The Combination of MoS2/WO3 and Its Adsorption Properties of Methylene Blue at Low Temperatures
Abstract
1. Introduction
2. Results and Discussion
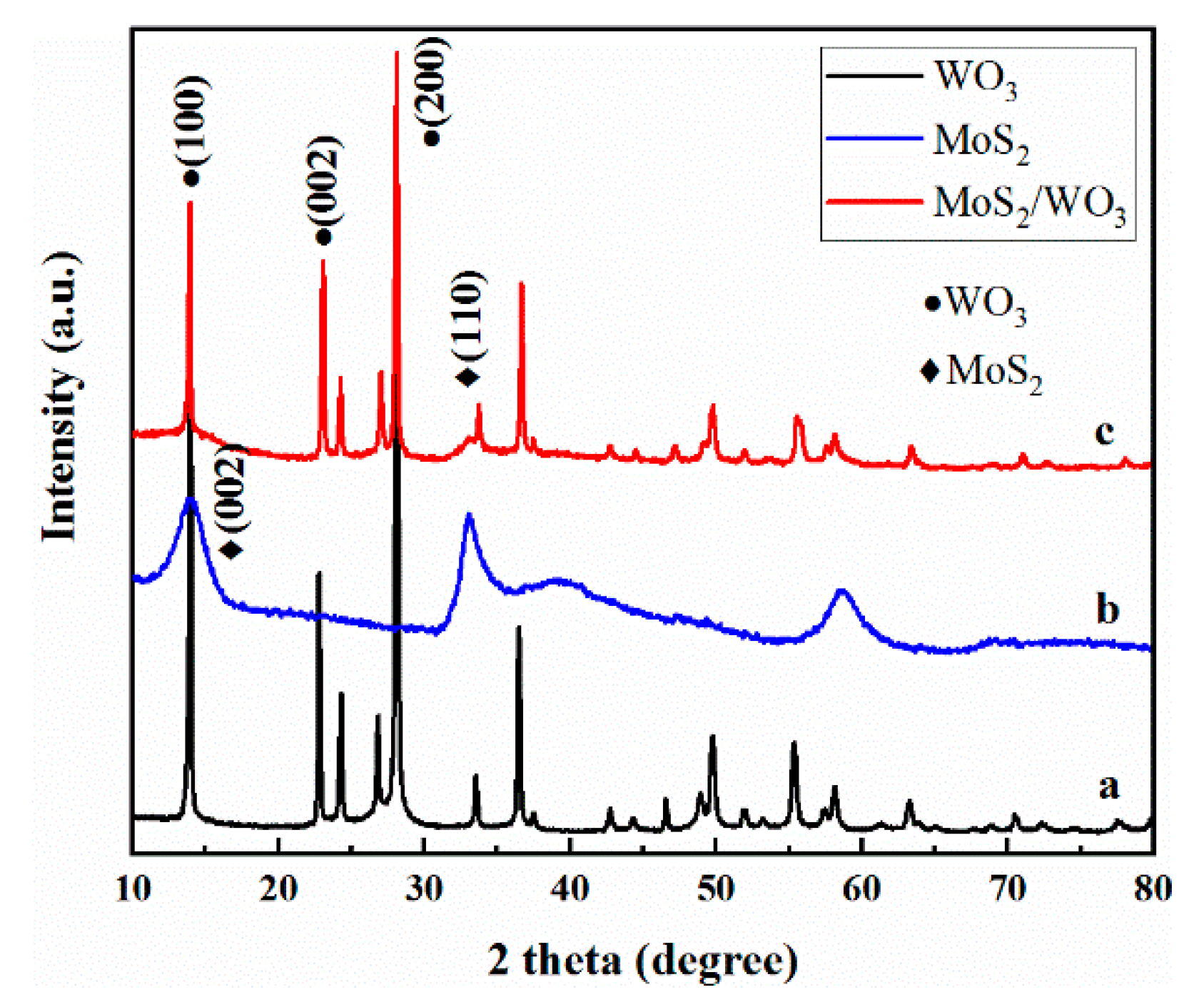
2.1. The Phase of Samples
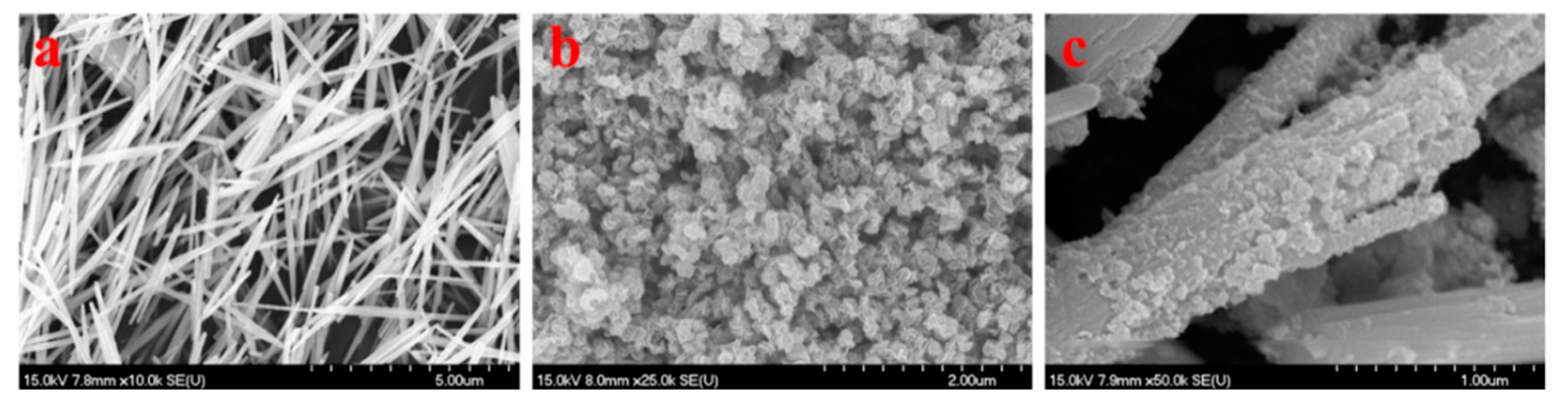
2.2. The Morphology of Samples
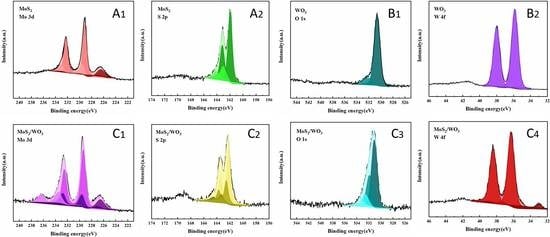
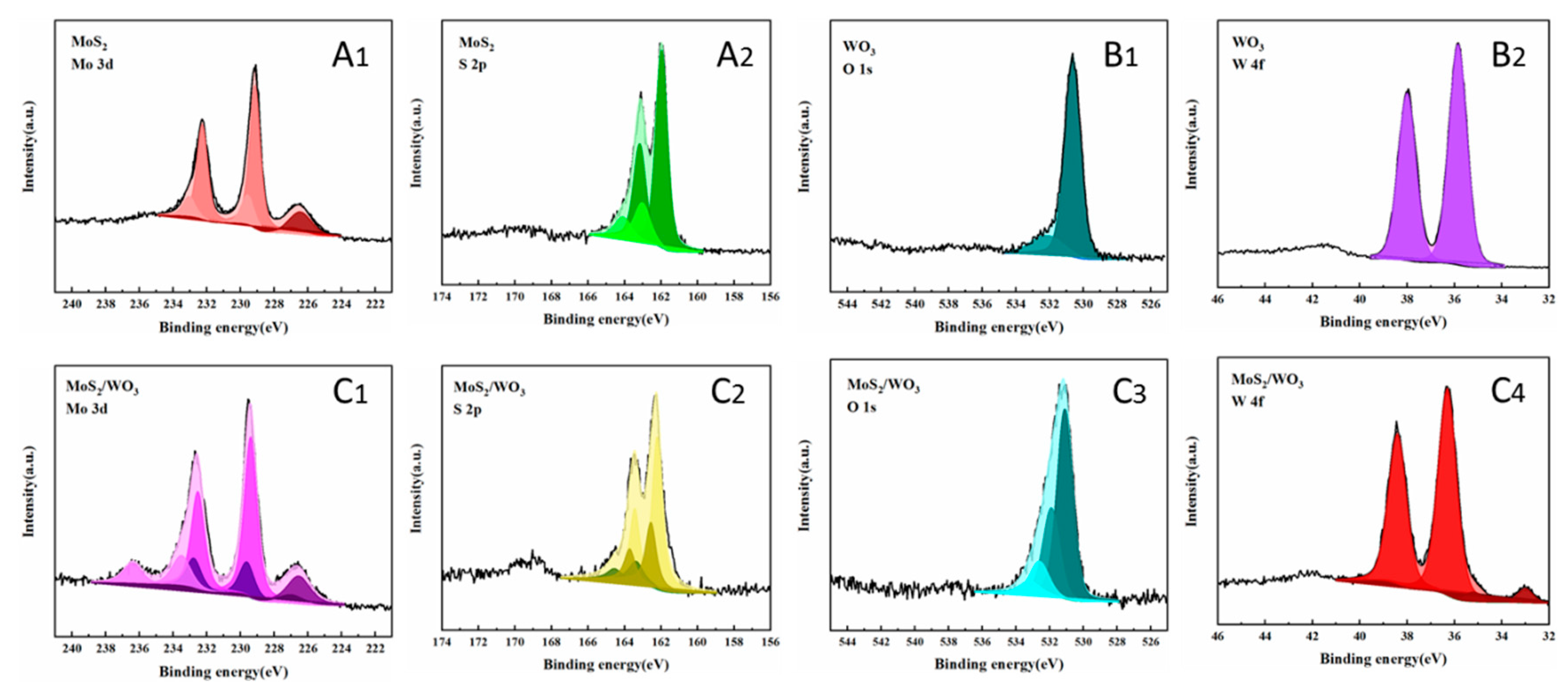
2.3. The Elemental Chemical States Analysis of Samples
2.4. Effect of Temperature on Adsorption Properties
2.5. Adsorption Kinetics
2.6. Adsorption Thermodynamics
3. Materials and Methods
3.1. Fabrication of WO3 Nanorods
3.2. Synthesis of MoS2/WO3 Composites
3.3. Characterization
3.4. Adsorption Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ravikumar, K.; Deebika, B.; Balu, K. Decolourization of aqueous dye solutions by a novel adsorbent: Application of statistical designs and surface plots for the optimization and regression analysis. J. Hazard. Mater. 2005, 1, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; Mcmullan, G.; Marchant, R.; Nigam, p. Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 3, 247–255. [Google Scholar] [CrossRef]
- Ritika; Manjot, K.; Ahmad, U.; Surinder, K.; Surinder, S.; Sushil, K.; Fouad, H.; Othman, Y. Rapid Solar-Light Driven Superior Photocatalytic Degradation of Methylene Blue Using MoS2-ZnO Heterostructure Nanorods Photocatalyst. Mater. 2018, 11, 2254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Sun, Y.; Sun, W.; Shen, H.; Zheng, H.; Xu, Y.; Zhao, J.; Wu, H.; Liu, C. Advanced treatment of actual textile dye wastewater by Fenton-flocculation process. Can. J. Chem. Eng. 2017, 7, 1245–1252. [Google Scholar] [CrossRef]
- Malik, P.K.; Saha, S.K. Oxidation of direct dyes with hydrogen peroxide using ferrous ion as catalyst. Sep. Purif. Technol. 2003, 3, 241–250. [Google Scholar] [CrossRef]
- Negar, D.K.; Reza, K.; Saeideh, K.E.; Saeid, M.; Seeram, R.; Hu, J. Visible Light Driven Heterojunction Photocatalyst of CuO–Cu2O Thin Films for Photocatalytic Degradation of Organic Pollutants. Nanomater. 2019, 9, 1011. [Google Scholar] [CrossRef]
- Martinezhuitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. 2009, 3, 105–145. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, M.; Wang, L.; Liu, X. The Adsorption of Methylene Blue by an Amphiphilic Block Co-Poly (Arylene Ether Nitrile) Microsphere-Based Adsorbent: Kinetic, Isotherm, Thermodynamic and Mechanistic Studies. Nanomater. 2019, 9, 1356. [Google Scholar] [CrossRef]
- Jiri, K.; Vojtech, A.; Ondrej, Z. Fabrication of Graphene/Molybdenum Disulfide Composites and Their Usage as Actuators for Electrochemical Sensors and Biosensors. Molecules 2019, 24, 3374. [Google Scholar] [CrossRef]
- Shang, S.; Lindwall, G.; Wang, G.Y.; Redwing, J.M.; Anderson, T.; Liu, Z. Lateral Versus Vertical Growth of Two-Dimensional Layered Transition-Metal Dichalcogenides: Thermodynamic Insight into MoS2. Nano Lett. 2016, 9, 5742–5750. [Google Scholar] [CrossRef]
- Wei, T.; Lau, W.; An, X.; Yu, X. Interfacial Charge Transfer in MoS2/TiO2 Heterostructured Photocatalysts: The Impact of Crystal Facets and Defects. Moleclus 2019, 24, 1769. [Google Scholar] [CrossRef] [PubMed]
- Sabarinathan, M.; Harish, S.; Archana, J.; Navaneethan, J.; Ikeda, H.; Hayakawa, Y. Controlled exfoliation of monodispersed MoS2 layered nanostructures by a ligand-assisted hydrothermal approach for the realization of ultrafast degradation of an organic pollutant. RSC Adv. 2016, 111, 109495–109505. [Google Scholar] [CrossRef]
- Hou, J.; Zhao, Q.; Li, T.; Hu, J.; Li, P.; Li, G. Spherical MoS2/WO3 Composite Semiconductor: Preparation and Photocatalytic Performance for RhB. Chin. J. Inorg. Chem. 2017, 9, 1527–1536. [Google Scholar] [CrossRef]
- Li, J.; Zhang, M.; Li, Q.; Yang, J. Enhanced visible light activity on direct contact Z-scheme g-C3N4-TiO2 photocatalyst. Appl. Surf. Sci. 2017, 184–193. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Li, Y.; Ho, W.; Yu, J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl. Surf. Sci. 2017, 175–183. [Google Scholar] [CrossRef]
- Wang, S.; Yang, X.; Zhang, X.; Ding, X.; Yang, Z.; Dai, K. A plate-on-plate sandwiched Z-scheme heterojunction photocatalyst: BiOBr-Bi2MoO6 with enhanced photocatalytic performance. Appl. Surf. Sci. 2017, 194–201. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, B.; Yu, J. A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure. Phys. Chem. Chem. Phys. 2016, 45, 31175–31183. [Google Scholar] [CrossRef]
- He, K.; Xie, J.; Luo, X.; Wen, J.; Ma, S.; Li, X.; Fang, Y.; Zhang, X. Enhanced visible light photocatalytic H2 production over Z-scheme g-C3N4 nansheets/WO3 nanorods nanocomposites loaded with Ni(OH)x cocatalysts. Chin. J. Catal. 2017, 2, 240–252. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Yang, R.; Hong, D.; Jiang, Y.; Shen, C.; Liang, K.; Xu, A. Enhanced visible-light photocatalytic activity of Z-scheme graphitic carbon nitride/oxygen vacancy-richzinc oxide hybrid photocatalysts. Chin. J. Catal. 2015, 12, 2135–2144. [Google Scholar] [CrossRef]
- Ying, J.; Wang, J.; Yang, Q.; Hu, X.; Zheng, Y.; Huang, W. Synthesis and adsorption properties of MoS2/WO3 nanocomposite. Funct. Mater. 2018, 7, 7062–7069. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Lin, H.; Li, X.; Chan, H.; Yang, L.; Gao, Q. MoS2–Ni3S2 Heteronanorods as Efficient and Stable Bifunctional Electrocatalysts for Overall Water Splitting. ACS Catals. 2017, 4, 2357–2366. [Google Scholar] [CrossRef]
- Li, A.; Feng, J.; Zhang, Y.; Wang, R.; Liu, H.; Wang, G.; Cheng, F.; Xi, P. Epitaxial Heterogeneous Interfaces on N-NiMoO4/NiS2 Nanowires/Nanosheets to Boost Hydrogen and Oxygen Production for Overall Water Splitting. Adv. Funct. Mater. 2019, 1, 1805298. [Google Scholar] [CrossRef]
- Cui, Z.; Sun, Y.G. From tremella-like MoS2 to α-MoO3 nanoplates: Sintering synthesis and adsorption properties. Micro Nano Lett. 2017, 9, 652–665. [Google Scholar] [CrossRef]
- Gao, M.; Liang, J.; Zheng, J.; Xu, Y.; Jiang, J.; Gao, Q.; Li, J.; Yu, S. An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation. Nat. Commun. 2015, 1, 5982. [Google Scholar] [CrossRef]
- Li, Y.; Yin, K.; Wang, K.; Lu, X.; Zhang, Y.; Liu, Y.; Yan, D.; Song, D.; Luo, S. Engineering MoS2 nanomesh with holes and lattice defects for highly active hydrogen evolution reaction. Appl. Catal. 2018, 239, 537–544. [Google Scholar] [CrossRef]
- Sun, M.Y.; Alan, E.N.; John, A. A DFT study of WS2, NiWS, and CoWS hydrotreating catalysts: Energeties and surface structures. J. Catal. 2004, 226, 41–53. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Zhu, T. Application of activated carbon derived from scrap tires for adsorption of Rhodamine, B. J. Environ. Sci. 2010, 8, 1273–1280. [Google Scholar] [CrossRef]
- Eftekhari, S.; Habibi-Yangjeh, A.; Sohrabnezhad, S. Application of AlMCM-41 for competitive adsorption of methylene blue and rhodamine B: Thermodynamic and kinetic studies. J. Hazard. Mater. 2010, 1, 349–355. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Bo, J.; Chow, J.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2010, 2, 354–364. [Google Scholar] [CrossRef]
- Motsa, M.M.; Mamba, B.B.; Thwala, J.M.; Msagati, T.A.M. Preparation, characterization, and application of polypropylene–clinoptilolite composites for the selective adsorption of lead from aqueous media. J. Colloid Inter. Sci. 2011, 359, 210–219. [Google Scholar] [CrossRef]
- Liu, T.H.; Li, Y.H.; Du, Q.J.; Sun, J.K.; Jiao, Y.Q.; Yang, G.M.; Wang, Z.H.; Xia, Y.Z.; Zhang, W.; Wang, K.L.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surfaces B 2012, 90, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Du, Q.J.; Liu, T.H.; Peng, X.J.; Wang, J.J.; Sun, J.K.; Wang, Y.H.; Wu, S.L.; Wang, Z.H.; Xia, Y.Z.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Sun, Q. Rapid and Selective Adsorption of Cationic Dyes by a Unique Metal-organic Framework with Decorated Pore Surface. Appl. Surf. Sci. 2018, 1219–1226. [Google Scholar] [CrossRef]
- Wang, T.; Kailasam, K.; Xiao, P.; Chen, G.; Chen, G.; Wang, L.; Li, J.; Zhu, J. Adsorption removal of organic dyes on covalent triazine framework (CTF). Microporous Mesoporous Mater. 2014, 187, 63–70. [Google Scholar] [CrossRef]
- Viegas, R.M.C.; Campinas, M.; Costa, H.; Rosa, M.J. How do the HSDM and Boyd’s model compare for estimating intraparticle diffusion coefficients in adsorption processes. Adsorption 2014, 20, 737–746. [Google Scholar] [CrossRef]
- Mckay, G. The adsorption of dyestuffs from aqueous solution using activated carbon: Analytical solution for batch adsorption based on external mass transfer and. Chem. Eng. J. 1983, 3, 187–196. [Google Scholar] [CrossRef]
- Ding, L.; Bo, Z.; Gao, W.; Liu, Q.; Wang, Z.; Guo, Y.; Wang, X.; Liu, Y. Adsorption of Rhodamine-B from aqueous solution using treated rice husk-based activated carbon. Colloid. Surf. A. 2014, 446, 1–7. [Google Scholar] [CrossRef]
- Sun, P.; Xu, L.; Li, J.; Zhai, P.; Zhang, H.; Zhang, Z.; Zhu, Z. Hydrothermal synthesis of mesoporous Mg3Si2O5(OH)4 microspheres as high-performance adsorbents for dye removal. Chem. Eng. J. 2018, 377–388. [Google Scholar] [CrossRef]
- Olu-Owolabi, B.I.; Diagboya, P.N.; Adebowale, K.O. Evaluation of pyrene sorption-desorption on tropical soils. J. Env. Manag. 2014, 137, 1–9. [Google Scholar] [CrossRef]
- Mckay, G.; Blair, H.S.; Gardner, J.R. Adsorption of dyes on chitin. I. Equilib. Stud. J. Appl. Poly. Sci. 1982, 8, 3043–3057. [Google Scholar] [CrossRef]
- De Sá, A.; Abreu, A.S.; Moura, I.; Machado, A.V. Polymeric materials for metal sorption from hydric resources. Water Purif. 2017, 289–322. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Adekola, F.A.; Olatunji, G.A. Kinetics, Isotherms and thermodynamic modeling of liquid phase adsorption of Rhodamine B dye onto Raphia hookerie fruit epicarp. Water Resour. Indust. 2016, 15, 14–27. [Google Scholar] [CrossRef]
- Feng, M.; You, W.; Wu, Z.; Chen, Q.; Zhan, H. Mildly alkaline preparation and methyleneblue adsorption capacity of hierarchical flower-like sodium titanate. Appl. Mater. Inter. 2013, 23, 12654–12662. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumaar, S.; Varadarajan, P.R.; Porkodi, K.; Subbhuraam, C.V. Adsorption of methylene blue onto jute fiber carbon: Kinetics and equilibrium studies. J. Colloid Interface Sci. 2005, 284, 78–82. [Google Scholar] [CrossRef]
- Acemioglu, B. Batch kinetic study of sorption of methylene blue by perlite. Chem. Eng. J. 2005, 1, 73–81. [Google Scholar] [CrossRef]
- Ozer, A.; Dursun, G. Removal of methylene blue from aqueous solution by dehydrated wheat bran carbon. J. Hazard. Mater. 2007, 1–2, 262–269. [Google Scholar] [CrossRef]
- Li, Z.; Meng, M.; Zhang, Z. Equilibrium and kinetic modelling of adsorption of Rhodamine B on MoS2. Mater. Res. Bull. 2019, 238–244. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available. |

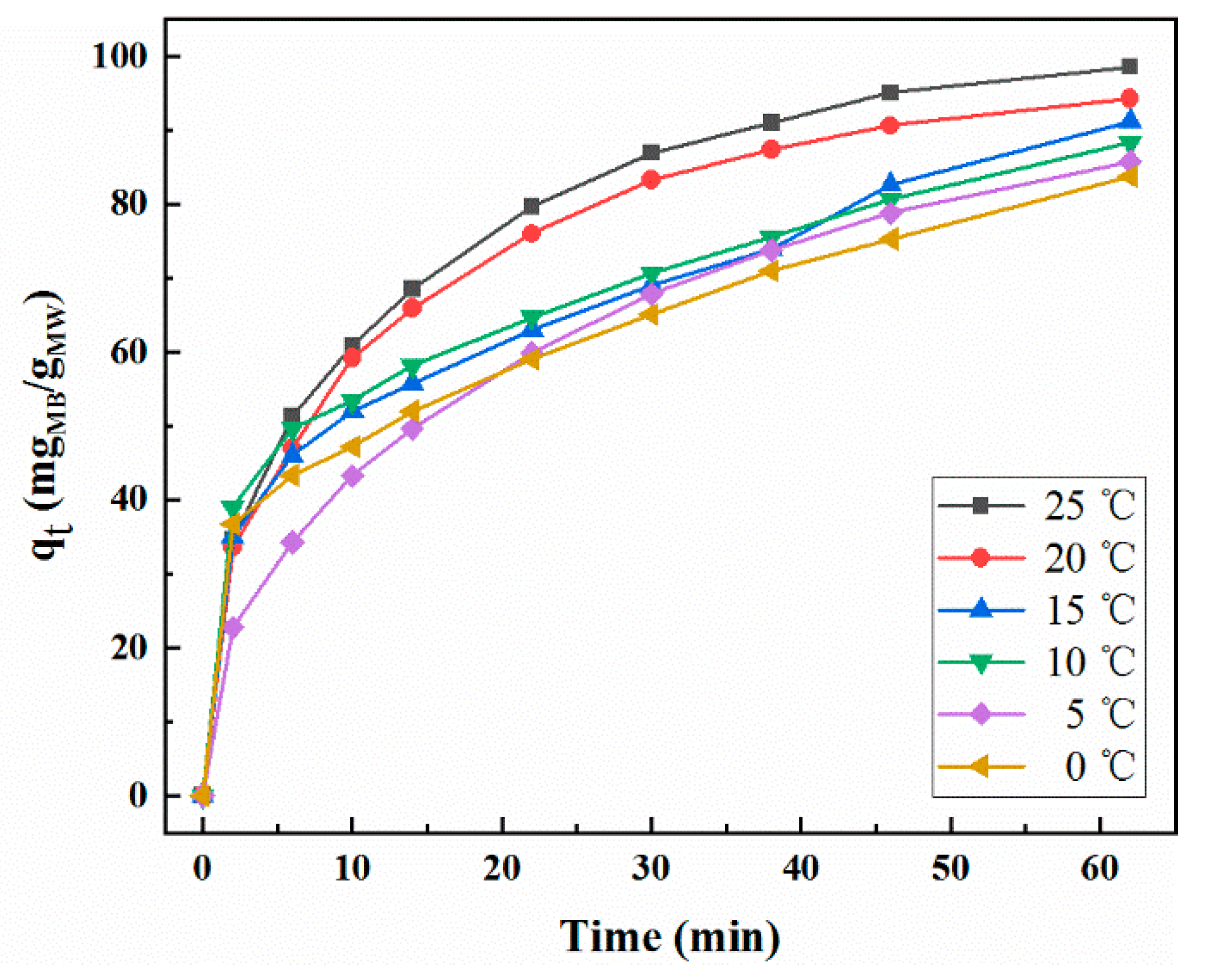
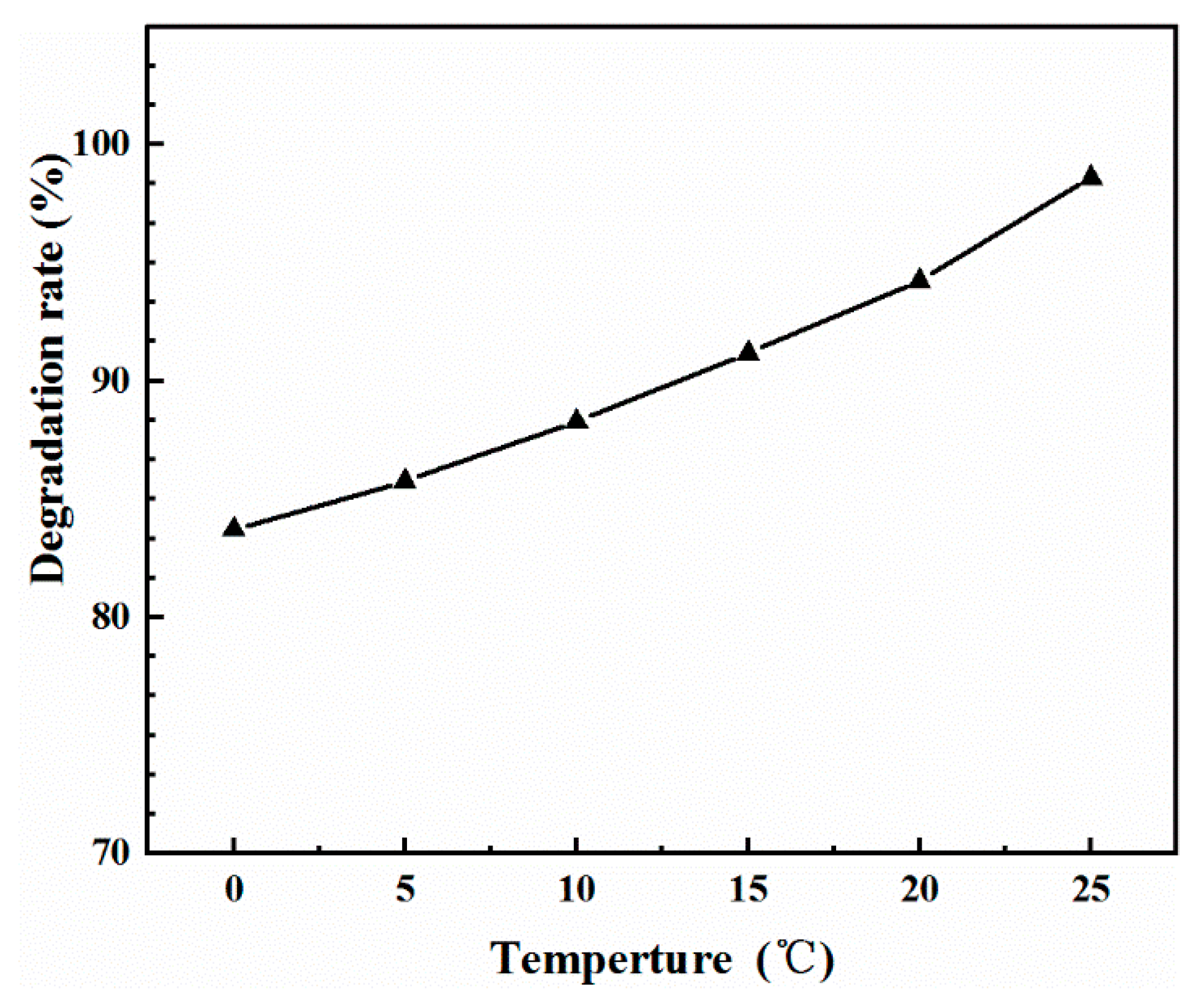
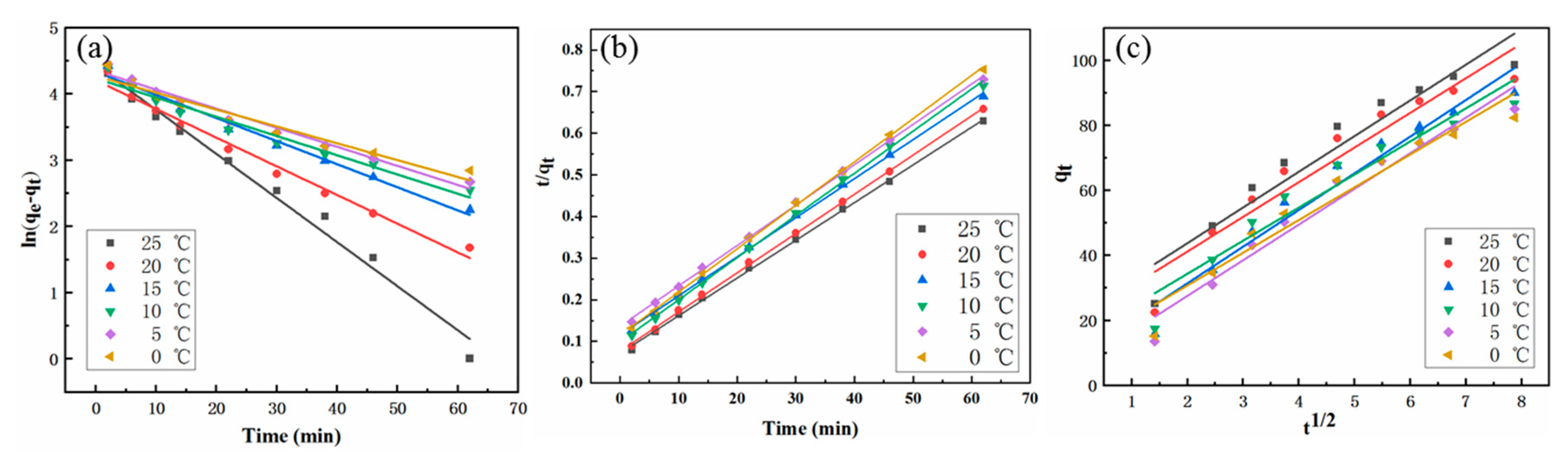
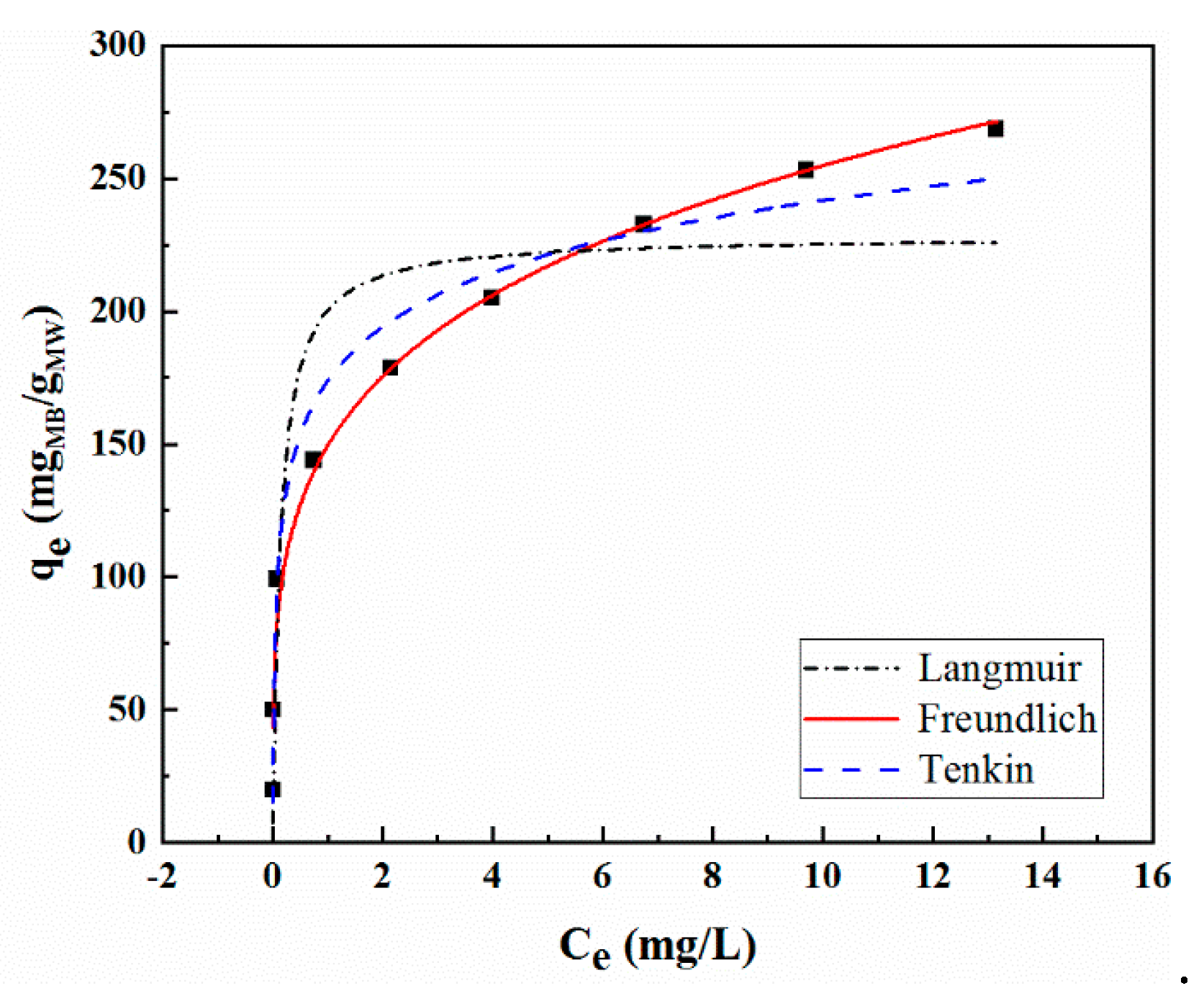
| Temperature (°C) | qe exp (mg/g) | Pseudo-First Order Model | Pseudo-Second Order Model | Intra-Particle Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe cal (mg/g) | R2 | k2·103 (g·mg−1·min−1) | qe cal (mg/g) | R2 | kp (g·g−1·min1/2) | Ci | R2 | ||
| 25 | 99.599 | 0.0665 | 83.713 | 0.9853 | 1.1506 | 110.497 | 0.9992 | 10.9769 | 21.8190 | 0.9802 |
| 20 | 99.588 | 0.0433 | 67.078 | 0.9826 | 1.1562 | 106.383 | 0.9995 | 10.6596 | 19.8856 | 0.9806 |
| 15 | 99.559 | 0.0349 | 76.256 | 0.9858 | 0.7608 | 106.610 | 0.9992 | 11.2756 | 8.8740 | 0.9912 |
| 10 | 99.575 | 0.0290 | 69.030 | 0.9598 | 1.0374 | 98.814 | 0.9991 | 10.1768 | 14.0003 | 0.9851 |
| 5 | 99.565 | 0.0288 | 77.676 | 0.9772 | 0.6904 | 103.093 | 0.9992 | 10.9661 | 5.6302 | 0.9934 |
| 0 | 99.559 | 0.0254 | 71.621 | 0.9511 | 0.9474 | 96.061 | 0.9994 | 10.0647 | 10.6341 | 0.9397 |
| Isotherms | Constants | MoS2/WO3 |
|---|---|---|
| Langmuir | Qm | 228.2849 |
| b | 7.1529 | |
| R2 | 0.8718 | |
| Freundlich | Ki | 161.5729 |
| 1/m | 0.2052 | |
| R2 | 0.9898 | |
| Temkin | A | 29.5634 |
| B | 354.7873 | |
| R2 | 0.9779 |
| Adsorbents | Q0 (mg/g) | Reference |
|---|---|---|
| Flower-like sodium titanate | 58.0 | [43] |
| Jute fiber carbon | 74 | [44] |
| Perlite | 94 | [45] |
| Dehydrated wheat bran | 122.0 | [46] |
| MoS2 | 136.99 | [47] |
| MoS2/WO3 | 268.42 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, J.; Wang, Y.; Zhou, H.; Pu, Z.; Yang, Q.; Huang, W. The Combination of MoS2/WO3 and Its Adsorption Properties of Methylene Blue at Low Temperatures. Molecules 2020, 25, 2. https://doi.org/10.3390/molecules25010002
Zheng Y, Wang J, Wang Y, Zhou H, Pu Z, Yang Q, Huang W. The Combination of MoS2/WO3 and Its Adsorption Properties of Methylene Blue at Low Temperatures. Molecules. 2020; 25(1):2. https://doi.org/10.3390/molecules25010002
Chicago/Turabian StyleZheng, Yifan, Jingjing Wang, Yedan Wang, Huan Zhou, Zhiying Pu, Qian Yang, and Wanzhen Huang. 2020. "The Combination of MoS2/WO3 and Its Adsorption Properties of Methylene Blue at Low Temperatures" Molecules 25, no. 1: 2. https://doi.org/10.3390/molecules25010002
APA StyleZheng, Y., Wang, J., Wang, Y., Zhou, H., Pu, Z., Yang, Q., & Huang, W. (2020). The Combination of MoS2/WO3 and Its Adsorption Properties of Methylene Blue at Low Temperatures. Molecules, 25(1), 2. https://doi.org/10.3390/molecules25010002