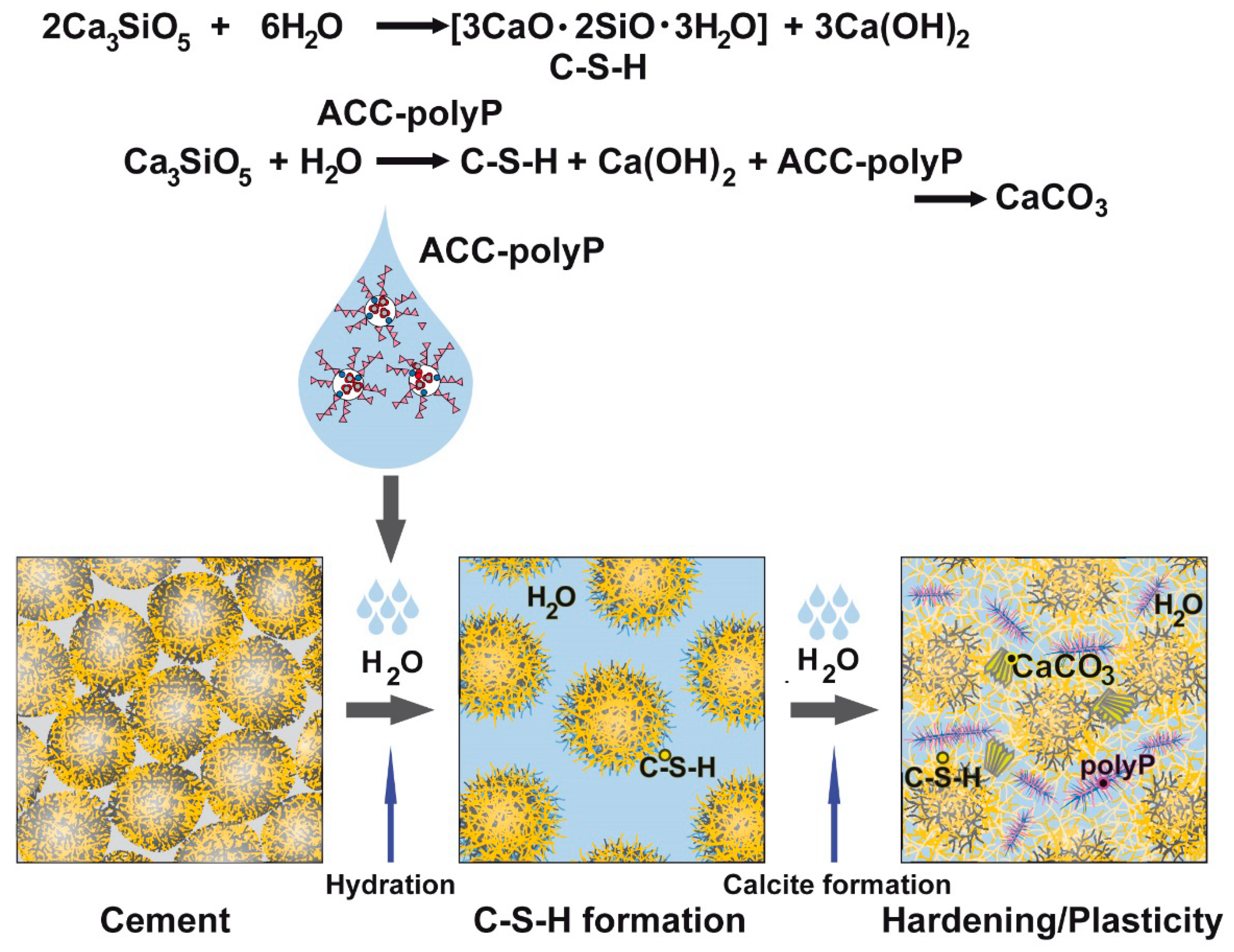
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Amorphous Ca-Carbonate Microparticles
4.3. Preparation of Cement Samples
4.4. Microscopic Analyses
4.5. Fourier Transformed Infrared Spectroscopy
4.6. X-Ray Diffraction
4.7. Compression Testing
4.8. Mechanical Toughness: 3-Point Bend Testing
4.9. Microcrack Formation and Self-healing Analysis
4.10. Determination of Mechanical Properties within Self-healed Microcracks
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hewlett, P.; Liska, M. Lea’s Chemistry of Cement and Concrete, 5th ed.; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Dunuweera, S.P.; Rajapakse, R.M.G. Cement types, composition, uses and advantages of nanocement, environmental impact on cement production, and possible solutions. Adv. Mater. Sci. Eng. 2018. [Google Scholar] [CrossRef]
- Davies, R.; Teall, O.; Pilegis, M.; Kanellopoulos, A.; Sharma, T.; Jefferson, A.; Gardner, D.; Al-Tabbaa, A.; Paine, K.; Lark, R. Large scale application of self-healing concrete: Design, construction, and testing. Front. Mater. 2018, 5. [Google Scholar] [CrossRef]
- De Rooij, M.; Tittelboom, K.V.; Belie, N.D.; Schlangen, E. Self-Healing Phenomena in Cement-Based Materials: State-of-the-Art Report of RILEM Technical Committee 221-SHC: Self-Healing Phenomena in Cement-Based Materials; Springer Science & Business Media: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Isaacs, B.; Lark, R.; Jefferson, T.; Davies, R.; Dunn, S. Crack healing of cementitious materials using shrinkable polymer tendons. Struct. Concr. 2013, 14, 138–147. [Google Scholar] [CrossRef]
- Ghosh, S.K. Self-Healing Materials: Fundamentals, Design Strategies, and Applications; Wiley Online Library: Weinheim, Germany, 2009. [Google Scholar]
- Jacobsen, S.; Marchand, J.; Hornain, H. SEM observations of the microstructure of frost deteriorated and self-healed concretes. Cem. Concr. Res. 1995, 25, 1781–1790. [Google Scholar] [CrossRef]
- Granger, S.; Loukili, A.; Pijaudier-Cabot, G.; Chanvillard, G. Mechanical characterization of the self-healing effect of cracks in ultra high performance concrete (UHPC). In Proceedings of the 3rd International Conference on Construction Materials, Performance, Innovations and Structural Implications, ConMat, Vancouver, BC, Canada, 22–24 August 2005; Volume 5, pp. 5–22. [Google Scholar]
- Hearn, N. Self-sealing, autogenous healing and continued hydration: What is the difference? Mater. Struct. 1998, 31, 563–567. [Google Scholar] [CrossRef]
- Clear, C.A. The effects of autogenous healing upon the leakage of water through cracks in concrete; Cement and Concrete Association-Wexham Springs: Slough, UK, 1985. [Google Scholar]
- Edvardsen, C. Water permeability and autogenous healing of cracks in concrete. ACI Mater. J. 1999, 96, 448–454. [Google Scholar]
- Müller, W.E.G.; Tolba, E.; Wang, S.; Li, Q.; Neufurth, M.; Ackermann, M.; Muñoz-Espí, R.; Schröder, H.C.; Wang, X.H. Transformation of construction cement to a self-healing hybrid binder. Int. J. Mol. Sci.-Mater. Sci. 2019, 20, 2948. [Google Scholar]
- Müller, W.E.G.; Wang, S.; Tolba, E.; Neufurth, M.; Ackermann, M.; Muñoz-Espí, R.; Lieberwirth, I.; Glasser, G.; Schröder, H.C.; Wang, X.H. Transformation of amorphous polyphosphate nanoparticles into coacervate complexes: An approach for the encapsulation of mesenchymal stem cells. Small 2018, 14, e1801170. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Schröder, H.C.; Wang, X.H. Inorganic polyphosphates as storage for and generator of metabolic energy in the extracellular matrix. Chem. Rev. 2019, 119, 12337–12374. [Google Scholar] [CrossRef]
- Tolba, E.; Müller, W.E.G.; El-Hady, B.M.A.; Neufurth, M.; Wurm, F.; Wang, S.; Schröder, H.C.; Wang, X.H. High biocompatibility and improved osteogenic potential of amorphous calcium carbonate/vaterite. J. Mater. Chem. B 2016, 4, 376–386. [Google Scholar] [CrossRef]
- Fitzner, B.J. Investigation of weathering damage on stone monuments. Geonomos 2016, 24, 1–15. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Belkowitz, J.S.; Armentrout, D. An investigation of nano silica in the cement hydration process. Mater. Sci. 2009. [Google Scholar] [CrossRef]
- Curry, M.D.; Boston, P.J.; Spilde, M.N.; Baichtal, J.F.; Campbell, A.R. Cottonballs, a unique subaqeous moonmilk, and abundant subaerial moonmilk in Cataract Cave, Tongass National Forest, Alaska. Int. J. Speleol. 2009, 38, 111–128. [Google Scholar] [CrossRef][Green Version]
- Nguyen, H.A.; Chang, T.P.; Shih, J.Y.; Chen, C.T.; Nguyen, T.D. Sulfate resistance of low energy SFC no-cement mortar. Constr. Build. Mater. 2016, 102, 239–243. [Google Scholar] [CrossRef]
- Berger, R.L.; Frohnsdorff, G.J.C.; Harris, P.H.; Johnson, P.D. Application of X-ray diffraction to routine mineralogical analysis of Portland cement. In Symposium on Structure of Portland Cement Paste and Concrete–Highway Research Board Special Report: 90; Highway Research Board: Washington, DC, USA, 1966; pp. 234–253. [Google Scholar]
- Wolter, J.M.; Schmeide, K.; Huittinen, N.; Stumpf, T. Cm(III) retention by calcium silicate hydrate (C-S-H) gel and secondary alteration phases in carbonate solutions with high ionic strength: A site-selective TRLFS study. Sci. Rep. 2019, 9, 14255. [Google Scholar] [CrossRef]
- Prussin, A.J.; Garcia, E.B.; Marr, L.C. Total concentrations of virus and bacteria in indoor and outdoor air. Environ. Sci. Technol. Lett. 2015, 2, 84–88. [Google Scholar] [CrossRef]
- Lorenz, B.; Schröder, H.C. Mammalian intestinal alkaline phosphatase acts as highly active exopolyphosphatase. Biochim. Biophys. Acta 2001, 1547, 254–261. [Google Scholar] [CrossRef]
- Konrad, F.; Gallien, F.; Gerard, D.E.; Dietzel, M. Transformation of amorphous calcium carbonate in air. Cryst. Growth Des. 2016, 16, 6310–6317. [Google Scholar] [CrossRef]
- Thiery, M.; Villain, G.; Dangla, P.; Platret, G. Investigation of the carbonation front shape on cementitious materials: Effects of the chemical kinetics. Cem. Concr. Res. 2007, 37, 1047–1058. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Gao, X.F.; Tam, C.M. Environmental enhancement through use of recycled aggregate concrete in a two-stage mixing approach. Hum. Ecol. Risk Ass. 2006, 12, 277–288. [Google Scholar] [CrossRef][Green Version]
- Wen, H.; Kang, W.; Liu, X.; Li, W.; Zhang, L.; Zhang, C. Two-phase interface hydrothermal synthesis of binder-free SnS2/graphene flexible paper electrodes for high-performance Li-ion batteries. RSC Adv. 2019, 9, 23607. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Schlangen, E. Self-healing of cracked concrete: A bacterial approach. In High Performance Concrete, Brick Masonry and Environmental Aspects–(17.7.–22.7.2007 London); Carpinteri, A., Gambarova, P.G., Ferro, G., Plizzari, G.A., Eds.; Taylor & Francis: Leiden, The Netherlands, 2007; pp. 1821–1826. [Google Scholar]
- Radha, A.V.; Forbes, T.Z.; Killian, C.E.; Gilbert, P.U.; Navrotsky, A. Transformation and crystallization energetics of synthetic and biogenic amorphous calcium carbonate. Proc. Natl. Acad. Sci. USA 2010, 107, 16438–16443. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rashid, M.H.; Hossain, M.A.; Adrita, F.S.; Hossain, T. Mixing time effects on properties of self compacting concrete. J. Eng. Appl. Sci. 2011, 6, 108–114. [Google Scholar]
- Giacomini, A.; Ackermann, M.; Belleri, M.; Coltrini, D.; Nico, B.; Ribatti, D.; Konerding, M.A.; Presta, M.; Righi, M. Brain angioarchitecture and intussusceptive microvascular growth in a murine model of Krabbe disease. Angiogenesis 2015, 18, 499–510. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Tolba, E.; Schröder, H.C.; Wang, S.; Glaßer, G.; Muñoz-Espí, R.; Link, T.; Wang, X.H. A new polyphosphate calcium material with morphogenetic activity. Mater. Lett. 2015, 148, 163–166. [Google Scholar] [CrossRef]
- Fischer, V.; Lieberwirth, I.; Jakob, G.; Landfester, K.; Muñoz-Espí, R. Metal oxide/polymer hybrid nanoparticles with versatile functionality prepared by controlled surface crystallization. Adv. Funct. Mater. 2013, 23, 451–466. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Lee, S.H.; Teramoto, Y.; Wang, S.; Pharr, G.M.; Rials, T.G. Nanoindentation of biodegradable cellulose diacetate-graft-poly(l-lactide) copolymers: Effect of molecular composition and thermal aging on mechanical properties. J. Polym. Sci. B 2007, 45, 1114–1121. [Google Scholar] [CrossRef]
- Klein, C.A. Anisotropy of Young’s modulus and Poisson’s ratio in diamond. Mater. Res. Bull. 1992, 27, 1407–1414. [Google Scholar] [CrossRef]
- Chu, Y.H.; Yu, X.X.; Jin, X.; Wang, Y.T.; Zhao, D.J.; Zhang, P.; Sun, G.M.; Zhang, Y.H. Purification and characterization of alkaline phosphatase from lactic acid bacteria. RSC Adv. 2019, 9, 354. [Google Scholar] [CrossRef]
- Wang, X.; Xu, K.; Li, Y.; Guo, S. Dissolution and leaching mechanisms of calcium ions in cement based materials. Constr. Build. Mater. 2018, 180, 103–108. [Google Scholar] [CrossRef]
- Pinto, O.A.; Tabaković, A.; Goff, T.M.; Liu, Y.; Adair, J.H. Calcium phosphate and calcium phosphosilicate mediated drug delivery and imaging. In Intracellular Delivery: Fundamentals and Applications, Fundamental Biomedical Technologies; Prokop, A., Ed.; Springer Science+Business Media B.V.: Berlin, Germany, 2011; pp. 713–744. [Google Scholar]
- Eanes, E.D.; Meyer, J.L. The maturation of crystalline calcium phosphates in aqueous suspensions at physiologic pH. Calcif. Tissue Res. 1977, 23, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, F.C.; Cölfen, H. Controlling mineral morphologies and structures in biological and synthetic systems. Chem. Rev. 2008, 108, 4332–4432. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the nanoparticles, are available from the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolba, E.; Wang, S.; Wang, X.; Neufurth, M.; Ackermann, M.; Muñoz-Espí, R.; Abd El-Hady, B.M.; Schröder, H.C.; Müller, W.E.G. Self-Healing Properties of Bioinspired Amorphous CaCO3/Polyphosphate-Supplemented Cement. Molecules 2020, 25, 2360. https://doi.org/10.3390/molecules25102360
Tolba E, Wang S, Wang X, Neufurth M, Ackermann M, Muñoz-Espí R, Abd El-Hady BM, Schröder HC, Müller WEG. Self-Healing Properties of Bioinspired Amorphous CaCO3/Polyphosphate-Supplemented Cement. Molecules. 2020; 25(10):2360. https://doi.org/10.3390/molecules25102360
Chicago/Turabian StyleTolba, Emad, Shunfeng Wang, Xiaohong Wang, Meik Neufurth, Maximilian Ackermann, Rafael Muñoz-Espí, Bothaina M. Abd El-Hady, Heinz C. Schröder, and Werner E. G. Müller. 2020. "Self-Healing Properties of Bioinspired Amorphous CaCO3/Polyphosphate-Supplemented Cement" Molecules 25, no. 10: 2360. https://doi.org/10.3390/molecules25102360
APA StyleTolba, E., Wang, S., Wang, X., Neufurth, M., Ackermann, M., Muñoz-Espí, R., Abd El-Hady, B. M., Schröder, H. C., & Müller, W. E. G. (2020). Self-Healing Properties of Bioinspired Amorphous CaCO3/Polyphosphate-Supplemented Cement. Molecules, 25(10), 2360. https://doi.org/10.3390/molecules25102360









