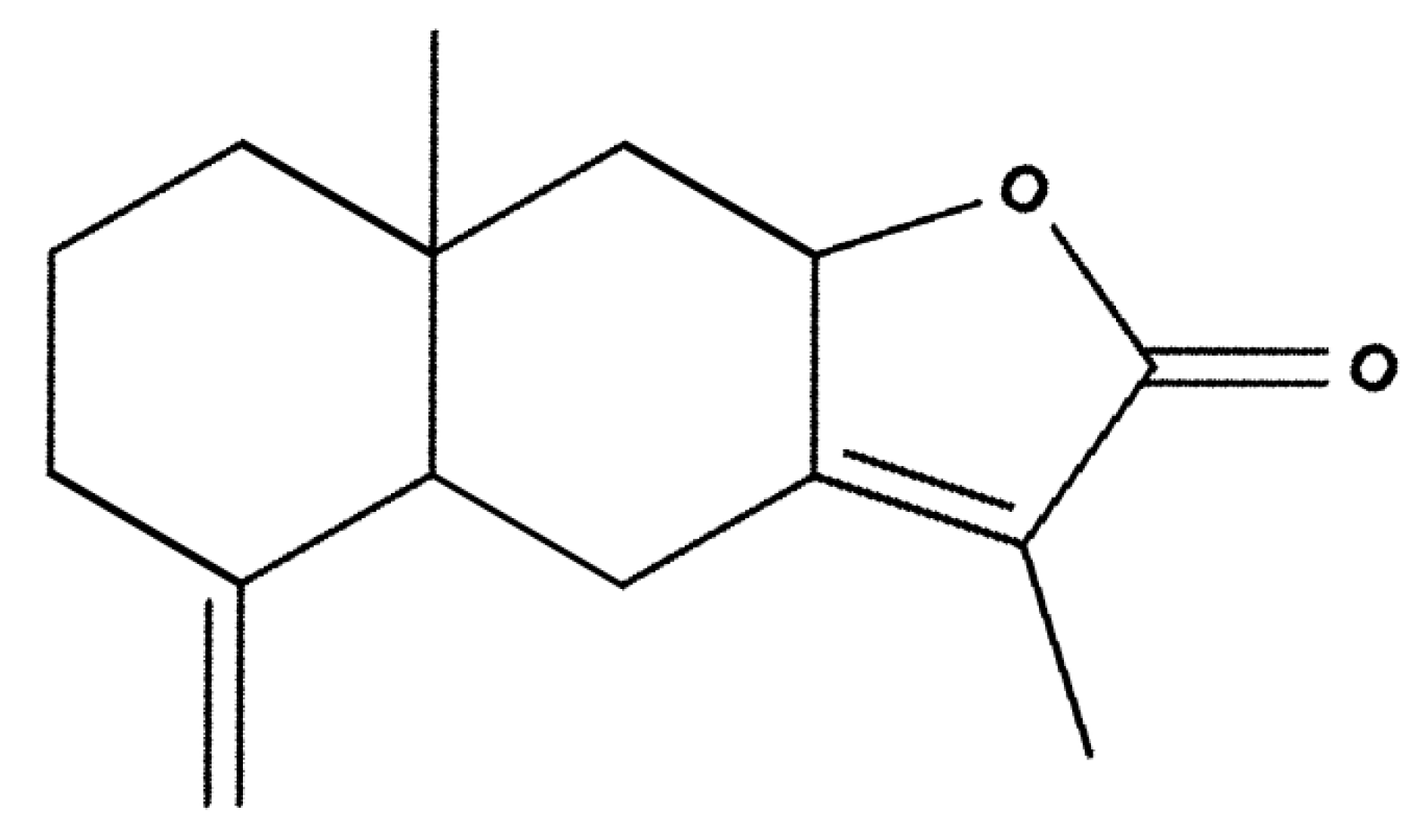
Anti-Tumor Activity of Atractylenolide I in Human Colon Adenocarcinoma In Vitro
Abstract
1. Introduction
2. Results
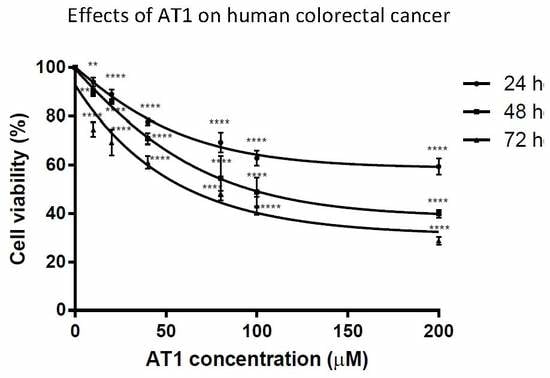
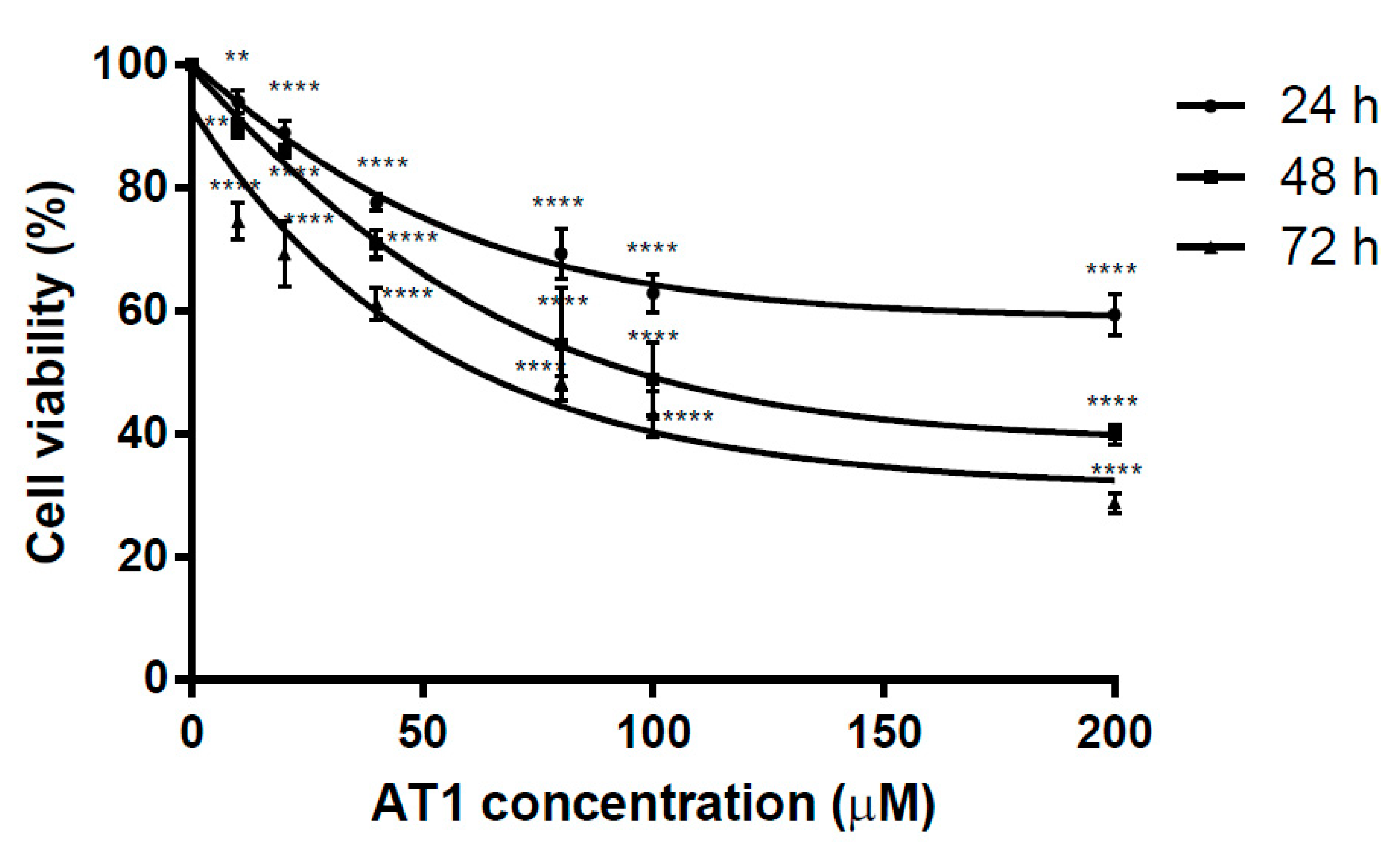
2.1. Anti-Proliferative Effects of AT-I on the HT-29 Cell Line
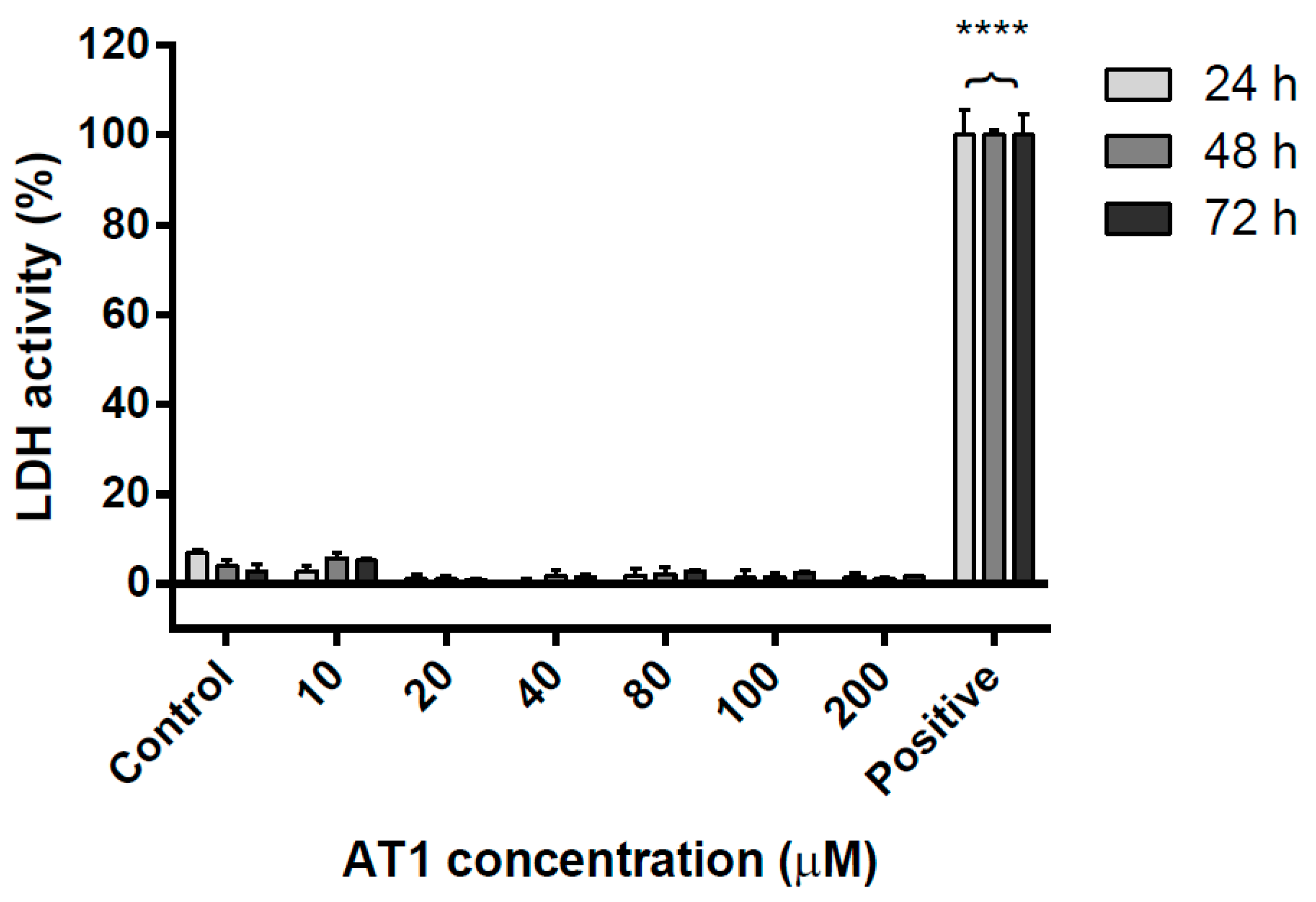
2.2. Necrotic Effects of AT-I on the HT-29 Cell Line
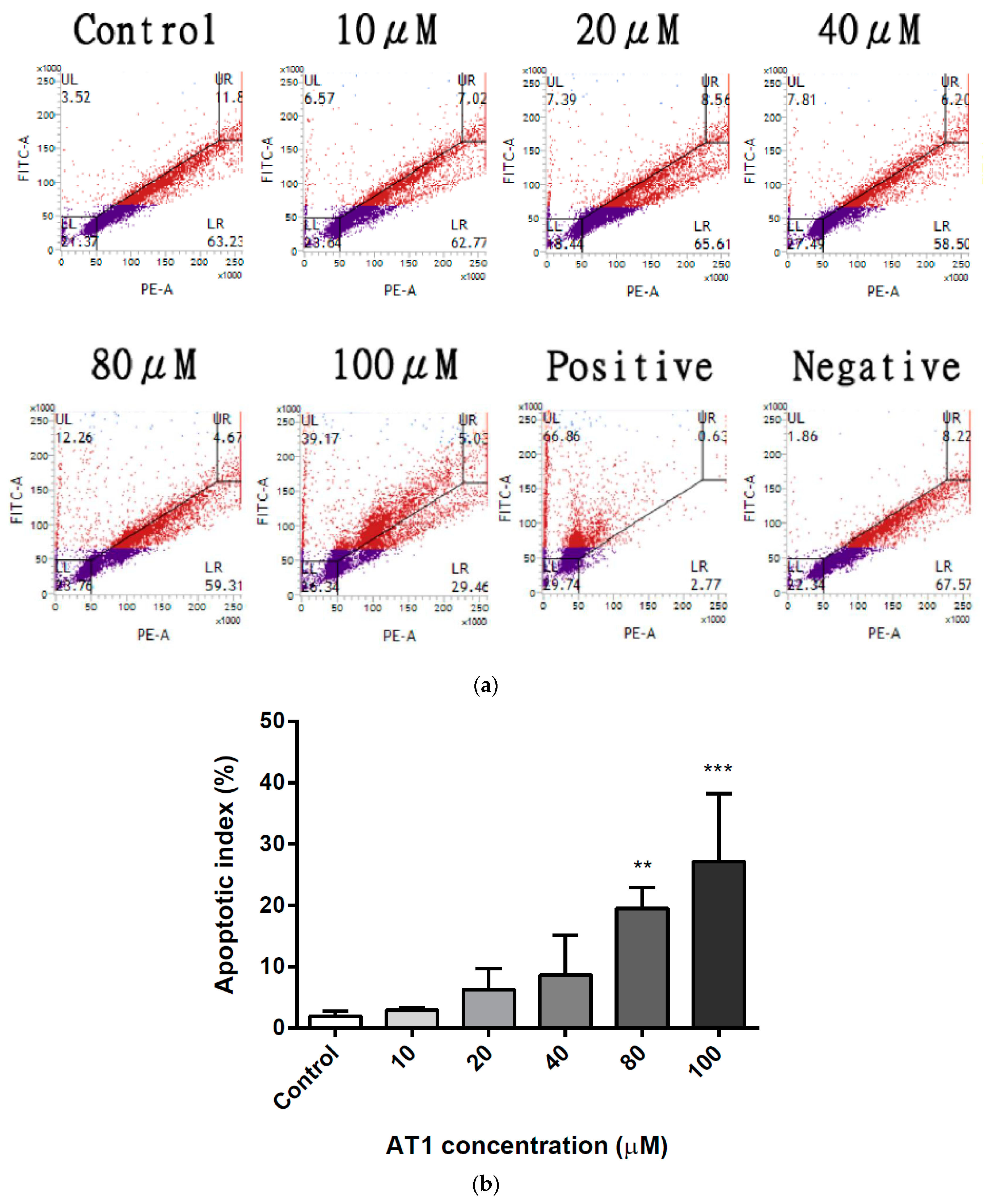
2.3. Effects of AT-I on DNA Fragmentation in the HT-29 Cell Line
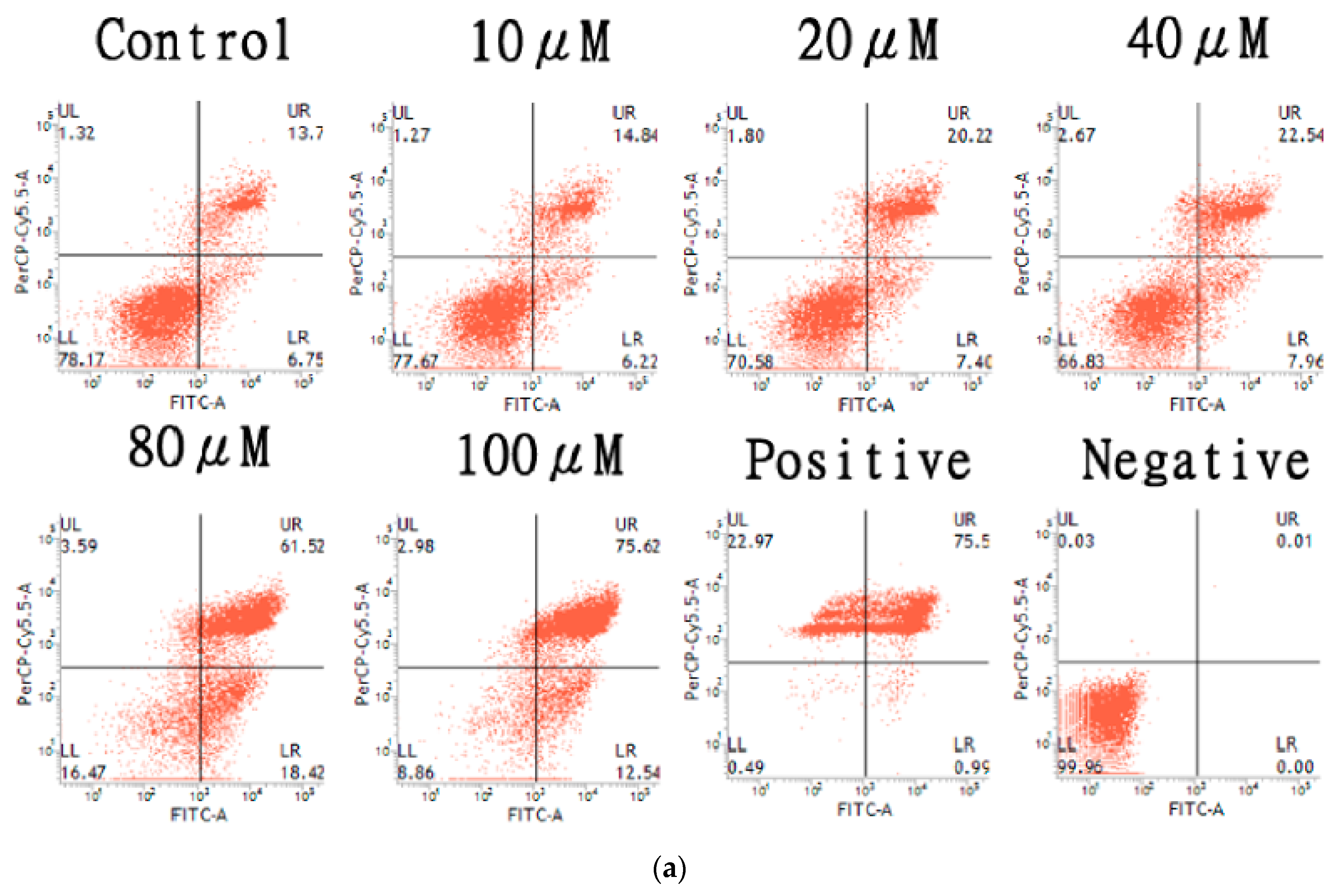
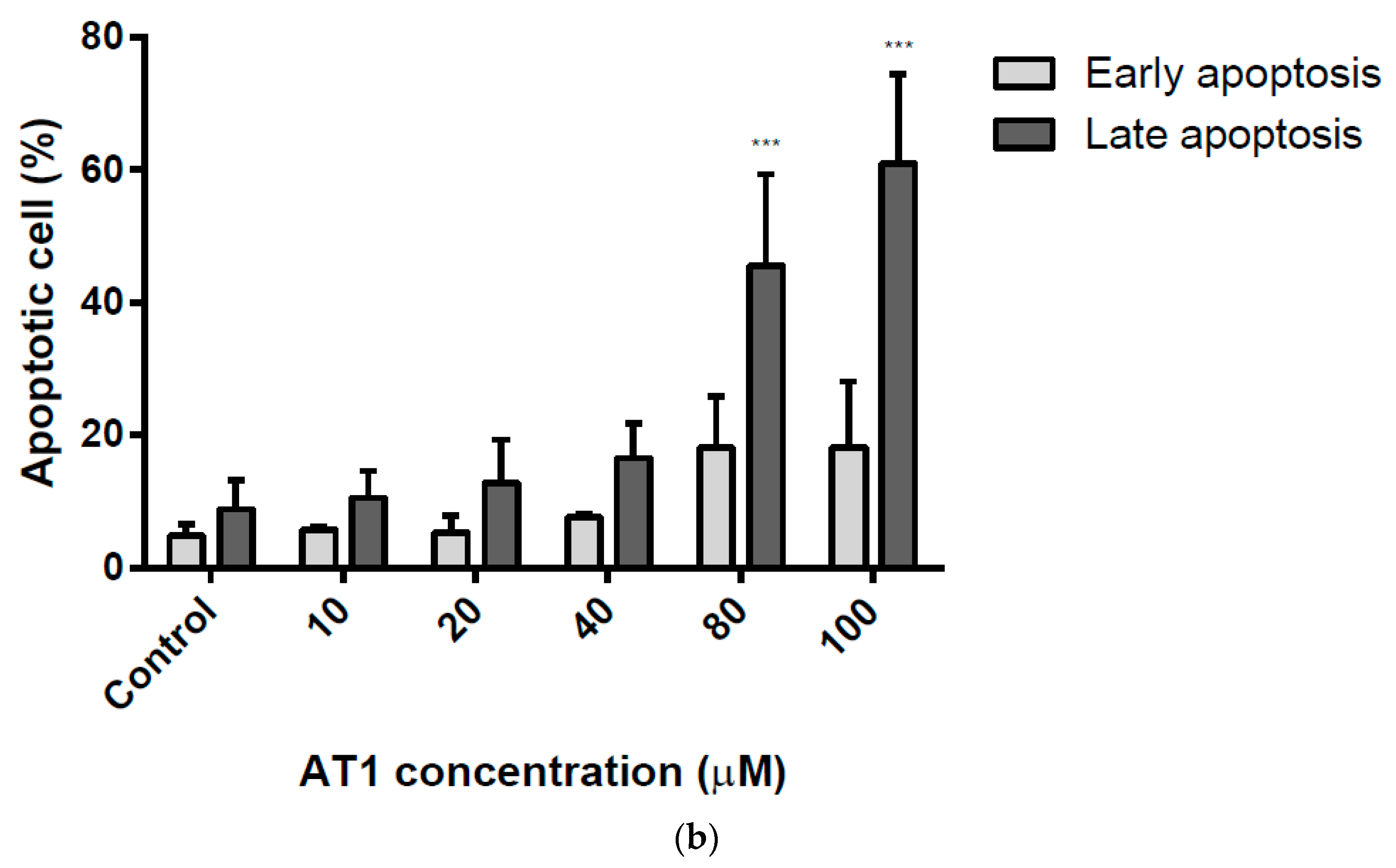
2.4. Apoptosis Induction of the HT-29 Cell Line by AT-I
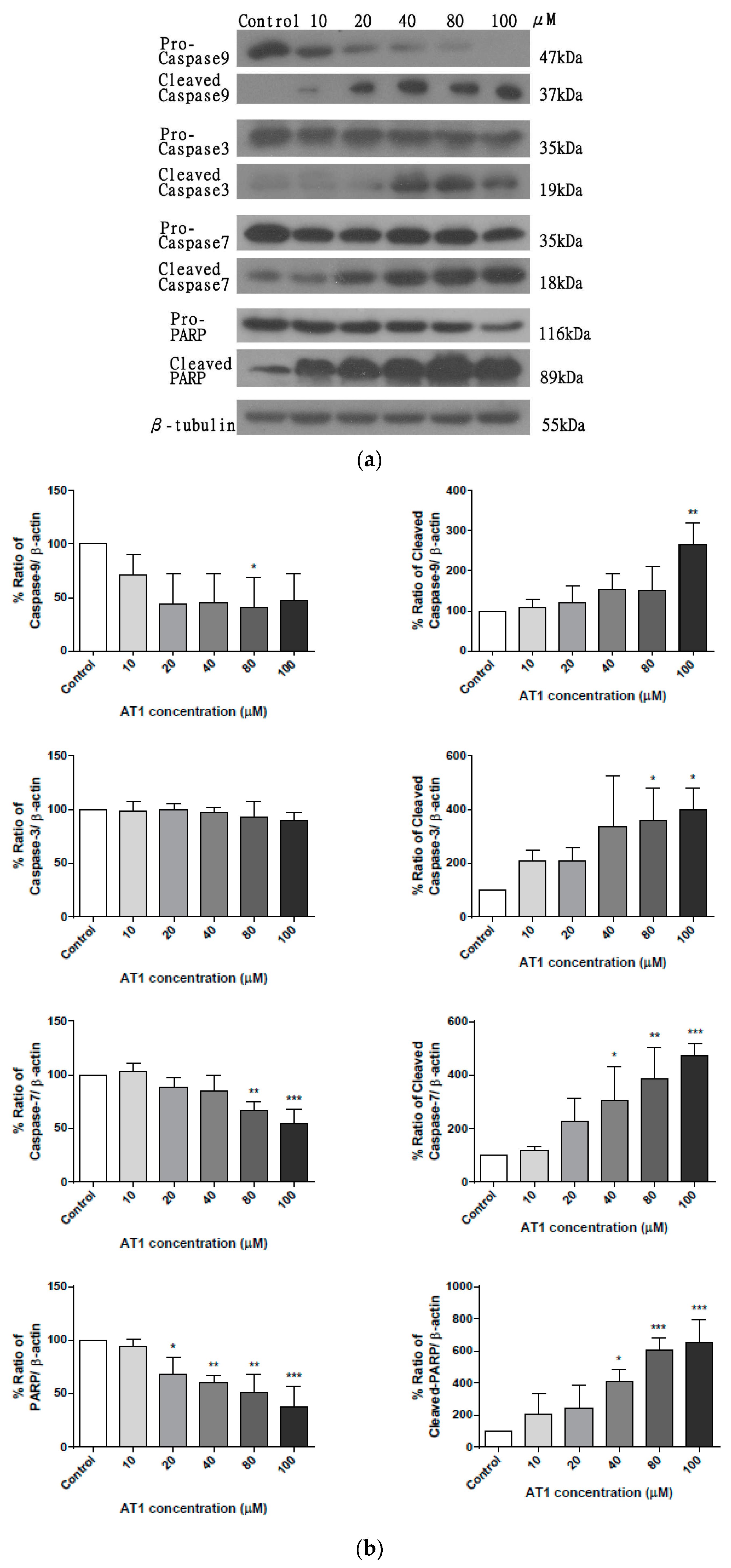
2.5. The effect of AT-I on the Expression Level of Caspases in the HT-29 Cell Line
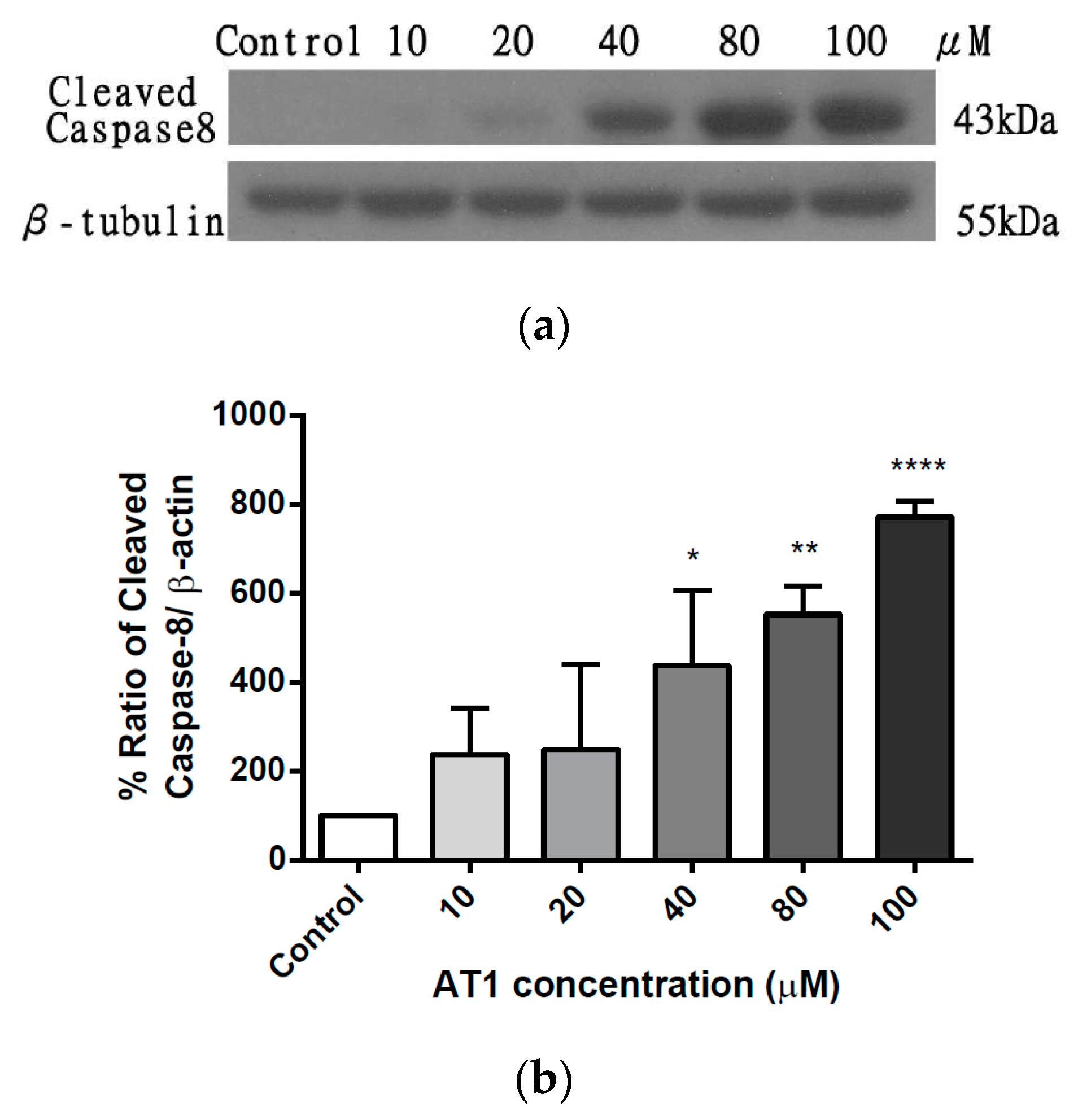
2.6. The effect of AT-I on the Expression Level of Caspase 8 in HT-29 Cell Line
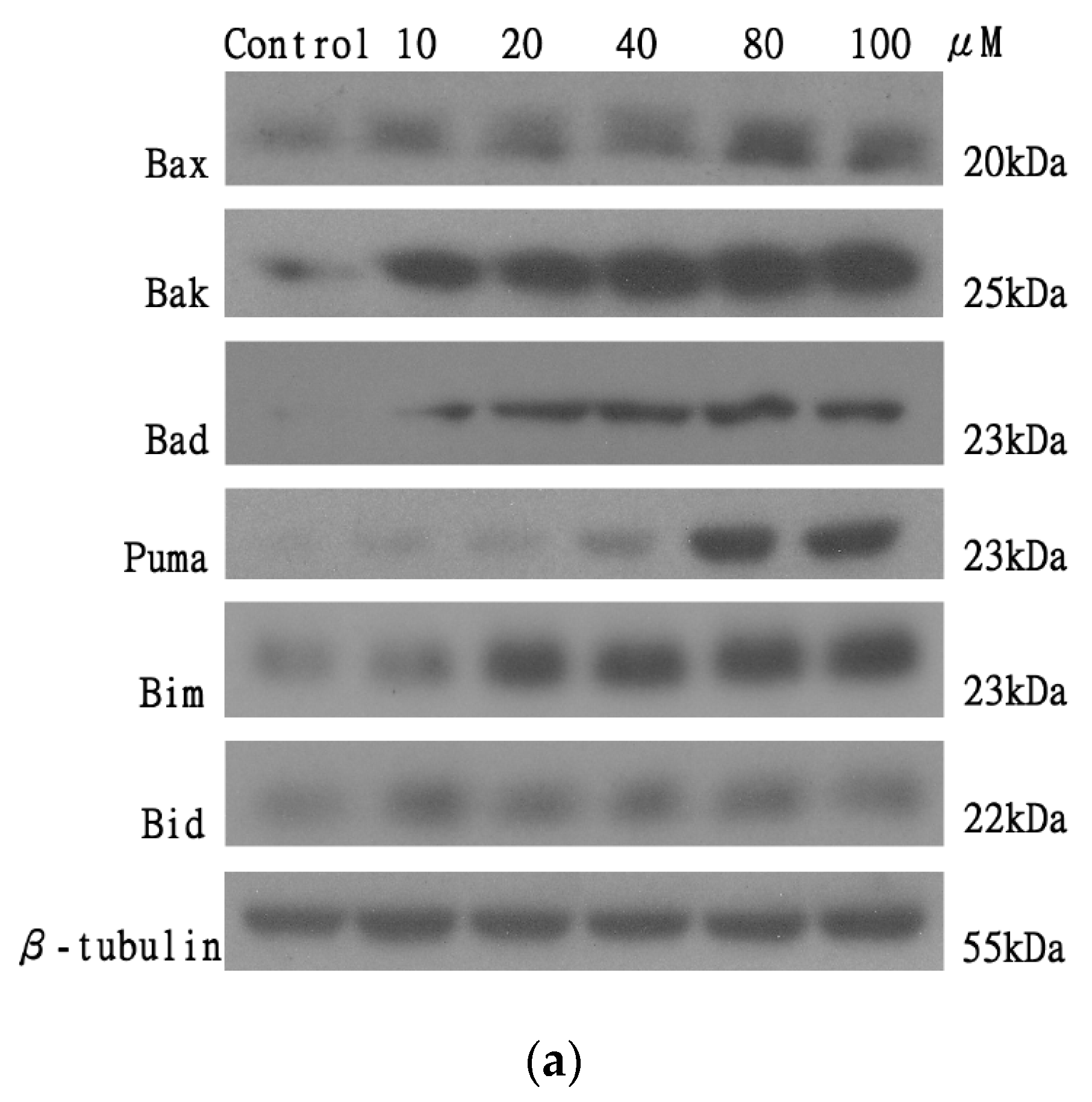
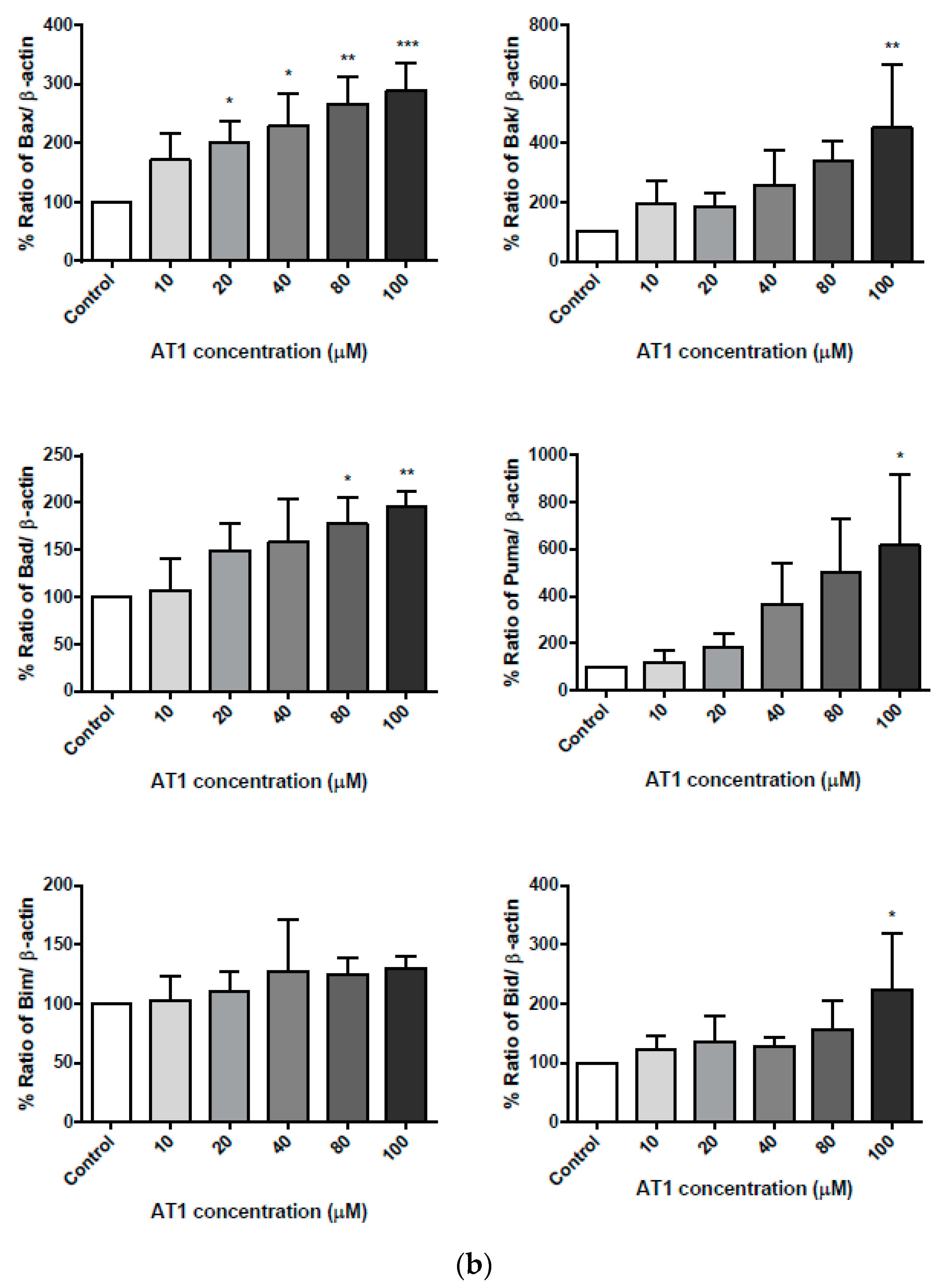
2.7. The Effect of AT-I on the Expression Level of the Pro-Apoptotic Bcl-2 Family Proteins in the HT-29 Cell Line
2.8. The Effect of AT-I on the Expression Level of the Pro-Survival Bcl-2 Family of Proteins in the HT-29 Cell Line
3. Discussion
4. Materials and Methods
4.1. Materials and Chemical Reagents
4.2. Cell Line and Cell Culture
4.3. Cell Viability Evaluation by MTT Assay
4.4. Cell Necrosis Evaluation by LDH Assay
4.5. DNA Fragmentation Analysis by TUNEL Assay
4.6. Cell Apoptosis Quantification by Annexin V-FITC/PI Double Staining Assay
4.7. Caspase 3/7 Activity Assay
4.8. Western Blot Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2016, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Pourhoseingholi, M.A. Increased burden of colorectal cancer in Asia. World J. Gastrointest. Oncol. 2012, 4, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; You, S.L.; Lin, L.H.; Hsu, W.L.; Yang, Y.W. Cancer epidemiology and control in Taiwan: A brief review. Jpn. J. Clin. Oncol. 2002, 32, 66–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Byeon, J.S.; Yang, S.K.; Kim, T.I.; Kim, W.H.; Lau, J.Y.W.; Leung, W.K.; Fujita, R.; Makharia, G.K.; Abdullah, M.; Hilmi, I.; et al. Colorectal neoplasm in asymptomatic Asians: A prospective multinational multicenter colonoscopy survey. Gastrointest. Endosc. 2007, 65, 1015–1022. [Google Scholar] [CrossRef]
- Benard, F.; Barkun, A.N.; Martel, M.; Renteln, D. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J. Gastroenterol. 2018, 24, 124–138. [Google Scholar] [CrossRef]
- Stracci, F.; Zorzi, M.; Grazzini, G. Colorectal Cancer Screening: Tests, Strategies, and Perspectives. Front. Public Health 2014, 2, 210. [Google Scholar] [CrossRef]
- Keane, M.G.; Johnson, G.J. Early diagnosis improves survival in colorectal cancer. Practitioner 2012, 256, 2. [Google Scholar]
- Sung, J.J.; Choi, S.Y.; Chan, F.K.; Ching, J.Y.; Lau, J.T.; Griffiths, S. Obstacles to colorectal cancer screening in Chinese: A study based on the health belief model. Am. J. Gastroenterol. 2008, 103, 974–981. [Google Scholar] [CrossRef]
- Sung, J.J.; Lau, J.Y.; Goh, K.L.; Leung, W.K. Increasing incidence of colorectal cancer in Asia: Implications for screening. Lancet Oncol. 2005, 6, 871–876. [Google Scholar] [CrossRef]
- Theodoratou, E.; Timofeeva, M.; Li, X.; Meng, X.; Ioannidis, J.P. Nature, Nurture, and Cancer Risks: Genetic and Nutritional Contributions to Cancer. Ann. Rev. Nutr. 2017, 37, 293–320. [Google Scholar] [CrossRef]
- Beaver, C.C.; Magnan, M.A. Managing Chemotherapy Side Effects: Achieving Reliable and Equitable Outcomes. Clin. J. Oncol. Nurs. 2016, 20, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G. Global harmonisation of herbal health claims. J. Nutr. 2001, 131, 162–196. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Li, A.; Inagaki, Y.; Gao, J.; Li, J.; Kokudo, N.; Li, X.K.; Tang, W. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci. Trends 2010, 4, 297–307. [Google Scholar]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and Antinociceptive Constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar] [CrossRef]
- Le, S.H.; Tran, M.H.; Lee, J.S.; Ngo, Q.M.; Woo, M.H.; Min, B.S. Inflammatory Inhibitory Activity of Sesquiterpenoids from Atractylodes macrocephala Rhizomes. Chem. Pharm. Bull. (Tokyo) 2016, 64, 507–511. [Google Scholar]
- Pejin, B.; Iodice, C.; Bogdanovic, G.; Kojic, V.; Tesevic, V. Stictic acid inhibits cell growth of human colon adenocarcinoma HT-29 cells. Arab J. Chem. 2017, 10, S1240–S1242. [Google Scholar] [CrossRef]
- Li, W.; Wen, H.; Zhang, A.; Ge, J.; Wu, H. Study on quality of Atractylodes macrocephala Koidz—determination of 2 atractylenolides by HPLC. Chin. J. Pharm. Anal. 2001, 21, 170–172. [Google Scholar]
- Liu, H.; Zhu, Y.; Zhang, T.; Zhao, Z.; Zhao, Y.; Cheng, P.; Li, H.; Gao, H.; Su, X. Anti-tumor effects of atractylenolide I isolated from Atractylodes macrocephala in human lung carcinoma cell lines. Molecules 2013, 18, 13357–13368. [Google Scholar] [CrossRef]
- Pejin, B.; Iodice, C.; Kojic, V.; Jakimov, D.; Lazovic, M.; Tommonaro, G. In vitro evaluation of cytotoxic and mutagenic activity of avarol. Nat. Prod. Res. 2016, 30, 1293–1296. [Google Scholar] [CrossRef]
- Huang, J.M.; Zhang, G.N.; Shi, Y.; Zha, X.; Zhu, Y.; Wang, M.M.; Lin, Q.; Wang, W.; Lu, H.Y.; Ma, S.Q.; et al. Atractylenolide-I sensitizes human ovarian cancer cells to paclitaxel by blocking activation of TLR4/ MyD88-dependent pathway. Sci. Rep. 2014, 4, 3840. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Yu, B.X.; Chen, J.F.; Lv, X.Y.; Yan, Z.J.; Cheng, Y.; Ma, Q. Anti-tumor effects of Atractylenolide I on bladder cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 40. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Park, H.H.; Chung, J.Y.; Lin, S.C.; Lo, Y.C.; da Graca, L.S.; Jiang, X.; Wu, H. Caspase-9 holoenzyme is a specific and optimal procaspase-3 processing machine. Mol. Cell 2006, 22, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Decker, P.; Muller, S. Modulating Poly (ADP-Ribose) Polymerase Activity: Potential for the Prevention and Therapy of Pathogenic Situations Involving DNA Damage and Oxidative Stress. Curr. Pharm. Biotechnol. 2002, 3, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Coelho, D.; Dufour, P.; Bergerat, J.P.; Denis, J.M.; Gueulette, J.; Bischoff, P. Caspase 8-mediated cleavage of the pro-apoptotic BCL-2 family member BID in p53-dependent apoptosis. Biochem. Biophys. Res. Commun. 2003, 306, 516–522. [Google Scholar] [CrossRef]
- Kantari, C.; Walczak, H. Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta 2011, 1813, 558–563. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.W.K.; Chung, H.Y.; Ho, W.S. Anti-Tumor Activity of Atractylenolide I in Human Colon Adenocarcinoma In Vitro. Molecules 2020, 25, 212. https://doi.org/10.3390/molecules25010212
Chan KWK, Chung HY, Ho WS. Anti-Tumor Activity of Atractylenolide I in Human Colon Adenocarcinoma In Vitro. Molecules. 2020; 25(1):212. https://doi.org/10.3390/molecules25010212
Chicago/Turabian StyleChan, Ka Woon Karen, Hau Yin Chung, and Wing Shing Ho. 2020. "Anti-Tumor Activity of Atractylenolide I in Human Colon Adenocarcinoma In Vitro" Molecules 25, no. 1: 212. https://doi.org/10.3390/molecules25010212
APA StyleChan, K. W. K., Chung, H. Y., & Ho, W. S. (2020). Anti-Tumor Activity of Atractylenolide I in Human Colon Adenocarcinoma In Vitro. Molecules, 25(1), 212. https://doi.org/10.3390/molecules25010212