Study on the Emulsifying Properties of Pomegranate Peel Pectin from Different Cultivation Areas
Abstract
1. Introduction
2. Results and Discussion
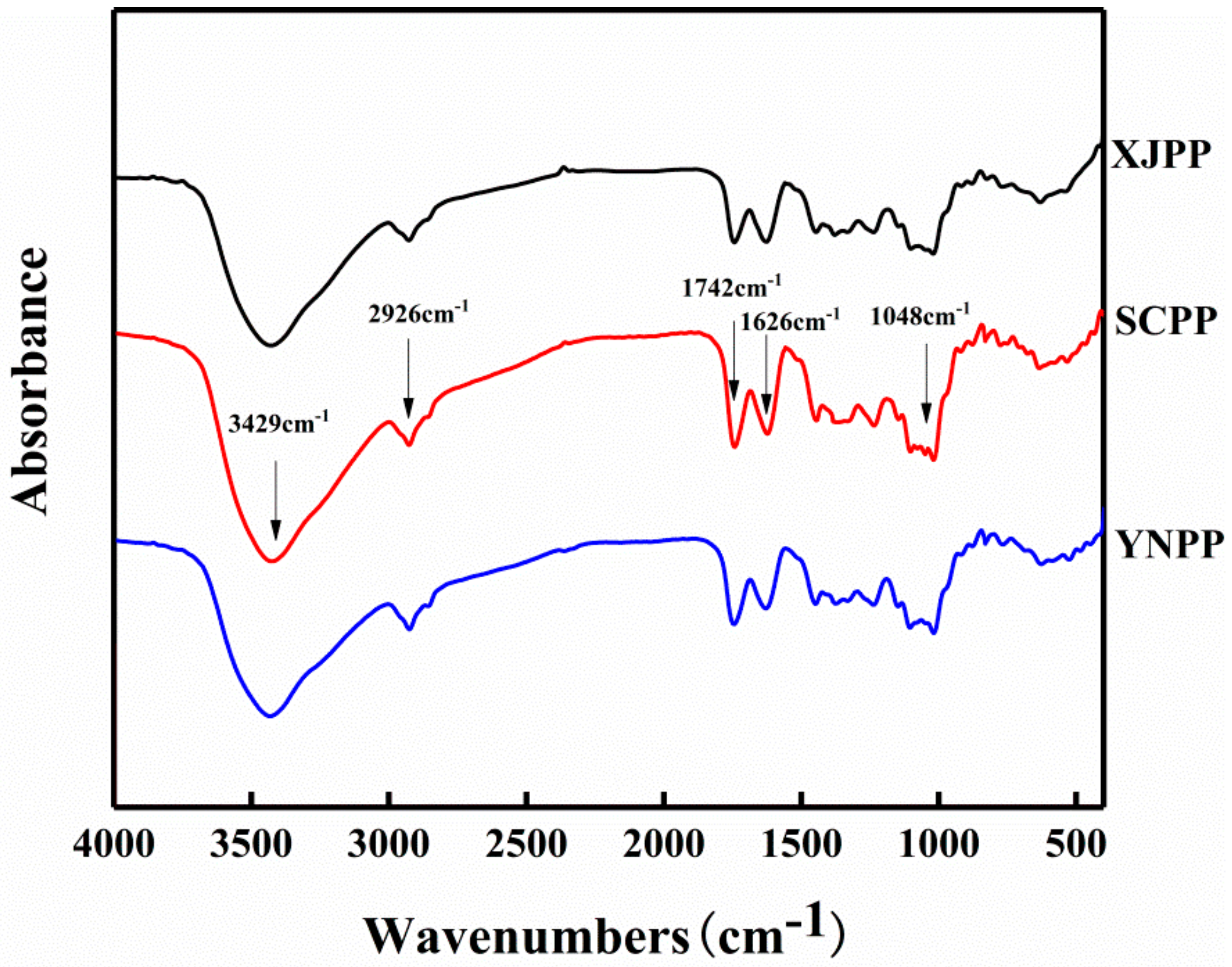
2.1. Composition and Structure Characterization of Pomegranate Peel Pectin
2.2. Analysis of Emulsion Properties
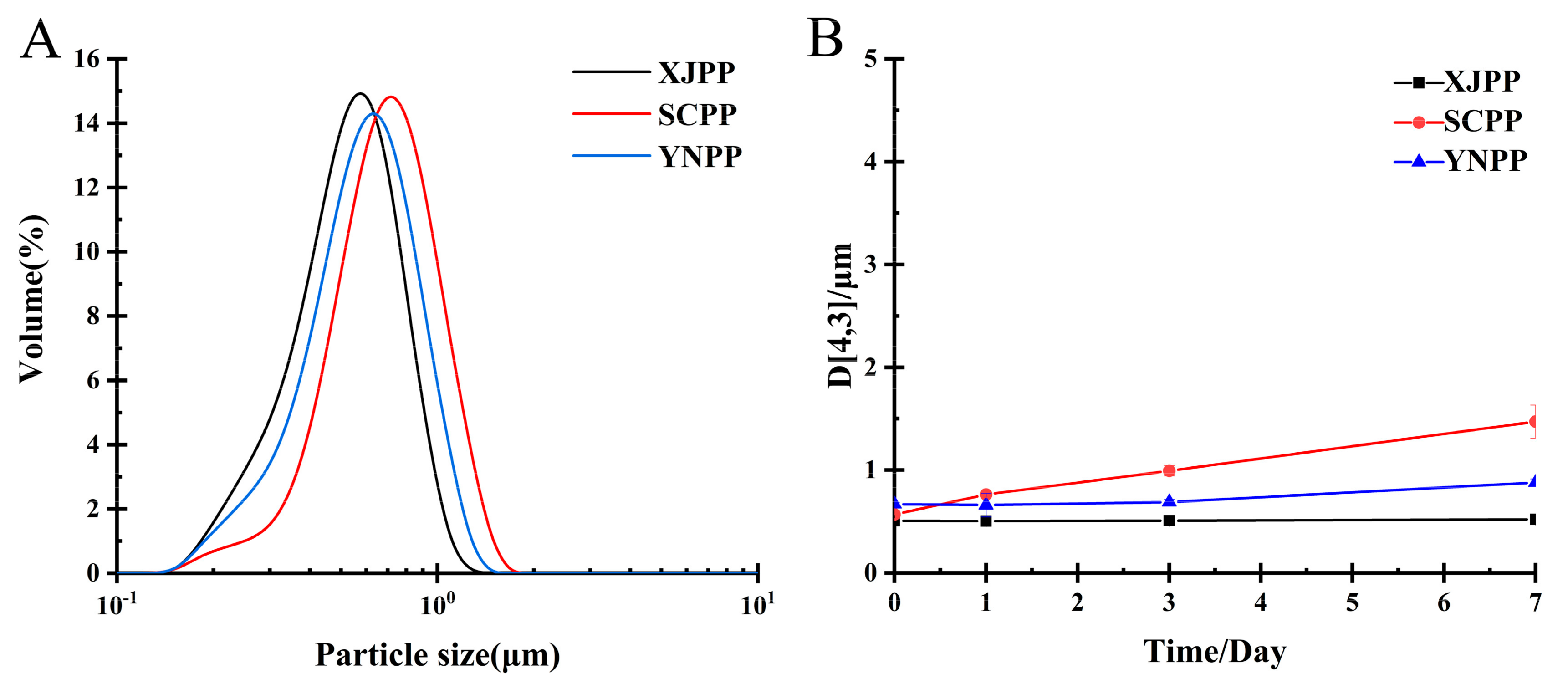
2.2.1. Analysis of Emulsification and Storage Stability of Pectin
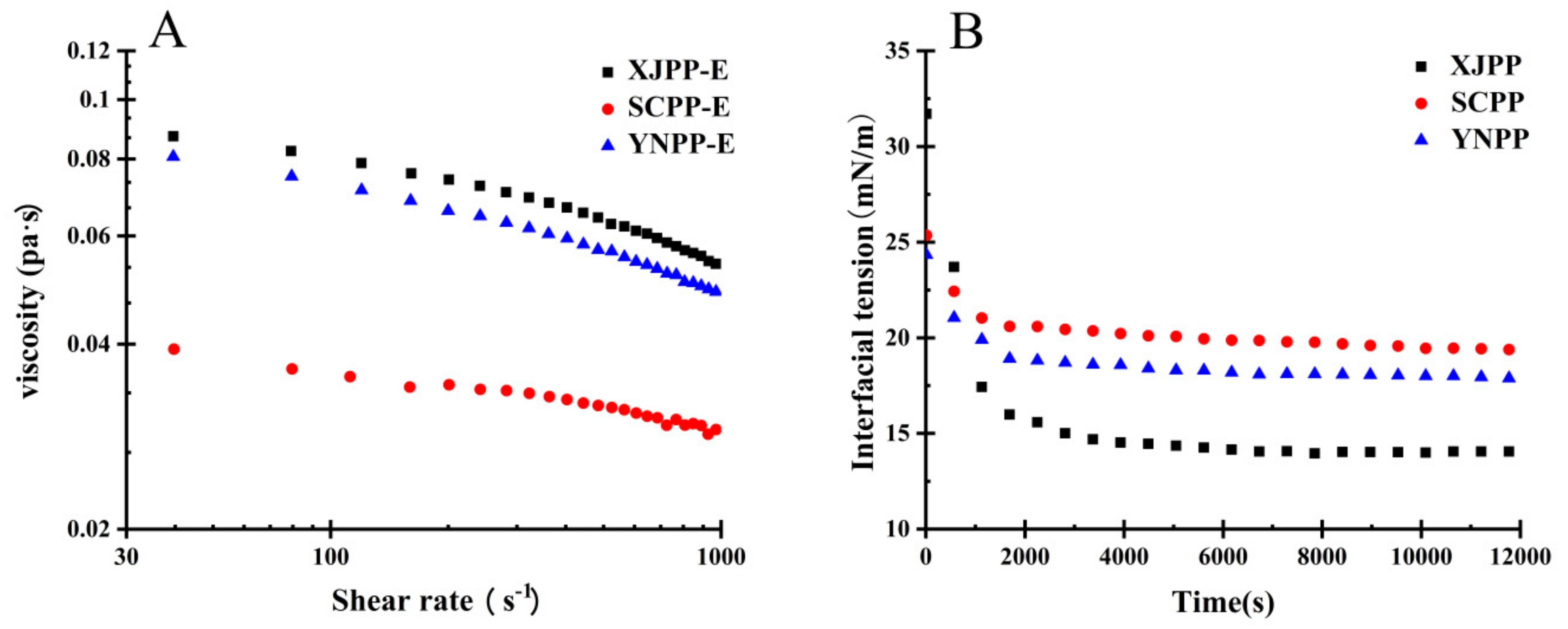
2.2.2. Rheology Analysis
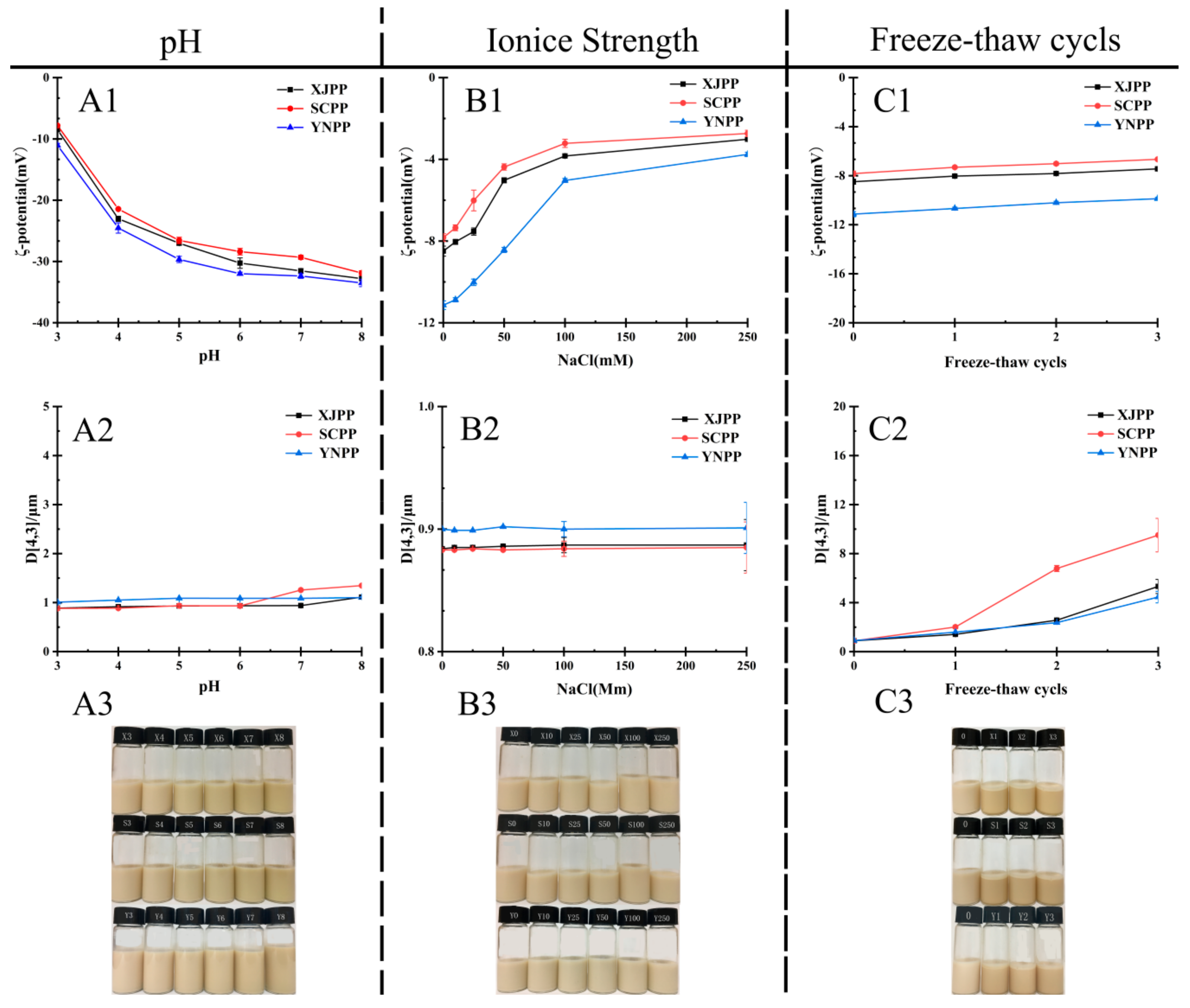
2.2.3. Influence of Environmental Conditions on Stability of Pomegranate Peel Pectin Emulsion
2.3. Analysis of Emulsification of Pomegranate Peel Pectin
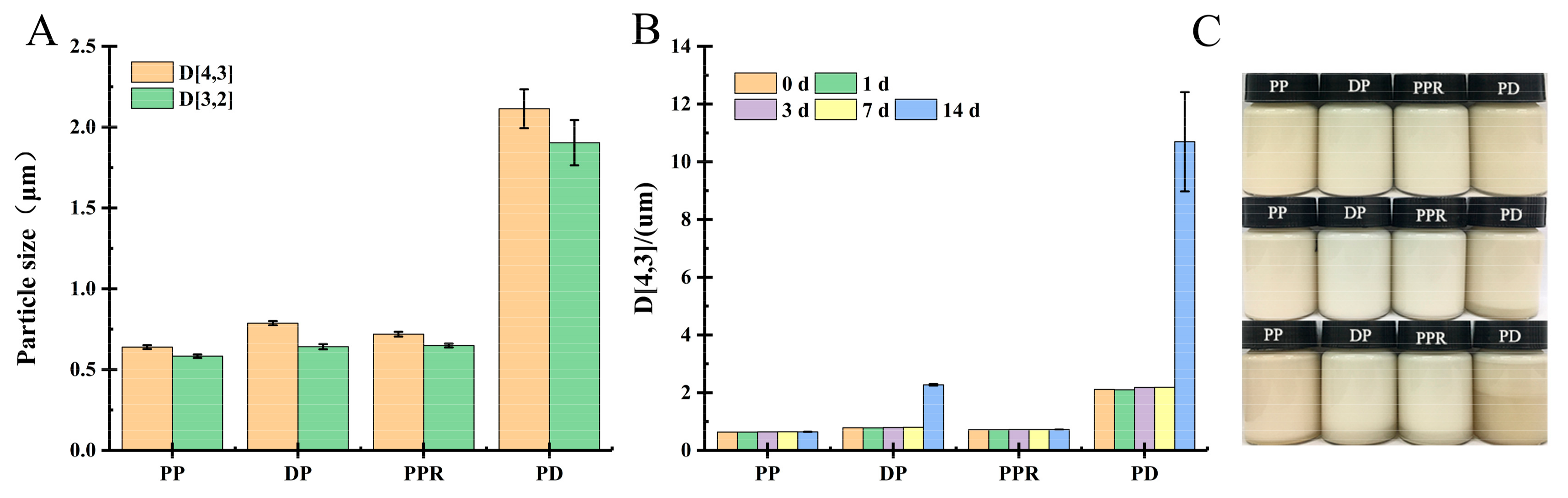
2.3.1. Influence of Purification Treatment on Emulsification and Storage Stability of PP Emulsion
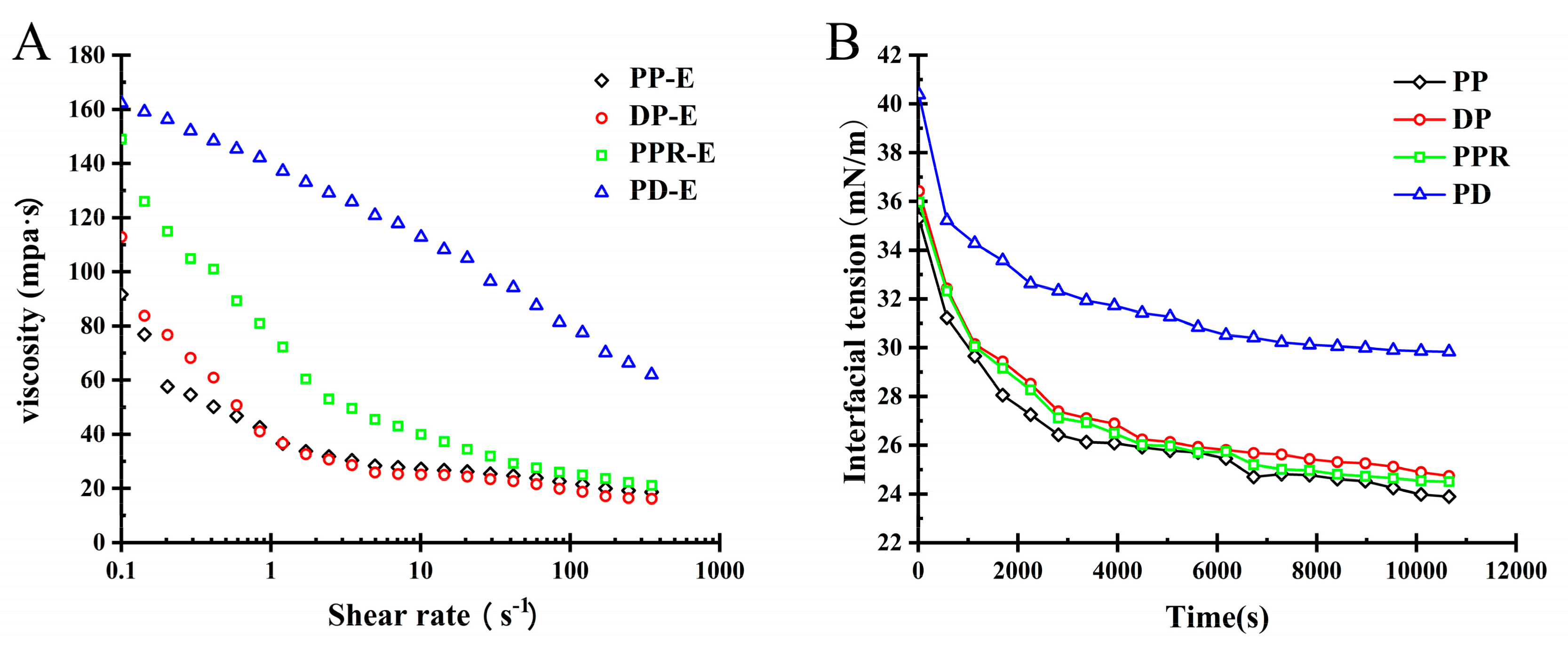
2.3.2. Influence of Purification Treatment on the Viscosity of PP Emulsion
2.3.3. Influence of Purification Treatment on Rheological Behaviour of PP Interface
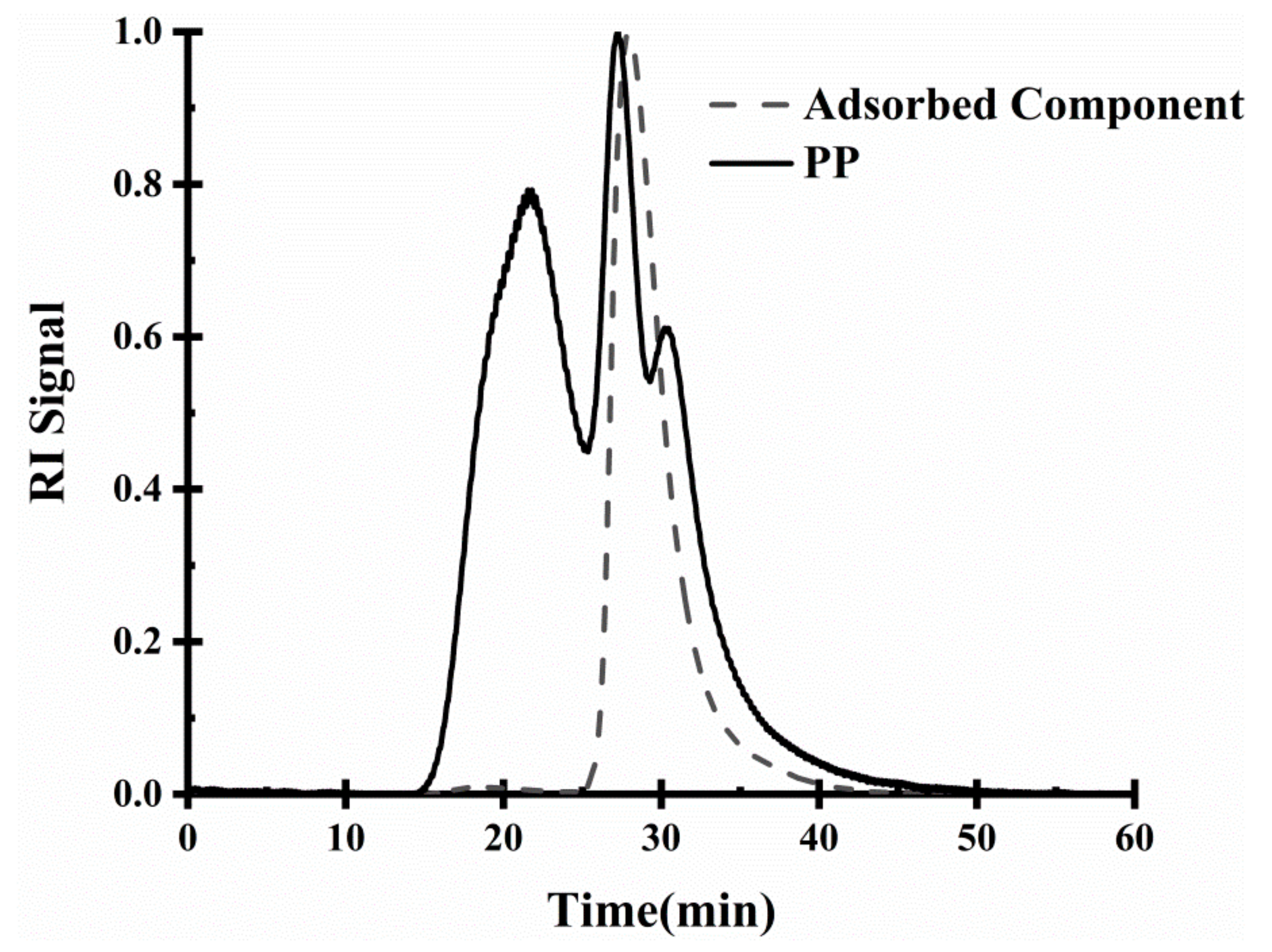
2.3.4. Analysis of Interface Adsorption Components
3. Materials and Methods
3.1. Materials
3.2. Preparation of Pomegranate Peel Pectin
3.2.1. Extraction of Pomegranate Peel Pectin
3.2.2. Purification of Pomegranate Peel Pectin
3.3. Determination of the Degree of Acetylation of Pectin Esterification
3.4. FTIR Analysis
3.5. GPC-MALLS Characterization
3.6. Determination of the Composition of Pomegranate Peel Pectin Monosaccharides
3.7. Emulsion Properties
3.7.1. Preparation of Emulsion
3.7.2. Emulsion Droplet Size
3.7.3. Determination of the Zeta Potential of the Emulsion
3.7.4. Analysis of Emulsion Rheology
3.7.5. Interface Properties
3.8. Analysis of Emulsion Stability in Extreme Environments
3.8.1. Influence of pH Values on Emulsion Stability
3.8.2. Influence of Ionic Strength on Emulsion Stability
3.8.3. Influence of Freeze-Thaw Cycle on Emulsion Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mualikrishna, G.; Tharanathan, R.N. Characterization of pectic polysaccharides from pulse husks. Food Chem. 1994, 50, 87–89. [Google Scholar] [CrossRef]
- Dea, I.C.M.; Madden, J.K. Acetylated pectic polysaccharides of sugar beet. Food Hydrocoll. 1986, 1, 71–88. [Google Scholar] [CrossRef]
- Mukhidinov, Z.K.; Kasymova, G.F.; Usmanova, S.R.; Murzagulova, K.B.; Kim, M.E.; Yanovich, A.V. Production of rifampicin-containing microcapsules based on apple pectin complexes with β-lactoglobulin. Pharm. Chem. J. 2012, 46, 306–309. [Google Scholar] [CrossRef]
- Cui, S.W.; Chang, Y.H. Emulsifying and structural properties of pectin enzymatically extracted from pumpkin. LWT—Food Sci. Technol. 2014, 58, 396–403. [Google Scholar] [CrossRef]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Extraction, structure, and emulsifying properties of pectin from potato pulp. Food Chem. 2017, 224, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Bernardino, J.C.; Lobato-Calleros, C.; Román-Guerrero, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Physicochemical characterisation of hawthorn pectins and their performing in stabilising oil-in-water emulsions. React. Funct. Polym. 2016, 103, 63–71. [Google Scholar] [CrossRef]
- Petkowicz, C.L.O.; Vriesmann, L.C.; Williams, P.A. Pectins from food waste: Extraction, characterization and properties of watermelon rind pectin. Food Hydrocoll. 2016, 65, 57–67. [Google Scholar] [CrossRef]
- Akhtar, M.; Dickinson, E.; Mazoyer, J.; Langendorff, V. Emulsion stabilizing properties of depolymerized pectin. Food Hydrocoll. 2002, 16, 249–256. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Alba, K.; Ritzoulis, C.; Georgiadis, N.; Kontogiorgos, V. Okra extracts as emulsifiers for acidic emulsions. Food Res. Int. 2013, 54, 1730–1737. [Google Scholar] [CrossRef]
- Williams, P.A.; Sayers, C.; Viebke, C.; Senan, C.; Mazoyer, J.; Boulenguer, P. Elucidation of the Emulsification Properties of Sugar Beet Pectin. J. Agr. Food Chem. 2005, 53, 3592–3597. [Google Scholar] [CrossRef]
- Shakhmatov, E.G.; Makarova, E.N.; Belyy, V.A. Structural studies of biologically active pectin-containing polysaccharides of pomegranate Punica granatum. Int. J. Biol. Macromol. 2019, 122, 29–36. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Abid, M.; Cheikhrouhou, S.; Renard, C.M.G.C.; Bureau, S.; Cuvelier, G.; Attia, H.; Ayadi, M.A. Characterization of pectins extracted from pomegranate peel and their gelling properties. Food Chem. 2017, 215, 318–325. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Oliveira, T.Í.S.; Rosa, M.F.; Cavalcante, F.L.; Moates, G.K.; Wellner, N.; Waldron, K.W.; Azeredo, H.M.C. Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 2016, 88, 373–379. [Google Scholar] [CrossRef]
- Sachin, T.; Patti, A.F.; Vijayraghavan, R.; Arora, A. An integrated green biorefinery approach towards simultaneous recovery of pectin and polyphenols coupled with bioethanol production from waste pomegranate peels. Bioresour. Technol. 2018, 266, 322–334. [Google Scholar]
- Xi, Y.; Tanzeela, N.; Yanjie, H.; Xiaoju, G.; Lijun, S.; Yurong, G. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar]
- Pappas, C.S.; Malovikova, A.; Hromadkova, Z.; Tarantilis, P.A.; Ebringerova, A.; Polissiou, M.G. Determination of the degree of esterification of pectinates with decyl and benzyl ester groups by diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and curve-fitting deconvolution method. Carbohyd. Polym. 2004, 56, 465–469. [Google Scholar] [CrossRef]
- Chen, H.M.; Fu, X.; Luo, Z.G. Effect of molecular structure on emulsifying properties of sugar beet pulp pectin. Food Hydrocoll. 2016, 54, 99–106. [Google Scholar] [CrossRef]
- Koocheki, A.; Kadkhodaee, R.; Mortazavi, S.A.; Shahidi, F.; Taherian, A.R. Influence of Alyssum homolocarpum seed gum on the stability and flow properties of O/W emulsion prepared by high intensity ultrasound. Food Hydrocoll. 2009, 23, 2416–2424. [Google Scholar] [CrossRef]
- Garti, N.; Slavin, Y.; Aserin, A. Portulaca oleracea gum and casein interactions and emulsion stability. Food Hydrocoll. 1999, 13, 127–138. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2016, 68, 211–218. [Google Scholar] [CrossRef]
- Perez, A.A.; Sánchez, C.C.; Patino, J.M.R.; Rubiolo, A.C.; Santiago, L.G. Surface adsorption behaviour of milk whey protein and pectin mixtures under conditions of air–water interface saturation. Coll. Surf. B 2011, 85, 306–315. [Google Scholar] [CrossRef]
- Zouambia, Y.; Moulai-Mostefa, N.; Krea, M. Structural characterization and surface activity of hydrophobically functionalized extracted pectins. Carbohyd. Polym. 2009, 78, 841–846. [Google Scholar] [CrossRef]
- Funami, T.; Zhang, G.Y.; Hiroe, M.; Noda, S.; Nakauma, M.; Asai, I.; Cowman, M.K.; Alassaf, S.; Phillips, G.O. Effects of the proteinaceous moiety on the emulsifying properties of sugar beet pectin. Food Hydrocoll. 2007, 21, 1319–1329. [Google Scholar] [CrossRef]
- Su, C.; Feng, Y.; Ye, J.; Zhang, Y.; Gao, Z.; Zhao, M.; Yang, N.; Nishinari, K.; Fang, Y. Effect of sodium alginate on the stability of natural soybean oil body emulsions. RSC Adv. 2018, 8, 4731–4741. [Google Scholar] [CrossRef]
- Ghazy, R.; El-Baradie, B.; El-Shaer, A.; El-Mekawey, F. Static laser light scattering (SLLS) investigations of the scattering parameters of a synthetic polymer. Opt. Laser Technol. 1999, 31, 447–453. [Google Scholar] [CrossRef]
- Liu, Y.; Bo, S.; Zhu, Y.; Zhang, W. Determination of molecular weight and molecular sizes of polymers by high temperature gel permeation chromatography with a static and dynamic laser light scattering detector. Polymer 2003, 44, 7209–7220. [Google Scholar] [CrossRef]
- Shu, X.; Zhang, Y.; Jia, J.; Ren, X.; Wang, Y. Extraction, purification and properties of water-soluble polysaccharides from mushroom Lepista nuda. Int. J. Biol. Macromol. 2019, 128, 858–869. [Google Scholar] [CrossRef]
- Virk, B.S.; Sogi, D.S. Extraction and characterization of pectin from apple (Malus pumila. cv Amri) peal waste. Int. J. Food Prop. 2004, 7, 693–703. [Google Scholar] [CrossRef]
- Pinheiro, E.S.; Silva, I.M.; Gonzaga, L.V.; Amante, E.R.; Teófilo, R.F.; Ferreira, M.M.; Amboni, R.D. Optimization of extraction of high-ester pectin from passion fruit peel (Passiflora edulis flavicarpa) with citric acid by using response surface methodology. Bioresour. Technol. 2008, 99, 5561–5566. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Yao, X.; Zhang, W.; Zhang, K.; Fang, Y.; Nishinari, K. Gum Arabic-stabilized conjugated linoleic acid emulsions: Emulsion properties in relation to interfacial adsorption behaviors. Food Hydrocoll. 2015, 48, 110–116. [Google Scholar] [CrossRef]
- Sakni, N.H.; Majdoub, H.; Roudesli, S.; Picton, L.; Cerf, D.L.; Rihouey, C.; Morvan, C. Composition, structure and solution properties of polysaccharides extractedfrom leaves of Mesembryanthenum crystallinum. Eur. Polym. J. 2006, 42, 786–795. [Google Scholar]
- Nakauma, M.; Funami, T.; Noda, S.; Ishihara, S.; Al-Assaf, S.; Nishinari, K. Comparison of sugar beet pectin, soybean soluble polysaccharide, and gum arabic as food emulsifiers. 1. Effect of concentration, pH, and salts on the emulsifying properties. Food Hydrocoll. 2008, 22, 1254–1267. [Google Scholar] [CrossRef]
Sample Availability: Samples of the pomegranate peel pectin are available from the authors. |
| XJPP | SCPP | YNPP | |
|---|---|---|---|
| Monosaccharide composition (%) | |||
| Rha | 1.88 ± 0.06 | 1.24 ± 0.11 | 1.35 ± 0.09 |
| Ara | 9.16 ± 0.15 | 10.79 ± 0.22 | 6.57 ± 0.14 |
| Gal | 7.78 ± 0.07 | 8.02 ± 0.19 | 4.13 ± 0.16 |
| Glu | 21.08 ± 0.11 | 20.52 ± 0.43 | 26.81 ± 0.23 |
| Gala | 60.11 ± 0.25 | 59.43 ± 0.76 | 61.15 ± 0.38 |
| HG | 58.23 | 58.19 | 59.80 |
| RG-I | 20.70 | 21.29 | 13.40 |
| DA(%) | 14.58 ± 1.37 | 13.21 ± 0.74 | 12.24 ± 1.21 |
| DM(%) | 52.27 ± 1.07 | 54.36 ± 0.82 | 58.74 ± 0.27 |
| Protein(%) | 2.41 ± 0.21 | 2.01 ± 0.13 | 2.82 ± 0.38 |
| Mw (g/mol) | 4.103 × 105 | 3.618 × 105 | 4.384 × 105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, H.; Chu, S.; Wang, P.; Zhou, B.; Han, L.; Yu, X.; Fu, Q.; Li, S. Study on the Emulsifying Properties of Pomegranate Peel Pectin from Different Cultivation Areas. Molecules 2019, 24, 1819. https://doi.org/10.3390/molecules24091819
Zhuang H, Chu S, Wang P, Zhou B, Han L, Yu X, Fu Q, Li S. Study on the Emulsifying Properties of Pomegranate Peel Pectin from Different Cultivation Areas. Molecules. 2019; 24(9):1819. https://doi.org/10.3390/molecules24091819
Chicago/Turabian StyleZhuang, Hu, Shang Chu, Ping Wang, Bin Zhou, Lingyu Han, Xiongwei Yu, Qinli Fu, and Shugang Li. 2019. "Study on the Emulsifying Properties of Pomegranate Peel Pectin from Different Cultivation Areas" Molecules 24, no. 9: 1819. https://doi.org/10.3390/molecules24091819
APA StyleZhuang, H., Chu, S., Wang, P., Zhou, B., Han, L., Yu, X., Fu, Q., & Li, S. (2019). Study on the Emulsifying Properties of Pomegranate Peel Pectin from Different Cultivation Areas. Molecules, 24(9), 1819. https://doi.org/10.3390/molecules24091819





