Abstract
Plants of the genus Callicarpa are known to possess several medicinal effects. The constituents of the Taiwan endemic plant Callicarpa hypoleucophylla have never been studied. Therefore, C. hypoleucophylla was selected for our phytochemical investigation. Two new clerodane-type diterpenoids, named callihypolins A (1) and B (2), along with seven known compounds were isolated from the leaves and twigs of the Lamiaceae plant C. hypoleucophylla and then characterized. The structures of compounds 1 and 2 were elucidated by spectroscopic data analysis, specifically, two-dimension nuclear magnetic resonance (NMR). The anti-inflammatory activity of compounds 1–9 based on the suppression of superoxide anion generation and elastase release was evaluated. Among the isolates, compounds 2–4 showed anti-inflammatory activity (9.52−32.48% inhibition at the concentration 10 μm) by suppressing superoxide anion generation and elastase release. Our findings not only expand the description of the structural diversity of the compounds present in plants of the genus Callicarpa but also highlight the possibility of developing anti-inflammatory agents from Callicarpa endemic species.
1. Introduction
Callicarpa (family Lamiaceae) is a genus of about 190 species of herbaceous plants. The plant is geographically found throughout east and southeast Asia, Australia, Madagascar, southeast North America, and South America [1]. Folkloric usage of various parts of Callicarpa includes preparations used as fish poisons [2,3], insect repellents [1], and for some medical indications [3]. The phytochemical investigation of this genus has resulted in the identification of diterpenoids, phenylethanoids, phenypropanoids, and flavonoids. These components display various biological effects, such as anti-inflammatory [4,5,6], anti-platelet aggregation [7], hemostatic [8], antioxidative [9,10], cytotoxic [6,11,12], and neuroprotective [13], antitubercular [14], hepatoprotective [15,16], antimicrobial [17], anti-arthritic [18], as well as analgesic properties [19]. From the above-mentioned phytochemical and biological studies, we know this genus may offer a rich supply of bioactive phytochemicals. Because the phytochemical profile of the Taiwanese endemic plant Callicarpa hypoleucophylla has never been analyzed, we carried out an investigation of the constituents and bioactivity of C. hypoleucophylla. A meticulous separation of an ethanolic extract of C. hypoleucophylla led to the isolation of two new clerodane-type diterpenoids that we named callihypolins A and B (1 and 2), together with seven known analogues (3–9). The anti-inflammatory evaluation of these isolates is also presented in this paper.
2. Results and Discussion
The leaves and twigs of C. hypoleucophylla were extracted with 95% ethanol; the yielded extracts were suspended in H2O and extracted with ethyl acetate (EtOAc). The EtOAc-soluble part was further partitioned with hexanes/methanol (MeOH)/H2O (4:3:1) to obtain a MeOH layer. The MeOH layer was subjected to extensive chromatography by normal- and reversed-phase HPLC, using a normal-phase silica gel open column and a Sephadex LH-20 resin column, supplying callihypolins A and B (1 and 2) as well as seven known compounds (4aR,5S,6R,8aR)-5-[2-(2,5-dihydro-5-methoxy-2-oxofuran-3-yl)ethyl]-3,4,4a,5,6,7,8,8a-octahydro-5,6,8a-trimethylnaphthalene-1-carboxylic acid (3) [20], patagonic acid (4) [21], limbatolide F (5) [22], limbatolide A (6) [23], methyl (4aR,5S,6R,8S,8aR)-3,4,4a,5,6,7,8,8a-octahydro-8-hydroxy-5,6,8a-trimethyl-5-[2-(2-oxo-2,5-dihydrofuran-3-yl)ethyl]naphthalene-1-carboxylate (7) [24], clerodermic acid (8) [25], and visclerodol acid (9) [26] (Figure 1).
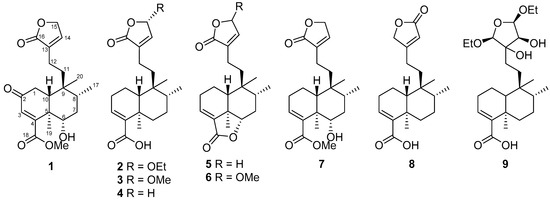
Figure 1.
Structures of compounds 1–9 isolated from Callicarpa hypoleucophylla.
The molecular formula of compound 1 was established to be C21H28O6 on the basis of the [M + Na]+ peak at m/z 399.17785 (calcd. 399.17781 for C21H28O6Na) obtained from high-resolution electrospray ionization mass spectrometry (HRESIMS) (Figure S13). The IR absorption bands of compound 1 indicated the presence of hydroxy (3451 cm−1), α,β-unsaturated-γ-lactone (1739 cm−1), and carboxyl (1678 cm−1) functionalities. The 13C and distortionless enhancement by polarization transfer (DEPT)-135 NMR data (Figure S2) showed the presence of 21 carbons divided into 7 quaternary carbons (including 3 carbonyls), 5 methines, 5 methylenes, and 4 methyls. The 1H (Figure S1) and 13C NMR signals of compound 1 showed some characteristic peaks such as an olefinic methine singlet (δH 5.96, δC 126.6, C-3), two tertiary methyls (δH 1.33, δC 14.0, Me-19; δH 0.84, δC 17.3, Me-20), a secondary methyl (δH 0.90, J = 6.8 Hz, δC 15.3, Me-17), as well as a butenolide unit (δC 134.0, C-13; δH 7.09, 1 H, quin, J = 1.7 Hz, δC 143.9, C-14; δH 4.77, 1H, dd, J = 3.8, 1.7 Hz, δC 70.2, C-15; δC 174.0, C-16). The above NMR data indicated that the structure of compound 1 was similar to that of dichrocephnoid E [27], a clerodane diterpenoid, except for a methylene corresponding to C-6 that was replaced by an oxymethine (δH 3.84, δC 72.5) and an additional methoxy (δH 3.81, δC 52.8) present in compound 1. The whole structure of compound 1 was then determined, starting from characteristic signals, by means of correlation spectroscopy (COSY), heteronuclear single quantum correlation (HSQC), and heteronuclear multiple bond correlation (HMBC) NMR correlations (Figures S3–S5). The COSY spectrum (Figure 2) showed cross-peaks with signals at H-1 (δH 2.43, 2.56)/H-10 (δH 2.00); H-6 (δH 3.84)/H-7 (δH 1.61, 1.70)/H-8 (δH 1.76)/Me-17 (δH 0.90); H-14 (δH 7.09)/H-15 (δH 4.77). Moreover, the key HMBC correlations (Figure 2) of H-1 with C-2, H-3 with C-1, C-4, C-5, and C-18; Me-19 with C-4, C-5, C-6, and C-10, Me-17 with C-7, C-8, and C-9, Me-20 with C-9, C-10, and C-11, and methoxy proton with C-18 led to the construction of the decalin core of compound 1, including a hydroxy group at C-6 and a methyl ester substituted at C-4. The linkage between C-12 and butanolide via C-13 was established by comparing the corresponding NMR data with those of similar analogues and confirmed by mass spectrometry analysis [22,24,27]. The planar structure of compound 1 is represented in Figure 2. The relative stereochemistry of compound 1 was deduced from nuclear overhauser effect spectroscopy (NOESY) correlations (Figure 2 and Figure S6) and by comparison of its spectroscopic data with those of clerodane analogues. The NOESY experiment showed correlations of H-6 (δH 3.84)/H-10 (δH 2.00)/H-8 (δH 1.76), which indicated protons located on the β face of the molecule. On the other hand, Me-20 presented NOESY correlations with Me-19 and Me-17, but neither Me-19 nor Me-20 correlated with H-10, suggesting that compound 1 is an ent-clerodane-type molecule with trans-decalin core [28]. The trans A/B ring junction was also evidenced by the carbon chemical shifts of C-19 (δC 14.0) and C-20 (δC 17.3) [29,30,31]. Thus, these correlations indicated that the hydroxy group at C-6 had an α-configuration, as confirmed by the coupling constants of H-6 with H-7α (J = 12.6 Hz) and H-7β (J = 4.4 Hz) [32,33]. All the spectral data appeared thus to be in agreement with the structure and stereochemistry of compound 1.
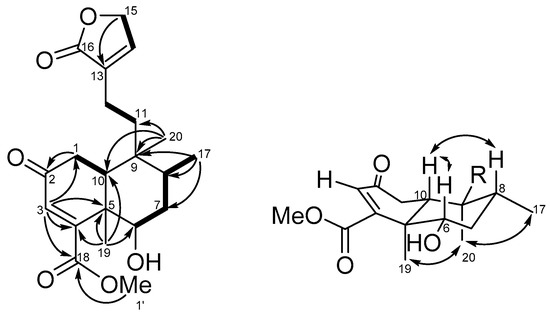
Figure 2.
COSY (bold bond), selected HMBC (arrow), and NOESY (left-right arrow) correlations of compound 1.
Callihypolin B (2) was isolated as a yellow oil. It possesses the molecular formula C22H32O5, corresponding to seven indices of hydrogen deficiency, as determined by the HRESIMS ion at m/z 399.21419 [M + Na]+ (calcd. 399.21420) (Figure S14) and 13C NMR data. The IR spectrum revealed the presence of ester (1768 cm−1) and conjugated carbonyl (1682 cm−1) groups. The 1H NMR data of compound 2 (Table 1, Figure S7) demonstrated the presence of one ethoxy [δH 3.94 (m) and 3.74 (m); 1.27 (t, J = 7.1 Hz)], one secondary methyl [δH 0.81 (d, J = 6.2 Hz)], two tertiary methyls (δH 0.76 and 1.23), and two olefinic methines [δH 6.85 (m), and 6.76 (d, J = 1.2)], together with one hemiacetal methine [δH 5.79 (brd, J = 1.2)]. The 13C NMR and DEPT spectra (Table 1, Figure S8) of compound 2 showed the presence of 22 carbon signals ascribable to 4 methyls, 7 methylenes (of which one was oxygenated), 2 olefinic methines, 3 aliphatic methines, 2 aliphatic quaternary carbons, 2 olefinic quaternary carbons, and 2 carbonyl carbons. Two carbonyls and two C=C double bonds accounted for four indices of hydrogen deficiency, so the remaining three indices suggested that compound 2 was a tricyclic compound. In the 1H-1H COSY spectrum (Figure S9), the correlations of H2-1/H2-2/H2-3, H2-6/H2-7/H-8/Me-17, H2-11/H2-12, H-14/H-15, and H2-1′/Me-2′ were used to establish the presence of five fragments, as shown in Figure 3. In the HMBC spectrum (Figure 3, Figure S11), the cross-peaks of H-3 with C-4 and C-18; of Me-19 with C-4, C-5, C-6, and C-10; and of H-10 with C-1 and C-5 revealed the presence of a cyclohexene ring (ring A), in which a carboxyl group and Me-19 were attached to C-4 and C-5, respectively. The presence of a cyclohexane ring (ring B) with Me-20 attached at C-9 was elucidated by the HMBC correlations of Me-20 to C-8, C-9, and C-10, as well as of H-10 to C-9. Additionally, both H3-20 and H-10 showed correlations with C-11 and indicated the linkage between ring B and C-11 via C-9. The HMBC cross-peaks of H-14 to C-13 (δC 139.0) and C-16 (δC 171.5); H-15 (δH 5.79) to C-16 and C-1′ (δC 66.0), as well as H2-12 to C-13 and C-16, revealed the presence of an α,β-unsaturated γ-lactone ring with an ethoxy group located at C-15. Thus, the planar structure of compound 2 could be established. The stereochemistry of compound 2 was determined by its NOESY spectrum, relative NMR data, and circular dichroism spectrum. The NOESY experiments (Figure 3 and Figure S12) carried out on compound 2 showed correlations of Me-19/Me-20/Me-17, and H-6β (δH 2.44)/H-10/H-8, whereas no correlation was revealed between H-10 and Me-19. These data, as well as the carbon chemical shift of Me-19 at δC 20.5 [29], indicated that compound 1 is characterized by a type TC clerodane skeleton under a chair conformation of ring B [34], a trans relationship between rings A and B, α-orientations of Me-17, Me-19, and Me-20, and β-orientation of H-10. The ethoxy group attached at C-15 in the butenolide moiety was assigned to the α-face by comparison with the circular dichroism (CD) data of known butenolides and by applying the octant rule. The CD spectrum showed a negative Cotton effect near 243 nm (π-π*) and supported the S configuration of C-15 [31,35,36]. Thus, the structure and stereochemistry of compound 2 were clearly determined.

Table 1.
1H and 13C NMR Data of compounds 1 and 2 in CDCl3.
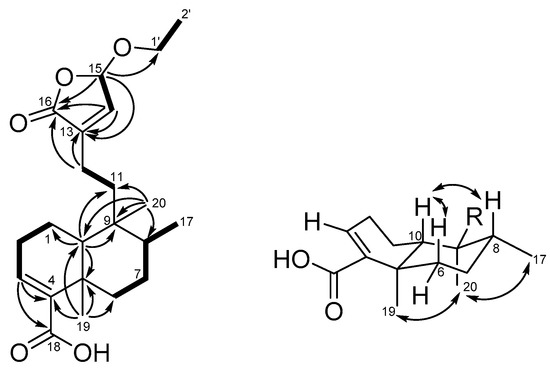
Figure 3.
COSY (bold bond), selected HMBC (arrow), and NOESY (left-right arrow) correlations of compound 2.
Compounds 1–9 were evaluated for their inhibitory activities on superoxide anion generation and elastase release in formyl-methionyl-leucyl-phenylalanine (fMLF)/cytochalasin (CB)-induced human neutrophils. The formyl peptide fMLF in combination with the priming agent CB serves as a stimulator that mimics the over-activation of neutrophils by a pathogen or an immune system reaction [37]. As shown in Table 2, compounds 2–4 exerted anti-inflammatory activity by suppressing superoxide anion generation and elastase release. The positive control genistein, which acts via inhibition of protein tyrosine kinases, showed a profound effect on the respiratory burst (89% inhibition of superoxide generation) and only a mild effect on degranulation (22.8% inhibition of elastase release). Among the tested samples, the new compound 2 showed the best activity, suppressing 32.2% of superoxide generation and 17.6% of elastase release. To exclude possible toxicity to the cells, the lactate dehydrogenase (LDH) release assay was employed, and none of the tested clerodane diterpenoids resulted toxic to human neutrophils (Figure 4). Clerodane diterpenes with an open lactone ring at C16 were previously reported to exert inhibitory effects on the function of neutrophils activated by fMLF/CB, including respiratory burst [38] and degranulation [39]. Thus, our results well correlate with the anti-inflammatory effects of previously isolated clerodane diterpenes and indicate the potential of the new compounds for the development of anti-inflammatory drugs targeting neutrophils.

Table 2.
Inhibitory effects of compounds 1–9 on superoxide anion generation and elastase release in formyl-methionyl-leucyl-phenylalanine (fMLF)/ cytochalasin (CB)-induced human neutrophils.
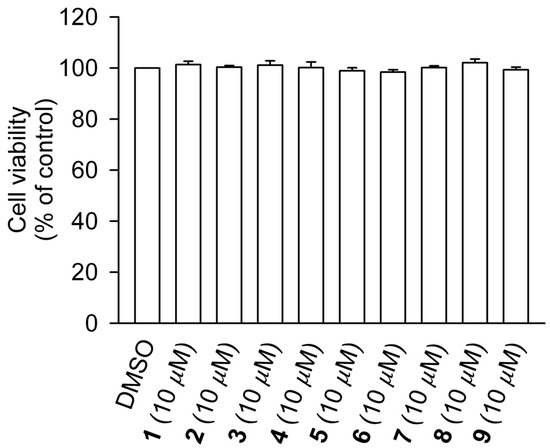
Figure 4.
Compounds 1–9 do not cause LDH release in human neutrophils. Human neutrophils were incubated with DMSO (as a control) or compounds 1–9 (10 μM) for 15 min. Cytotoxicity was evaluated by LDH release. All data are presented as the means ± S.E.M. (n = 3).
3. Experimental
3.1. General
Silica gel 60 (Merck) was used for open-column chromatography (CC). Luna C18 (5 m, 250 × 10 mm, Phenomenex), Luna CN (5 m, 250 10 mm, Phenomenex), and Luna phenyl-hexyl (5 m, 250 × 10 mm, Phenomenex) semi-preparative columns were used for high-performance liquid chromatography (HPLC). HPLC used a Shimadzu LC-10AT pump with an SPD-20A UV-Vis detector. The UV spectra were obtained by using a Jasco UV-530 ultraviolet spectrophotometer (Jasco, Tokyo, Japan), whereas the IR spectra were obtained on a Jasco FT-IR-4600 spectrophotometer (Jasco, Tokyo, Japan). Optical rotations were measured with a Jasco P-1020 digital polarimeter (Jasco, Tokyo, Japan). NMR spectra were obtained using JEOL JNM ECS 400 MHz (JEOL, Tokyo, Japan) and Varian 600 MHz NMR spectrometers (Varian, Palo Alto, CA, USA). ESI–MS data were collected on a VG Biotech Quattro 5022 mass spectrometer (VG Biotech, Altrincham, UK). High-resolution ESI–MS data were obtained with a Bruker APEX II spectrometer (Bruker, Bremen, Germany). Circular dichroism spectra were recorded on a JASCO J-810 spectrophotometer (Jasco, Tokyo, Japan).
3.2. Plant Material
The plant samples of C. hypoleucophylla were collected in Kaohsiung city, Taiwan, in May 2018. The plant material was identified by one of the authors, Dr. Ming-Hong Yen. A voucher sample (specimen code: CH001) was deposited at the Graduate Institute of Natural Products, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, Taiwan.
3.3. Extraction and Isolation
Air-dried leaves and twigs of C. hypoleucophylla (17.0 kg) were extracted three times with 95% ethanol at room temperature for 72 h each time. The extract was evaporated under reduced pressure to get a crude extract (3.6 kg). Next, the ethanol extract of C. hypoleucophylla was suspended and dissolved in H2O and then partitioned with ethyl acetate to obtain an ethyl acetate layer (118.3 g). The the ethyl acetate layer was further partitioned between hexanes and 75% MeOH to acquire hexanes and MeOH layers, respectively.
Due to the results of the cytotoxic assay, the MeOH layer (45.6 g) was selected for further isolation. At first, it was loaded on a normal-phase silica gel open column and was eluted by stepwise hexanes with ethyl acetate (1:0~0:1) followed by stepwise ethyl acetate with methanol (1:0~0:1) to obtain seven subfractions (CH1~7), according to TLC analysis. The third sub-fraction, CH3, was isolated on Sephadex LH-20 and eluted with MeOH to afford four subfractions (CH3-1–4). Then, repeated column chromatography isolation on CH3-3 yielded CH3-3-1–5 fractions. CH3-3-2 (500.3 mg) was separated by silica gel CC (dichloromethane/MeOH, 100:1→0:1) to afford more subfractions (CH3-3-2-1–6). Fr. CH3-3-2-6 was purified by normal-phase HPLC using a Phenomenex Luna-CN column (hexane/dichloromethane/methanol, 30:10:1, 1.5 mL/min) to give compounds 2 (33.1mg), 3 (7.2 mg), 4 (62.7 mg), and 5 (26.7 mg). Fr. CH3-3-2-4 was isolated by reverse-phase HPLC using a CN column and gave compounds 1 (1.9 mg) and 7 (0.7 mg). Fr. CH3-3-3 was subjected to silica gel CC (CH2Cl2/MeOH, 1:0→0:1) followed by NP-CN HPLC and elution with (hexane/dichloromethane/methanol, 40:10:1, 2.0 mL/min) to obtain compound 8 (9.8 mg). In addition, Fr. CH3-2 was separated by normal-phase silica gel CC with hexane/dichloromethane/methanol (100:40:1→0:0:1) to afford Frs. CH3-2-1–5. Fr. CH3-2-5 was purified by silica gel CC (CH2Cl2/MeOH, 1:0→0:1) followed by RP-phenyl-hexyl HPLC (methanol/H2O, 65/35, 2.0 mL/min) to give compounds 6 (2.5 mg) and 9 (7.0 mg).
3.4. Spectroscopic Data
Callihypolin A (1) yellow oily, −1.0° (c 0.05, MeOH); IR (neat) νmax 3452, 2956, 1768, 1682, 1376, 1342, 1202, 1141, 1018 cm−1; 1H-NMR and 13C-NMR (CDCl3, 600/150 MHz) see Table 1; HRESIMS m/z 399.17785 (calcd for C21H28O6Na, 399.17781).
Callihypolin B (2) yellow oily, −47.6° (c 0.05, MeOH); IR (neat) νmax 3451, 2930, 1739, 1678, 1450, 1253, 1072 cm−1; 1H-NMR and 13C-NMR (CDCl3, 400/100 MHz) see Table 1; HRESIMS m/z 399.21419 (calcd for C22H32O5Na, 399.21420).
3.5. Superoxide Anion Generation and Elastase Release Assays by Human Neutrophils
Human neutrophils were obtained from the venous blood of healthy adult volunteers (20−30 years old), following a reported procedure [37]. Superoxide anion generation by fMLF (0.1 μM)/CB (1μM)-activated neutrophils was evaluated based on the reduction of ferricytochrome c, as previously described [37,40]. Elastase release by the fMLF (0.1 μM)/CB (0.5 μM)-activated neutrophils was determined using N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitroanilide as the elastase substrate, according to a previous protocol [37,40]. The concentration was 10 μM for compounds 1–9. Genistein was used as a positive control.
3.6. Cytotoxicity Test
A lactate dehydrogenase (LDH) assay kit (Promega, Madison, WI, USA) was utilized to evaluate the cytotoxicity of the samples in human neutrophils. Human neutrophils were treated with DMSO or compounds 1–9 for 15 min at 37 °C. Cell-free supernatants were collected, and the amount of LDH was evaluated [37].
4. Conclusions
The first phytochemical investigation of the leaves and twigs of the Taiwanese endemic plant Callicarpa hypoleucophylla has resulted in the isolation of nine clerodane-type diterpenoids, compounds 1–9, including two new compounds designated callihypolins A and B (compounds 1 and 2). All isolates from C. hypoleucophylla possess a TC ent-clerodane skeleton, which is different from that of the phyllocladane and labdane diterpenoids that were identified as major components of the other well-studied species Callicarpa macrophylla Vahl, which is recorded in the Pharmacopoeia of the People’s Republic of China. These results reflect the unique properties of C. hypoleucophylla from the perspective of chemotaxonomy. Moreover, the anti-inflammatory activity of the isolated compounds highlights the potential of clerodane-type diterpenoids for further pharmaceutic development.
Supplementary Materials
The NMR spectra of compounds 1 and 2 are available online.
Author Contributions
Y.-B.C., C.-Y.C. and C.-H.W. conceived and designed the experiments; Y.-C.L. and J.-J.L. carried out the plant extraction and isolation of the compounds; T.-L.H., S.-Y.F., and M.K. conducted the biological studies; M.-H.Y. collected and identified the material; C.-Y.C., Y.-S.L., and T.-Y.W. assisted with the interpretation of various data; Y.-C.L., S.-R.C., Y.-B.C. contributed to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grants from the Ministry of Science and Technology of Taiwan (MOST-107-2628-B-037-001 and MOST-108-2320-B-037-013-MY3 awarded to Prof. Yuan-Bin Cheng; MOST 106-2320-B-255-003-MY3 and MOST 108-2320-B-255-003-MY3 awarded to Prof. Tsong-Long Hwang). This work was also supported by grants from Zuoying Branch of Kaohsiung Armed Forces General Hospital (KAFGH-ZY-A-109030).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tu, Y.; Sun, L.; Guo, M.; Chen, W. The medicinal uses of Callicarpa, L. in traditional Chinese medicine: An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2013, 146, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Kawazu, K.; Inaba, M.; Mitsui, T. Studies on fish-killing components of Callicarpa candicans. Agr. Biol. Chem. 1967, 31, 494–497. [Google Scholar]
- Cantrell, C.L.; Klun, J.A.; Bryson, C.T.; Kobaisy, M.; Duke, S.O. Isolation and identification of mosquito bite deterrent terpenoids from leaves of American (Callicarpa americana) and Japanese (Callicarpa japonica) beautyberry. J. Agric. Food Chem. 2005, 53, 5948–5953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, L.; Huang, J.; Liu, M.; Li, G.; Zhang, C.; Zhang, K.; Wang, J. 3,4-seco-Labdane diterpenoids from the leaves of Callicarpa nudiflora and their inhibitory effects on nitric oxide production. Fitoterapia 2013, 89, 218–223. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, L.; Zhang, X.; Liu, M.; Wang, J.; Wang, Y. Two new 3,4-seco-labdane diterpenoids from Callicarpa nudiflora and their inhibitory activities against nitric oxide production. Phytochem. Lett. 2014, 10, 127–131. [Google Scholar] [CrossRef]
- Cheng, H.H.; Cheng, Y.B.; Hwang, T.L.; Kuo, Y.H.; Chen, C.H.; Shen, Y.C. Randainins A–D, based on unique diterpenoid architectures, from Callicarpa randaiensis. J. Nat. Prod. 2015, 78, 1823–1828. [Google Scholar] [CrossRef]
- Zhou, Z.; Wei, X.; Fu, H.; Luo, Y. Chemical constituents of Callicarpa nudiflora and their anti-platelet aggregation activity. Fitoterapia 2013, 88, 91–95. [Google Scholar] [CrossRef]
- Wu, A.Z.; Zhai, Y.J.; Zhao, Z.X.; Zhang, C.X.; Lin, C.Z.; Zhu, C.C. Phenylethanoid glycosides from the stems of Callicarpa peii (hemostatic drug). Fitoterapia 2013, 84, 237–241. [Google Scholar] [CrossRef]
- Luo, Y.H.; Zhou, Z.Q.; Ma, S.C.; Fu, H.Z. Three new antioxidant furofuran lignans from Callicarpa nudiflora. Phytochem. Lett. 2014, 7, 194–197. [Google Scholar] [CrossRef]
- Cai, H.; Xie, Z.; Liu, G.; Sun, X.; Peng, G.; Lin, B.; Liao, Q. Isolation, identification and activities of natural antioxidants from Callicarpa kwangtungensis Chun. PLoS ONE 2014, 9, e93000. [Google Scholar] [CrossRef]
- Jones, W.P.; Lobo-Echeverri, T.; Mi, Q.; Chai, H.B.; Soejarto, D.D.; Cordell, G.A.; Swanson, S.M.; Kinghorn, A.D. Cytotoxic constituents from the fruiting branches of Callicarpa Americana collected in southern Florida. J. Nat. Prod. 2007, 70, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.L.; Han, Z.; Cui, H.B.; Zhao, Y.X.; Deng, Y.Y.; Dai, H.F. A new cytotoxic iridoid from Callicarpa nudiflora. Nat. Prod. Res. 2010, 24, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, Y.; Wang, M.; Ren, Q.; Li, S.; Wang, H.; Sun, X.; Jin, D.Q.; Sun, H.; Ohizumi, Y.; et al. Bioactive diterpenoids from the leaves of Callicarpa macrophylla. J. Nat. Prod. 2015, 78, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Wu, H.M.; Peng, C.F.; Chen, I.S.; Chu, S.D. seco-Abietane diterpenoids, a phenylethanoid derivative, and antitubercular constituents from Callicarpa pilosissima. J. Nat. Prod. 2009, 72, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Fu, H.Z.; Chen, W.K.; Luo, Y.H.; Ma, S.C. Hepatoprotective triterpenoid saponins from Callicarpa nudiflora. Chem. Pharm. Bull. 2014, 62, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Fu, H.Z.; Huang, B.; Chen, W.K.; Ma, S.C. Hepatoprotective iridoid glucosides from Callicarpa nudiflora. J. Asian. Nat. Prod. Res. 2016, 18, 274–279. [Google Scholar] [CrossRef]
- Chung, P.Y.; Chung, L.Y.; Navaratnam, P. Potential targets by pentacyclic triterpenoids from Callicarpa farinosa against methicillin-resistant and sensitive. Staphylococcus aureus. Fitoterapia 2014, 94, 48–54. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, A.; Gupta, A.K.; Pakash, D.V. In vitro anti-arthritic activity of ethanolic extract of Callicarpa Macrophylla flower. Int. Res. J. Pharm. 2013, 4, 160–162. [Google Scholar] [CrossRef]
- Yadav, V.; Jayalakshmi, S.; Singla, R.K.; Patra, A.; Khan, S. Assessment of anti-inflammatory and analgesic activities of Callicarpa macrophylla Vahl. roots extracts. Webmed Cent. Pharmacol. 2012, 3, WMC003366. [Google Scholar]
- Ahmad, V.U.; Farooq, U.; Abbaskhan, A.; Hussain, J.; Abbasi, M.A.; Nawaz, S.A.; Choudhary, M.I. Four new diterpenoids from Ballota limbata. Helv. Chim. Acta. 2004, 87, 682–689. [Google Scholar] [CrossRef]
- Pinto, M.E.F.; Silva, M.S.D.; Schindler, E.; Filho, J.M.B.; El-Bachá, R.D.S.; Castello-Branco, M.V.S.; Agra, M.D.F.; Tavares, J.F. 3′,8"-Biisokaempferide, a cytotoxic biflavonoid and other chemical constituents of Nanuza plicata (Velloziaceae). J. Braz. Chem. Soc. 2010, 21, 1819–1824. [Google Scholar] [CrossRef]
- Farooq, U.; Khan, A.; Ahmad, V.U.; Kousar, F.; Iqbal, S. Limbatolide F and G: Two new trans-clerodane diterpenoids from Otostegia limbata. Pol. J. Chem. 2005, 79, 1757–1762. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Khan, A.; Farooq, U.; Kousar, F.; Khan, S.S.; Nawaz, S.A.; Abbasi, M.A.; Choudhary, M.I. Three new cholinesterase-inhibiting cis-clerodane diterpenoids from Otostegia limbata. Chem. Pharm. Bull. 2005, 53, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Iqbal Choudhary, M.; Mohammad, M.Y.; Musharraf, S.G.; Onajobi, I.; Mohammad, A.; Anis, I.; Shah, M.R.; Atta-Ur-Rahman. Biotransformation of clerodane diterpenoids by Rhizopus stolonifer and antibacterial activity of resulting metabolites. Phytochemistry 2013, 90, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Raha, P.; Das, A.K.; Adityachaudhuri, N.; Majumder, P.L. Cleroinermin, aneo-clerodane diterpenoid from Clerodendron inermi. Phytochemistry 1991, 30, 3812–3814. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, M.Y.; Zhou, Z.Y.; Xu, D. Two new clerodane diterpenes from Dodonaea viscosa. Z. Naturforsch. 2010, 65b, 83–86. [Google Scholar] [CrossRef]
- Song, B.; Ding, G.; Tian, X.H.; Li, L.; Zhou, C.; Zhang, Q.B.; Wang, M.H.; Zhang, T.; Zou, Z.M. Anti-HIV-1 integrase diterpenoids from Dichrocephala benthamii. Phytochem. Lett. 2015, 14, 249–253. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Simozar, E.; Ahmadi, A.; Grenz, M.; Bohlmann, F. A hardwickiic acid derivative from Pulicaria gnaphalodes. Phytochemistry 1981, 20, 2772–2773. [Google Scholar] [CrossRef]
- Heymann, H.; Tezuka, Y.; Kikuchi, T.; Supriyatna, S. Constituents of Sindora sumatrana MIQ. III. new trans-clerodane diterpenoids from the dried pods. Chem. Pharm. Bull. 1994, 42, 1202–1207. [Google Scholar] [CrossRef]
- García, A.; Ramírez-Apan, T.; Cogordan, J.A.; Delgado, G. Absolute configuration assignments by experimental and theoretical approaches of ent-labdane- and cis-ent-clerodane-type diterpenes isolated from Croton glabellus. Can. J. Chem. 2006, 84, 1593–1602. [Google Scholar] [CrossRef]
- Chang, F.R.; Huang, S.T.; Liaw, C.C.; Yen, M.H.; Hwang, T.L.; Chen, C.Y.; Hou, M.F.; Yuan, S.S.; Cheng, Y.B.; Wu, Y.C. Diterpenes from Grangea maderaspatana. Phytochemistry 2016, 131, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Calderón, C.; De Ford, C.; Castro, V.; Merfort, I.; Murillo, R. Cytotoxic clerodane diterpenes from Zuelania guidonia. J. Nat. Prod. 2014, 77, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Oberlies, N.H.; Burgess, J.P.; Navarro, H.A.; Pinos, R.E.; Fairchild, C.R.; Peterson, R.W.; Soejarto, D.D.; Farnsworth, N.R.; Kinghorn, A.D.; Wani, M.C.; et al. Novel bioactive clerodane diterpenoids from the leaves and twigs of Casearia sylvestris. J. Nat. Prod. 2002, 65, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Tokoroyama, T. Synthesis of clerodane diterpenoids and related compounds-stereoselective construction of the decalin skeleton with multiple contiguous stereogenic centers. Synthesis 2000, 5, 611–633. [Google Scholar] [CrossRef]
- Wu, T.H.; Cheng, Y.Y.; Liou, J.R.; Way, T.D.; Chen, C.J.; Chen, Y.H.; Kuo, S.C.; El-Shazly, M.; Chang, F.R.; Wu, Y.C.; et al. Clerodane diterpenes from Polyalthia longifolia var. pendula protect SK-N-MC human neuroblastoma cells from β-amyloid insult. RSC Adv. 2014, 4, 23707–23712. [Google Scholar] [CrossRef]
- Itokawa, H.; Morita, H.; Katou, I.; Takeya, K.; Cavalheiro, A.J.; de Oliveira, R.C.; Ishige, M.; Motidome, M. Cytotoxic diterpenes from the rhizomes of Hedychium coronarium. Planta Med. 1988, 54, 311–315. [Google Scholar] [CrossRef]
- Liu, F.C.; Yu, H.P.; Chen, P.J.; Yang, H.W.; Chang, S.H.; Tzeng, C.C.; Cheng, W.J.; Chen, Y.R.; Chen, Y.L.; Hwang, T.L. A novel NOX2 inhibitor attenuates human neutrophil oxidative stress and ameliorates inflammatory arthritis in mice. Redox Biol. 2019, 26, 101273. [Google Scholar] [CrossRef]
- Chang, F.R.; Hwang, T.L.; Yang, Y.L.; Li, C.E.; Wu, C.C.; Issa, H.H.; Hsieh, W.B.; Wu, Y.C. Anti-inflammatory and cytotoxic diterpenes from formosan Polyalthia longifolia var. pendula. Planta Med. 2006, 72, 1344–1347. [Google Scholar] [CrossRef]
- Chang, H.L.; Chang, F.R.; Chen, J.S.; Wang, H.P.; Wu, Y.H.; Wang, C.C.; Wu, Y.C.; Hwang, T.L. Inhibitory effects of 16-hydroxycleroda-3,13(14)E-dien-15-oic acid on superoxide anion and elastase release in human neutrophils through multiple mechanisms. Eur. J. Pharmacol. 2008, 31, 332–339. [Google Scholar] [CrossRef]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol inhibits superoxide production, elastase release, and chemotaxis in formyl peptide-activated human neutrophils by blocking formyl peptide receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).