Symbiotic Aerogel Fibers Made via In-Situ Gelation of Aramid Nanofibers with Polyamidoxime for Uranium Extraction
Abstract
1. Introduction
2. Results and Discussion
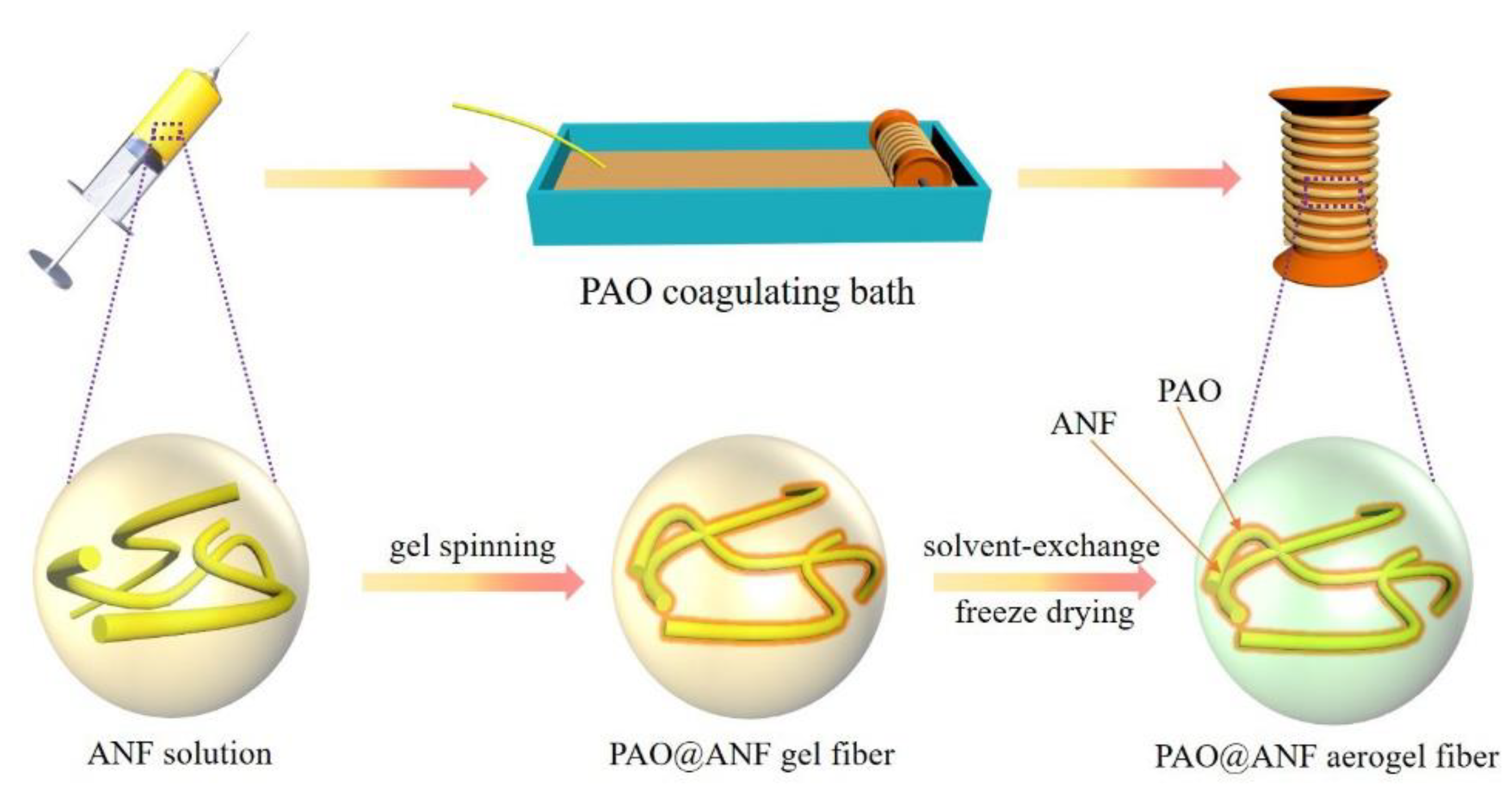
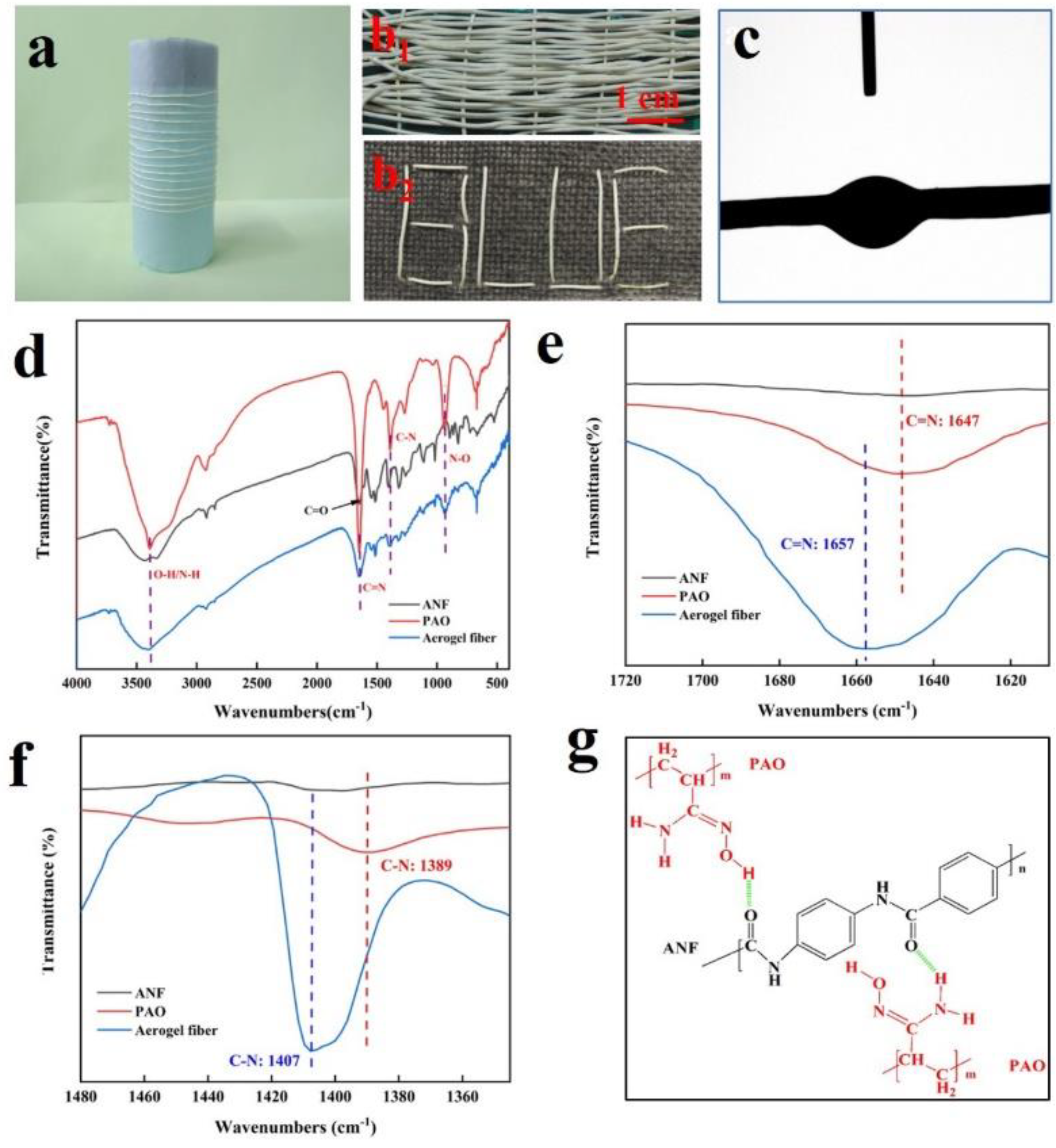
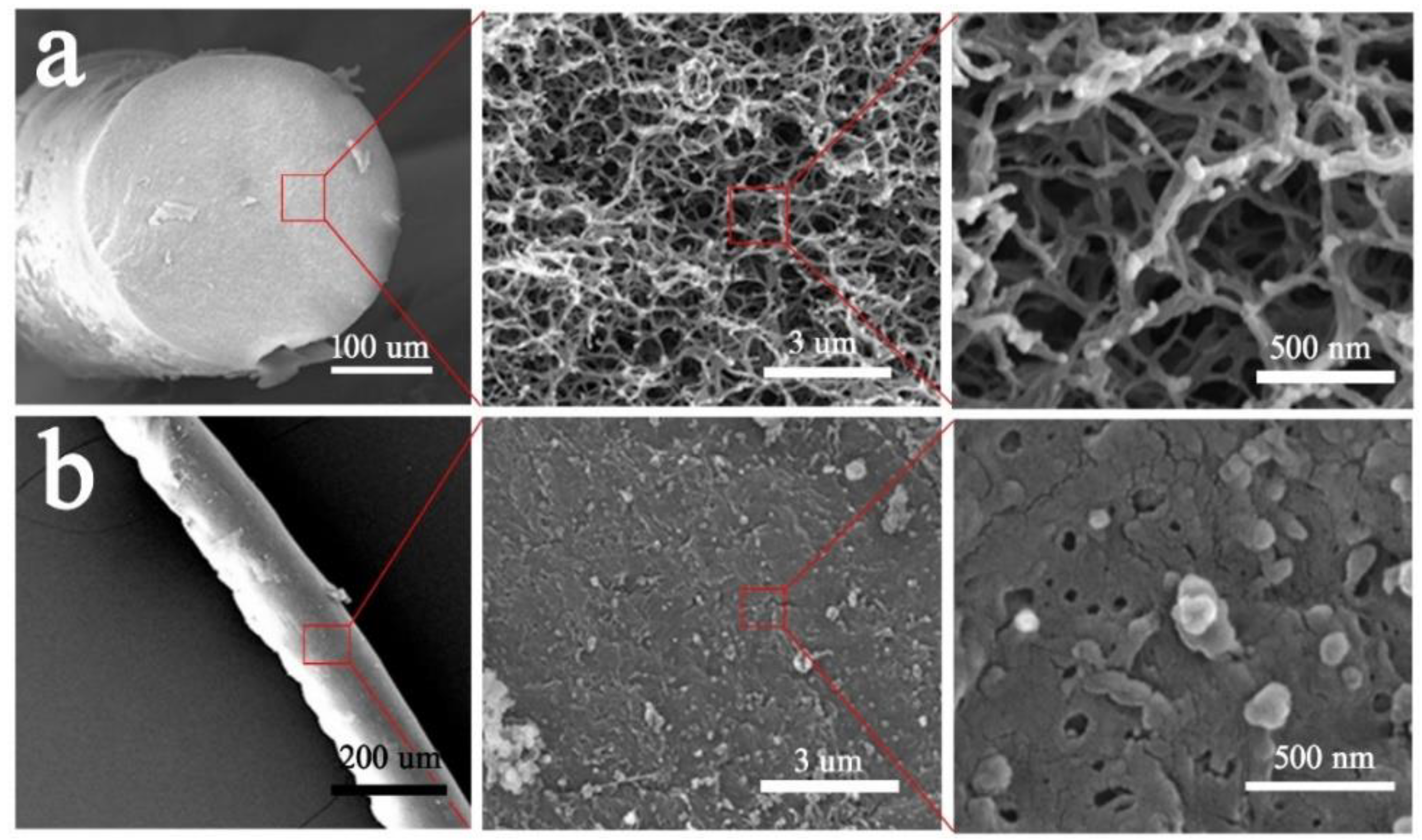
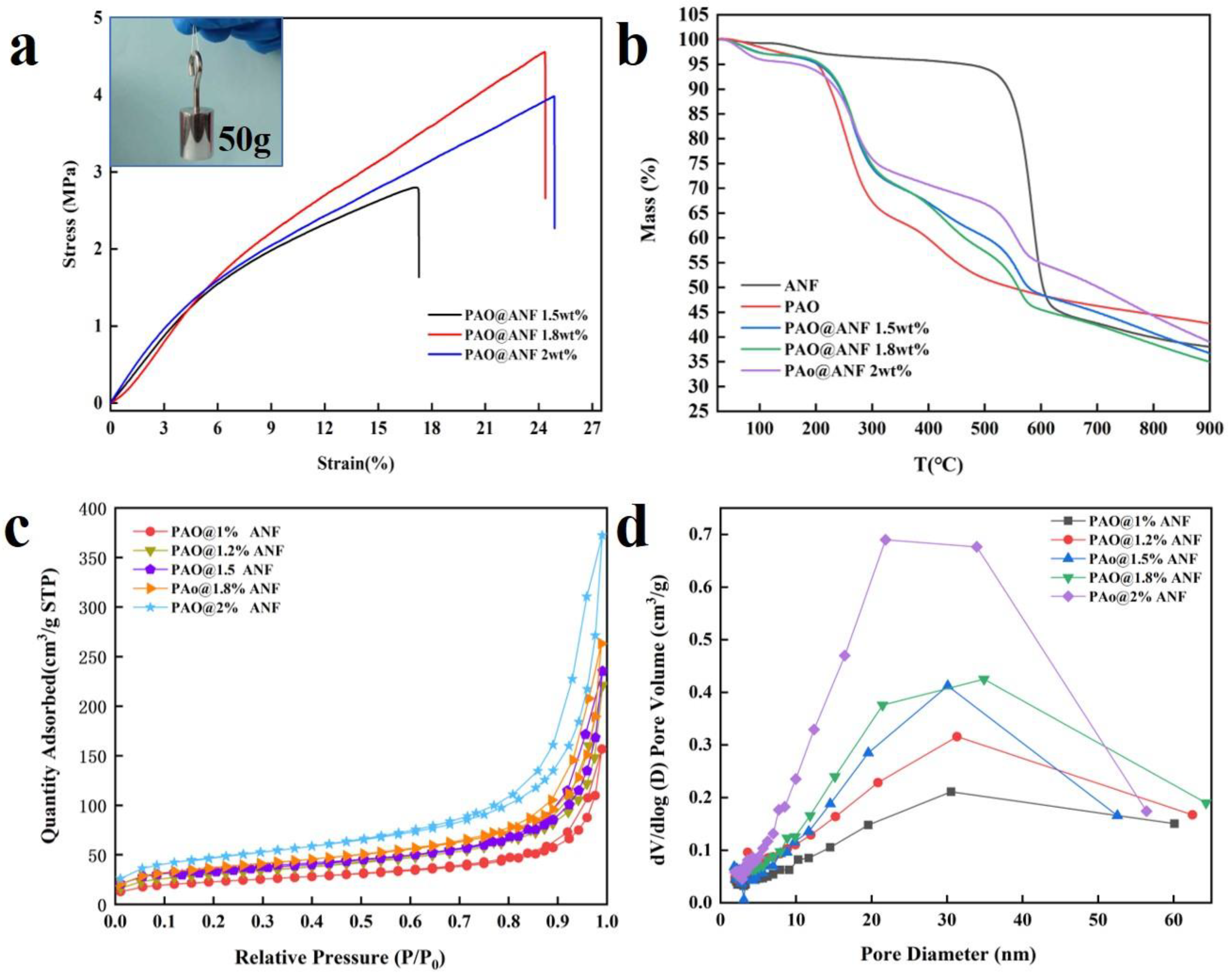
2.1. Synthesis and Characterization of Fibers
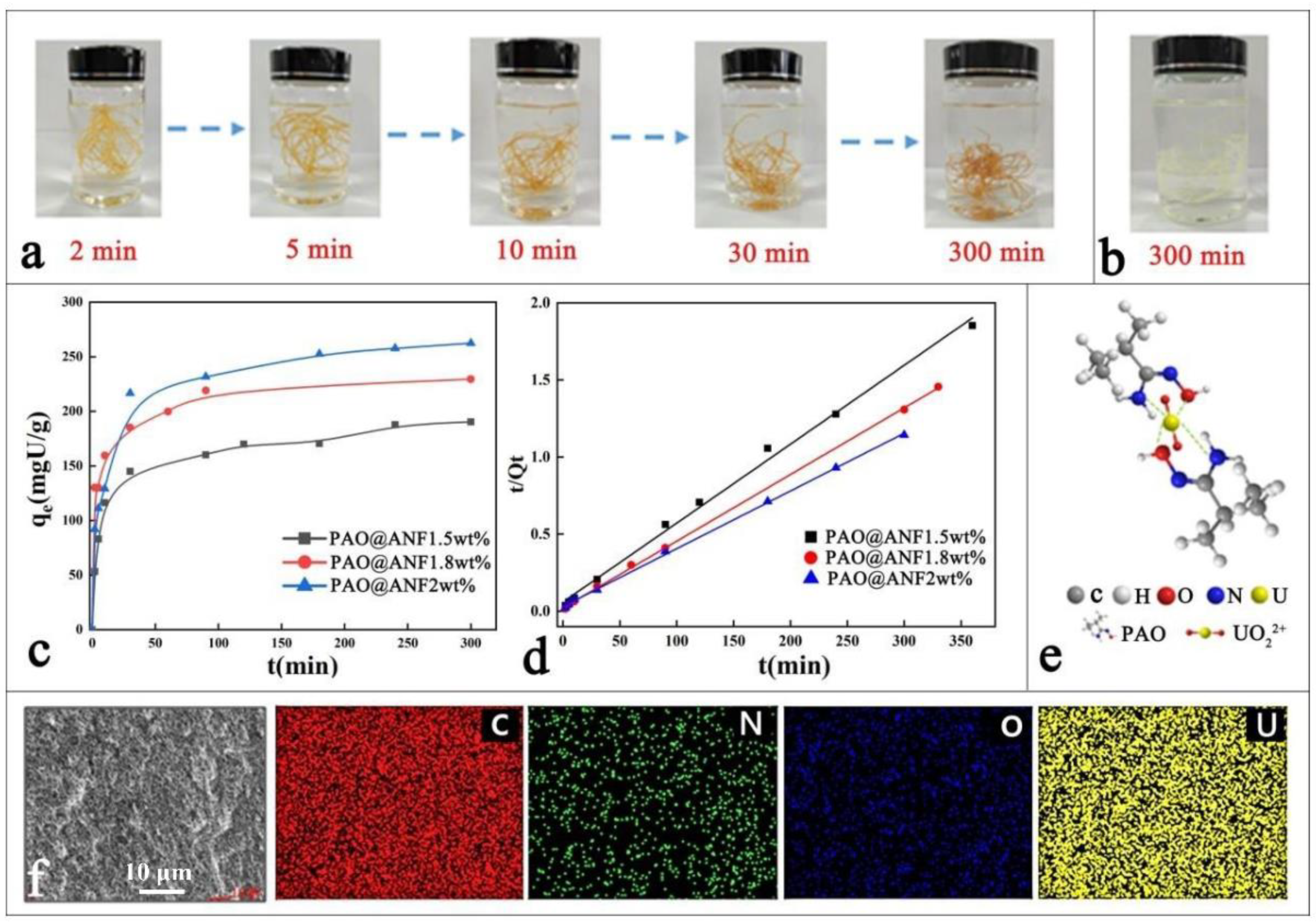
2.2. Effect of Contact Time and Adsorption Kinetics
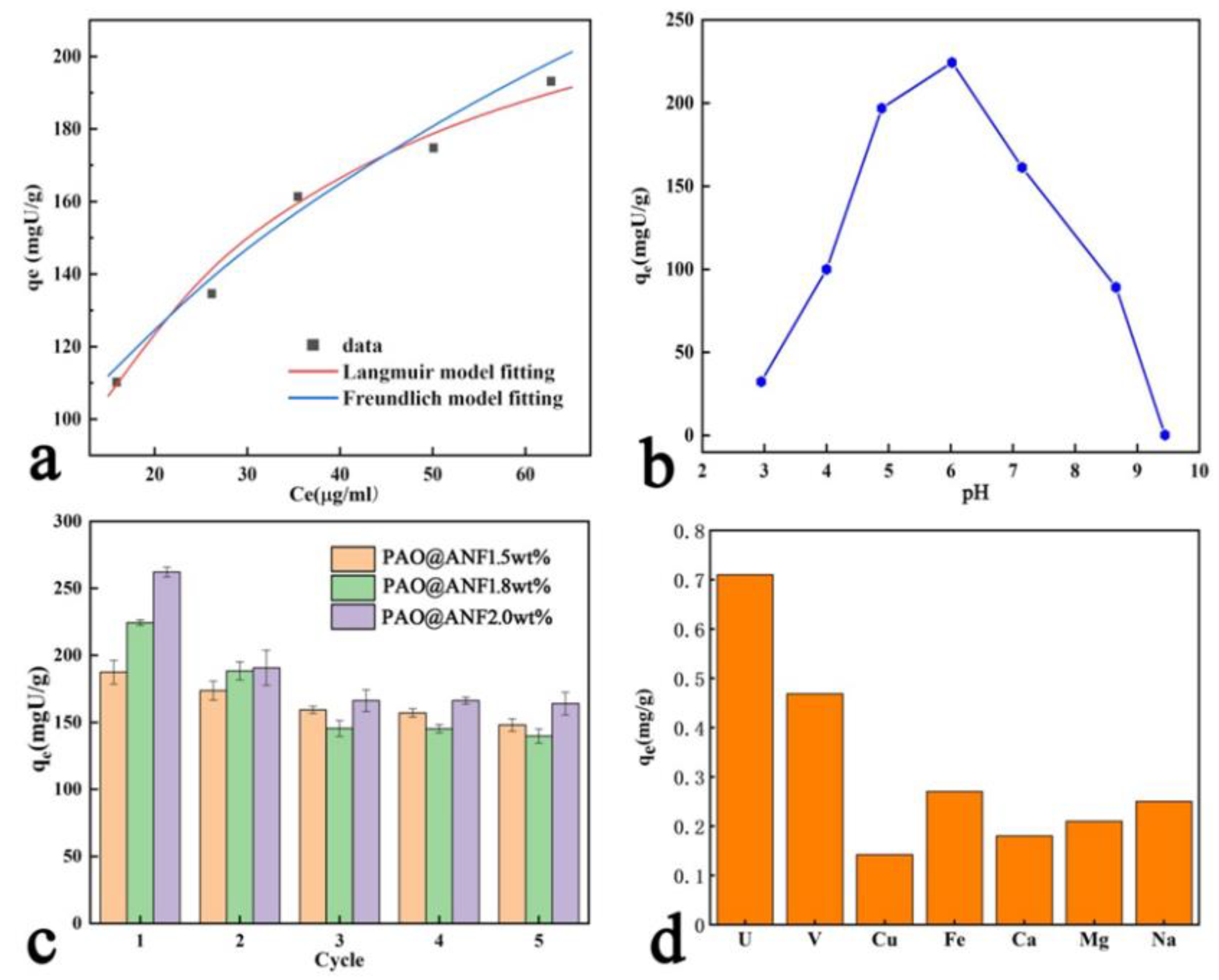
2.3. Equilibrium Adsorption
2.4. Effect of PH
2.5. Reusability
2.6. Selective Adsorption in Simulated Seawater
3. Materials and Methods
3.1. Materials
3.2. Preparation of the PAO Coagulating Bath
3.3. Preparation of PAO@ANF Symbiotic Aerogel Fibers
3.4. Characterization
3.5. Batch Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Luo, W.; Xiao, G.; Tian, F.; Richardson, J.J.; Wang, Y.; Zhou, J.; Guo, J.; Liao, X.; Shi, B.B. Engineering robust metal–phenolic network membranes for uranium extraction from seawater. Energ. Environ. Sci. 2019, 12, 607–614. [Google Scholar] [CrossRef]
- Hoffert, M.I.; Caldeira, K.; Benford, G.; Criswell, D.R.; Green, C.; Herzog, H.; Jain, A.K.; Kheshgi, H.S.; Lackner, K.S.; Lewis, J.S. Advanced Technology Paths to Global Climate Stability: Energy for a Greenhouse Planet. Science 2002, 298, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Kaltsoyannis, N.; Liddle, S. Catalyst: Nuclear Power in the 21st Century. Chem 2016, 1, 659–662. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Q.; Zhang, H.; Lu, Z.; Liu, J.; Chen, R.; Li, R.; Li, Z.; Liu, P.; Wang, J. Efficient removal of uranium (vi) from simulated seawater using amidoximated polyacrylonitrile/FeOOH composites. Dalton Tran. 2017, 46, 15746–15756. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Perman, J.; Ivanov, A.S.; Bryantsev, V.S.; Earl, L.D.; Abney, C.W.; Wojtas, L.; Ma, S.Q. Bio-inspired nano-traps for uranium extraction from seawater and recovery from nuclear waste. Nat. Commun. 2018, 9, 1644. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.F.; Mayes, R.T.; Jungseung, K.; Fulvio, P.F.; Sun, X.G.; Tsouris, C.; Chen, J.H.; dBrown, S.; Dai, S. Seawater uranium sorbents: Preparation from a mesoporous copolymer initiator by atom-transfer radical polymerization. Angew. Chem. Int. Ed. 2013, 52, 13458–13462. [Google Scholar] [CrossRef]
- Feng, M.L.; Sarma, D.; Qi, X.H.; Du, K.Z.; Huang, X.Y.; Kanatzidis, M.G. Efficient Removal and Recovery of Uranium by a Layered Organic-Inorganic Hybrid Thiostannate. J. Am. Chem. Soc. 2016, 138, 12578–12585. [Google Scholar] [CrossRef]
- Lu, Y. Uranium Extraction: Coordination chemistry in the ocean. Nat. Chem. 2014, 6, 175. [Google Scholar] [CrossRef]
- Abney, C.W.; Mayes, R.T.; Saito, T.; Dai, S. Materials for the Recovery of Uranium from Seawater. Chem. Rev. 2017, 117, 13935. [Google Scholar] [CrossRef]
- Li, W.T.; Chen, R.R.; Liu, Q.; Liu, J.Y.; Yu, J.; Zhang, H.S.; Li, R.M.; Zhang, M.L.; Wang, J. Hierarchical Ni–Al Layered Double Hydroxide in situ Anchored onto Polyethylenimine-Functionalized Fibers for Efficient U(VI) Capture. ACS Sustain. Chem. Engin. 2018, 6, 13385–13394. [Google Scholar] [CrossRef]
- Lindner, H.; Schneider, E. Review of cost estimates for uranium recovery from seawater. Energy Econ. 2015, 49, 9–22. [Google Scholar] [CrossRef]
- Yuan, Y.H.; Zhao, S.L.; Wen, J.; Wang, D.; Guo, X.W.; Xu, L.L.; Wang, X.L.; Wang, N. Rational Design of Porous Nanofiber Adsorbent by Blow-Spinning with Ultrahigh Uranium Recovery Capacity from Seawater. Adv. Funct. Mater. 2019, 29, 1805380. [Google Scholar] [CrossRef]
- Ma, S.L.; Huang, L.; Ma, L.J.; Shim, Y.; Islam, S.M.; Wang, P.L.; Zhao, L.D.; Wang, S.C.; Sun, G.B.; Yang, X.J.; et al. Efficient Uranium Capture by Polysulfide/Layered Double Hydroxide Composites. J. Am. Chem. Soc. 2015, 137, 3670–3677. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.Y.; Chen, T.; Duan, T.; Yao, W.T.; Zheng, K.; Dai, L.H.; Zhu, W.K. Bioassembly of fungal hypha/graphene oxide aerogel as high performance adsorbents for U(VI) removal. Chem. Eng. J. 2018, 347, 407–414. [Google Scholar] [CrossRef]
- Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Lebed, P.J.; Savoie, J.D.; Florek, J.; Bilodeau, F.; Lariviere, D.; Kleitz, F. Large Pore Mesostructured Organosilica-Phosphonate Hybrids as Highly Efficient and Regenerable Sorbents for Uranium Sequestration. Chem. Mater. 2012, 24, 4166–4176. [Google Scholar] [CrossRef]
- Das, S.; Liao, W.P.; Byers, M.F.; Tsouris, C.; Janke, C.J.; Mayes, R.T.; Schneider, E.; Kuo, L.J.; Wood, J.R.; Gill, G.A. Alternative Alkaline Conditioning of Amidoxime Based Adsorbent for Uranium Extraction from Seawater. Ind. Eng. Chem. Res. 2015, 55, 4303–4312. [Google Scholar] [CrossRef]
- Kuo, L.J.; Pan, H.B.; Wai, C.M.; Byers, M.F.; Schneider, E.; Strivens, J.E.; Janke, C.J.; Das, S.; Mayes, R.T.; Wood, J.R. Investigations into the Reusability of Amidoxime-Based Polymeric Adsorbents for Seawater Uranium Extraction. Ind. Eng. Chem. Res. 2017, 56, 11603–11611. [Google Scholar] [CrossRef]
- Piechowicz, M.; Abney, C.W.; Thacker, N.C.; Gilhula, J.C.; Wang, Y.F.; Veroneau, S.S.; Hu, A.G.; Lin, W.B. Successful Coupling of a Bis-Amidoxime Uranophile with a Hydrophilic Backbone for Selective Uranium Sequestration. ACS Appl. Mater. Interfaces 2017, 9, 27894–27904. [Google Scholar] [CrossRef]
- Satilmis, B.; Isık, T.; Demir, M.M.; Uyar, T. Amidoxime functionalized Polymers of Intrinsic Microporosity (PIM-1) electrospun ultrafine fibers for rapid removal of uranyl ions from water. Appl. Surf. Sci. 2019, 467, 648–657. [Google Scholar] [CrossRef]
- Wang, D.; Song, J.A.; Wen, J.; Yuan, Y.H.; Liu, Z.L.; Lin, S.; Wang, H.; Wang, H.Y.; Zhao, S.L.; Zhao, X.M.; et al. Significantly Enhanced Uranium Extraction from Seawater with Mass Produced Fully Amidoximated Nanofiber Adsorbent. Adv. Energy Mater. 2018, 8, 1802607. [Google Scholar] [CrossRef]
- Liu, C.; Hsu, P.C.; Xie, J.; Jie, J.Z.; Wu, T.; Wang, H.T.; Liu, W.; Zhang, J.S.; Chu, S.; Cui, Y. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2017, 2, 17007. [Google Scholar] [CrossRef]
- Atta, A.M.; Wahab, Z.H.A.E.; Shafey, Z.A.E.; Zidan, W.I.; Akl, Z.F. Uranyl Ions Uptake from Aqueous Solutions Using Crosslinked Ionic Copolymers Based on 2-Acrylamido-2-Methylpropane Sulfonic Acid Copolymers. J. Disper. Sci. Technol. 2010, 31, 1601–1610. [Google Scholar] [CrossRef]
- Awwad, N.S.; Daifullah, A.A.M. Preconcentration of U(VI) from aqueous solutions after sorption using Sorel’s cement in dynamic mode. J. Radioanal. Nucl. Chem. 2005, 264, 623–628. [Google Scholar] [CrossRef]
- Shuang, S.; Huang, S.Y.; Zhang, R.; Chen, Z.S.; Wen, T.; Wang, S.H.; Hayat, T.; Alsaedi, A.; Wang, X.K. Simultaneous removal of U(VI) and humic acid on defective TiO2–x investigated by batch and spectroscopy techniques. Chem. Eng. J. 2017, 325, 576–587. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, D.X.; Reda, A.T.; Liu, C.; Yang, Z.; Guo, S.S.; Xiao, S.T.; Ouyang, Y.G. Synthesis of amidoxime-grafted activated carbon fibers for efficient removal of uranium(VI) from aqueous solution. Ind. Eng. Chem. Res. 2017, 56, 11936–11947. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Wang, J.; Zhang, X.T. Surfactant-free synthesis of silica aerogel microspheres with hierarchically porous structure. J. Colloid Interface Sci. 2018, 515, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Du, R.; Zhang, X.T. Thermoresponsive Polyrotaxane Aerogels: Converting Molecular Necklaces into Tough Porous Monoliths. ACS Appl. Mater. Interfaces 2018, 10, 1468–1473. [Google Scholar] [CrossRef]
- Li, G.Y.; Zhang, X.T.; Wang, J.; Fang, J.H. From anisotropic graphene aerogels to electron- and photo-driven phase change composites. J. Mater. Chem. A 2016, 4, 17042–17049. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.C.; Zhang, X.T. Cyclic Molecule Aerogels: A Robust Cyclodextrin Monolith with Hierarchically Porous Structures for Removal of Micropollutants from water. J. Mater. Chem. A 2017, 5, 4308–4313. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.T. Binary crystallized supramolecular aerogels derived from host-guest inclusion complexes. ACS Nano 2015, 9, 11389–11397. [Google Scholar] [CrossRef]
- He, W.N.; Li, G.Y.; Zhang, S.Q.; Wei, Y.; Wang, J.; Li, Q.W.; Zhang, X.T. Polypyrrole/silver coaxial nanowire aero-sponges for temperature-independent stress sensing and stress-triggered Joule heating. ACS Nano 2015, 9, 4244–4451. [Google Scholar] [CrossRef]
- Yang, M.; Cao, K.Q.; Sui, L.; Qi, Y.; Zhu, J.; Waas, A.; Arruda, E.M.; Kieffer, J.; Thouless, M.D.; Kotov, N.A. Dispersions of aramid nanofibers: A new nanoscale building block. ACS Nano 2011, 5, 6945–6954. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.Y.; Liu, X.Y.; Zhang, B.W.; Ma, H.J.; Ling, C.J.; Yu, M.; Li, L.F.; Li, J.Y. Electrospun Nanofibrous Adsorbent for Uranium Extraction from Seawater. J. Mater. Chem. A 2015, 3, 2552–2558. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, Z.W.; Wu, X.H.; Li, G.Y.; Fang, D.; Zhang, X.T. Nanofibrous Kevlar aerogel films and their phase change composites for highly efficient Infrared Stealth. ACS Nano 2019, 13, 2236–2245. [Google Scholar] [CrossRef]
- Li, W.T.; Liu, Q.; Liu, J.Y.; Zhang, H.S.; Li, R.M.; Li, Z.S.; Jing, X.Y.; Wang, J. Removal U(VI) from artificial seawater using facilely and covalently grafted polyacrylonitrile fibers with lysine. Appl. Surf. Sci. 2017, 403, 378–388. [Google Scholar] [CrossRef]
- Shao, D.D.; Wang, X.L.; Ren, X.M.; Hu, S.; Wen, J.; Tan, Z.Y.; Xiong, J.; Asiri, A.M.; Marwani, H.M. Polyamidoxime functionalized with phosphate groups by plasma technique for effective U (VI) adsorption. J. Ind. Eng. Chem. 2018, 67, 380–387. [Google Scholar] [CrossRef]
- Raut, P.; Swanson, N.; Kulkarni, A.; Pugh, C.; Jana, S.C. Exploiting arene-perfluoroarene interactions for dispersion of carbon black in rubber compounds. Polymer 2018, 148, 247–258. [Google Scholar] [CrossRef]
- Raut, P.; Liang, W.; Chen, Y.M.; Zhu, Y.; Jana, S.C. Syndiotactic Polystyrene-Based Ionogel Membranes for High Temperature Electrochemical Applications. ACS Appl. Mater. Interfaces 2017, 9, 30933–30942. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hong, G.; Dong, D.; Song, W.; Zhang, X. Multiresponsive Graphene-Aerogel-Directed Phase-Change Smart Fibers. Adv. Mater. 2018, 30, 1801754. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; He, S.N.; Zhang, D.X.; Reda, A.T.; Liu, C.; Feng, J.; Yang, Z. Synthesis and characterization of amidoxime modified calix [8]arene for adsorption of U (vi) in low concentration uranium solutions. RSC Adv. 2016, 6, 101087–101097. [Google Scholar] [CrossRef]
- Lutfor, M.R.; Silong, S.; Zin, W.M.; Ab Rahman, M.Z.; Ahmad, M.; Haron, J. Preparation and characterization of poly(amidoxime) chelating resin from polyacrylonitrile grafted sago starch. Eur. Polym. J. 2000, 36, 2105–2113. [Google Scholar] [CrossRef]
- Hu, C.C.; Chen, L.; Gu, R.X.; Yu, J.R.; Zhu, J.; Hu, Z.M. Thermal Decomposition Behavior of a Heterocyclic Aramid Fiber. J. Macromol. Sci. Part B 2013, 52, 726–737. [Google Scholar] [CrossRef]
- Sa, R.N.; Yan, Y.; Wei, Z.H.; Zhang, L.Q.; Wang, W.C.; Tian, M. Surface modification of aramid fibers by bio-inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Li, P.; Gao, C. Strong, Conductive, Lightweight, Neat Graphene Aerogel Fibers with Aligned Pores. ACS Nano 2012, 6, 7103–7113. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, M.; Han, X.; Yang, S.; Hua, D. Petroleum pitch-based porous aromatic frameworks with phosphonate ligand for efficient separation of uranium from radioactive effluents. J. Hazard. Mater. 2019, 368, 214–220. [Google Scholar] [CrossRef]
- Yun, W.; Gu, Z.; Yang, J.; Liao, J.; Yang, Y.; Ning, L.; Tang, J. Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium(VI). Appl. Surface Sci. 2014, 320, 10–20. [Google Scholar] [CrossRef]
- Sun, Q.; Aguila, B.; Earl, L.D.; Abney, C.W.; Wojtas, L.; Thallapally, P.K.; Ma, S. Covalent Organic Frameworks as a Decorating Platform for Utilization and Affinity Enhancement of Chelating Sites for Radionuclide Sequestration. Adv. Mater. 2018, 30, 1705479. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chen, R.; Qi, L.; Liu, J.; Zhang, H.; Li, R.; Zhang, M.; Liu, P.; Wang, J. High efficiency extraction of U(VI) from seawater by incorporation of polyethyleneimine, polyacrylic acid hydrogel and Luffa cylindrical fibers. Chem. Eng. J. 2018, 345, 526–535. [Google Scholar] [CrossRef]
- Wei, Y.; Qian, J.; Huang, L.; Hua, D. Bifunctional polymeric microspheres for efficient uranium sorption from aqueous solution: Synergistic interaction of positive charge and amidoxime group. RSC Adv. 2015, 5, 64286–64292. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Li, J.X.; Zhao, L.P.; Zhang, S.W.; Huang, Y.S.; Wu, X.L.; Wang, X.K. Synthesis of amidoxime-functionalized Fe3O4@SiO2 core–shell magnetic microspheres for highly efficient sorption of U(VI). Chem. Eng. J. 2014, 235, 275–283. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Li, J.X.; Zhang, S.W.; Chen, H.; Shao, D. Efficient enrichment of uranium(VI) on amidoximated magnetite/graphene oxide composites. RSC Adv. 2013, 3, 18952–18959. [Google Scholar] [CrossRef]
- Das, S.; Brown, S.; Mayes, R.T.; Janke, C.J.; Tsouris, C.; Kuo, L.J.; Gill, G.; Dai, S. Novel poly(imide dioxime) sorbents: Development and testing for enhanced extraction of uranium from natural seawater. Chem. Eng. J. 2016, 298, 125–135. [Google Scholar] [CrossRef]
Sample Availability: Samples of PAO@ANF aerogel fibers are available from the authors. |
| Adsorbents | K (g·mg−1·min−1) | qe,exp (mg·g−1) | qe,cal (mg·g−1) | R2 |
|---|---|---|---|---|
| PAO@ANF 1.5wt% | 4.39 × 10−4 | 194.36 | 195.31 | 0.9953 |
| PAO@ANF 1.8wt% | 8.42 × 10−4 | 226.74 | 231.48 | 0.9995 |
| PAO@ANF 2.0wt% | 4.50 × 10−4 | 262.50 | 267.38 | 0.9991 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qmax (mg/g) | b (mL/μg) | R2 | KF ((mg/g) × (μg/mL) 1 − 1/n) | n | R2 |
| 251.89 | 0.0487 | 0.9967 | 37.39 | 2.505 | 0.9846 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, J.; Wang, W.; Zhang, X. Symbiotic Aerogel Fibers Made via In-Situ Gelation of Aramid Nanofibers with Polyamidoxime for Uranium Extraction. Molecules 2019, 24, 1821. https://doi.org/10.3390/molecules24091821
Li J, Wang J, Wang W, Zhang X. Symbiotic Aerogel Fibers Made via In-Situ Gelation of Aramid Nanofibers with Polyamidoxime for Uranium Extraction. Molecules. 2019; 24(9):1821. https://doi.org/10.3390/molecules24091821
Chicago/Turabian StyleLi, Juan, Jin Wang, Wei Wang, and Xuetong Zhang. 2019. "Symbiotic Aerogel Fibers Made via In-Situ Gelation of Aramid Nanofibers with Polyamidoxime for Uranium Extraction" Molecules 24, no. 9: 1821. https://doi.org/10.3390/molecules24091821
APA StyleLi, J., Wang, J., Wang, W., & Zhang, X. (2019). Symbiotic Aerogel Fibers Made via In-Situ Gelation of Aramid Nanofibers with Polyamidoxime for Uranium Extraction. Molecules, 24(9), 1821. https://doi.org/10.3390/molecules24091821






