Comparison of Sensory and Electronic Tongue Analysis Combined with HS-SPME-GC-MS in the Evaluation of Skim Milk Processed with Different Preheating Treatments
Abstract
1. Introduction
2. Results
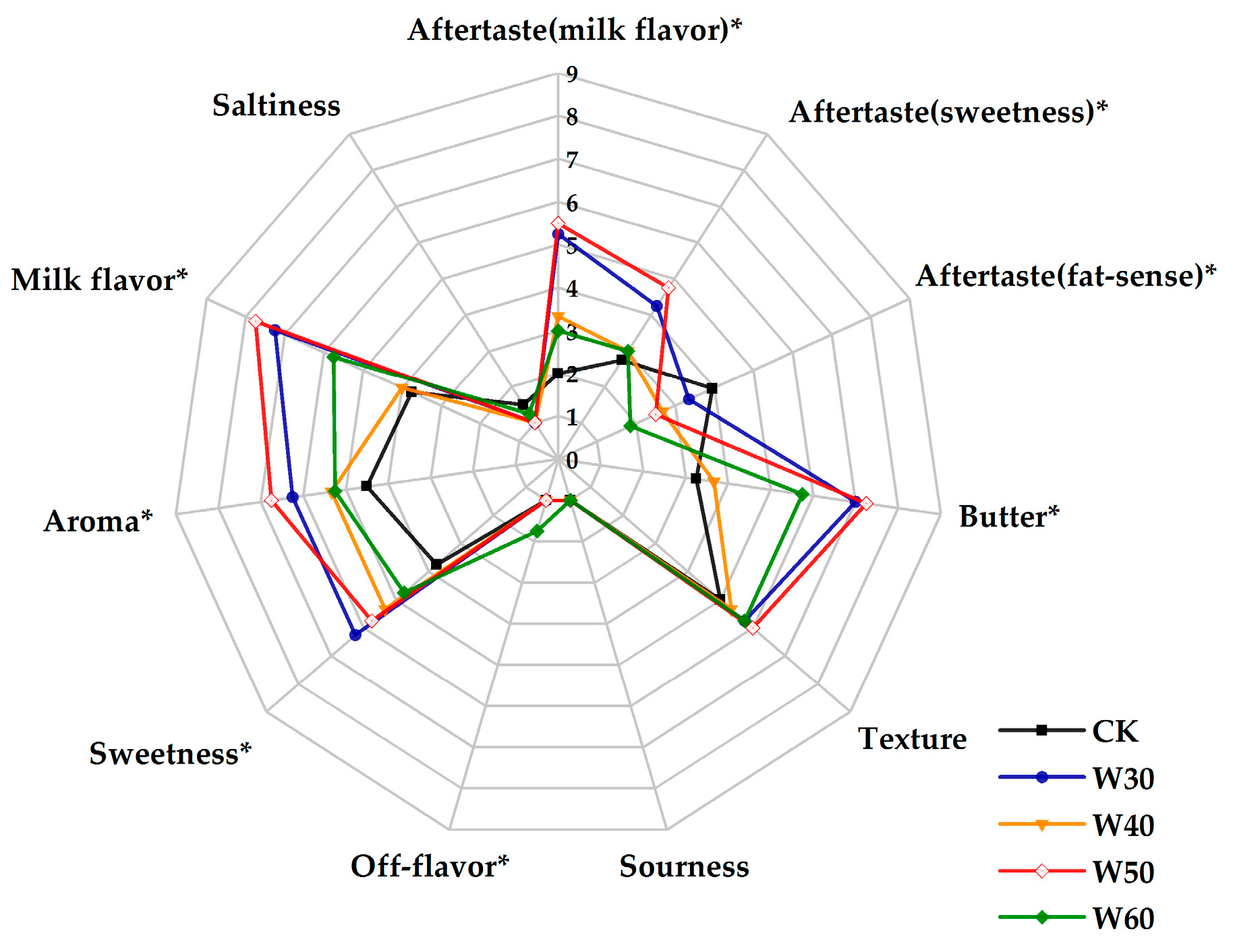
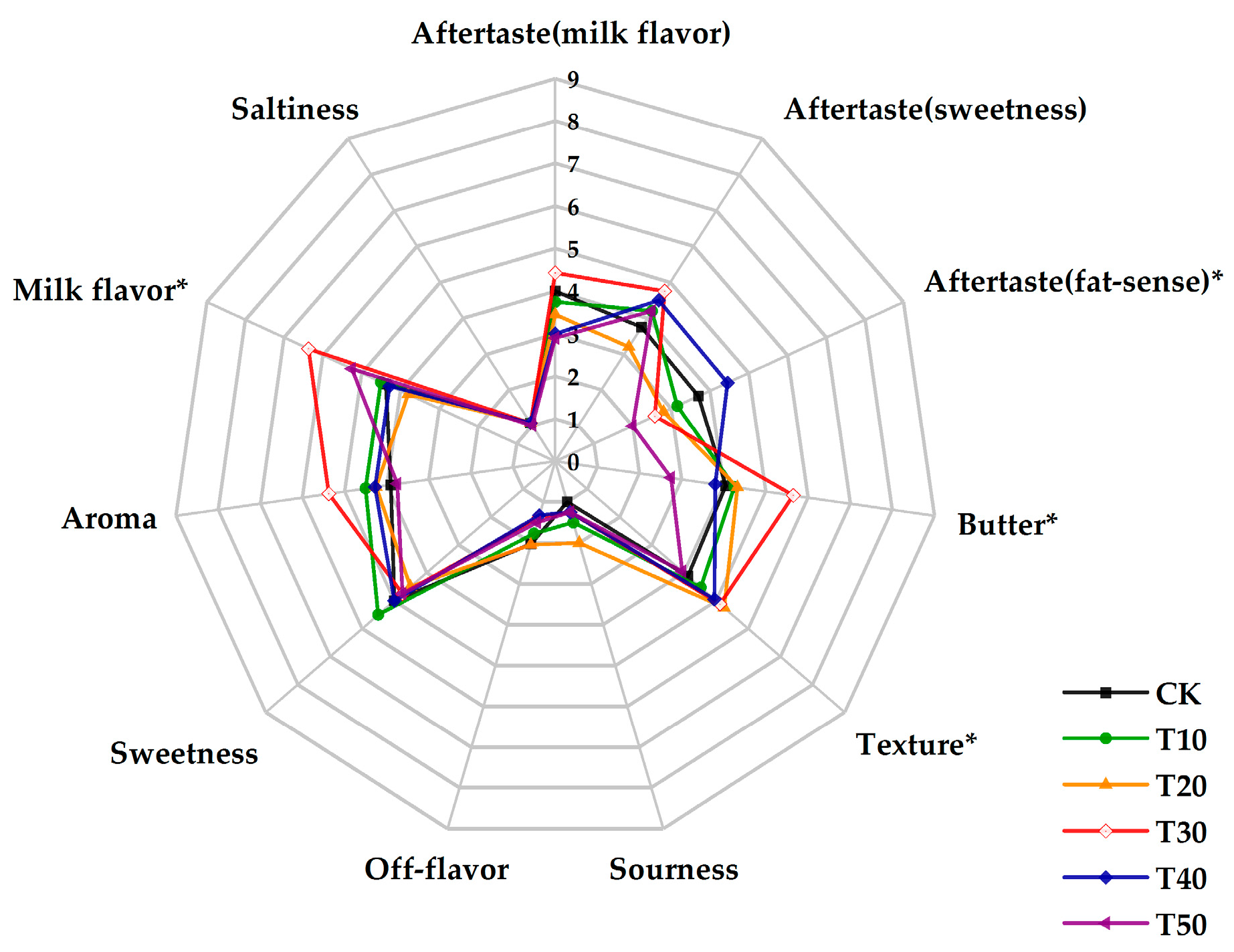
2.1. Sensory Evaluation Analysis
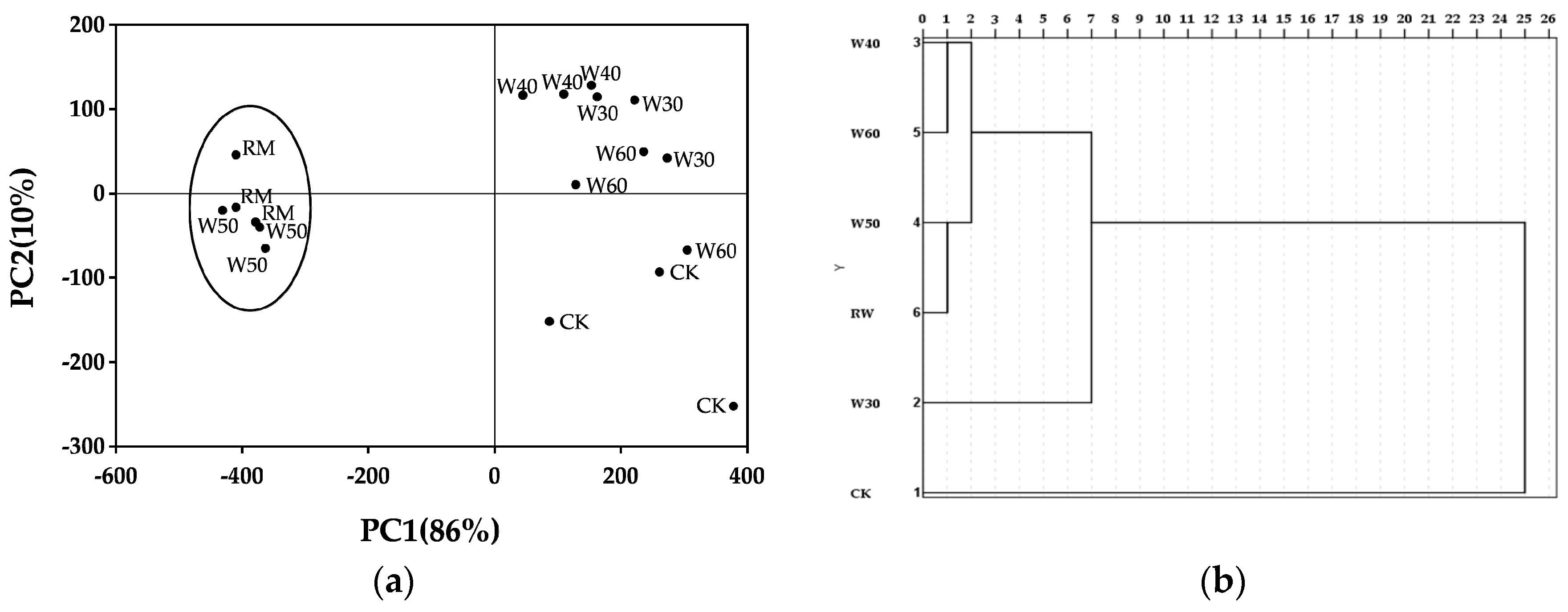
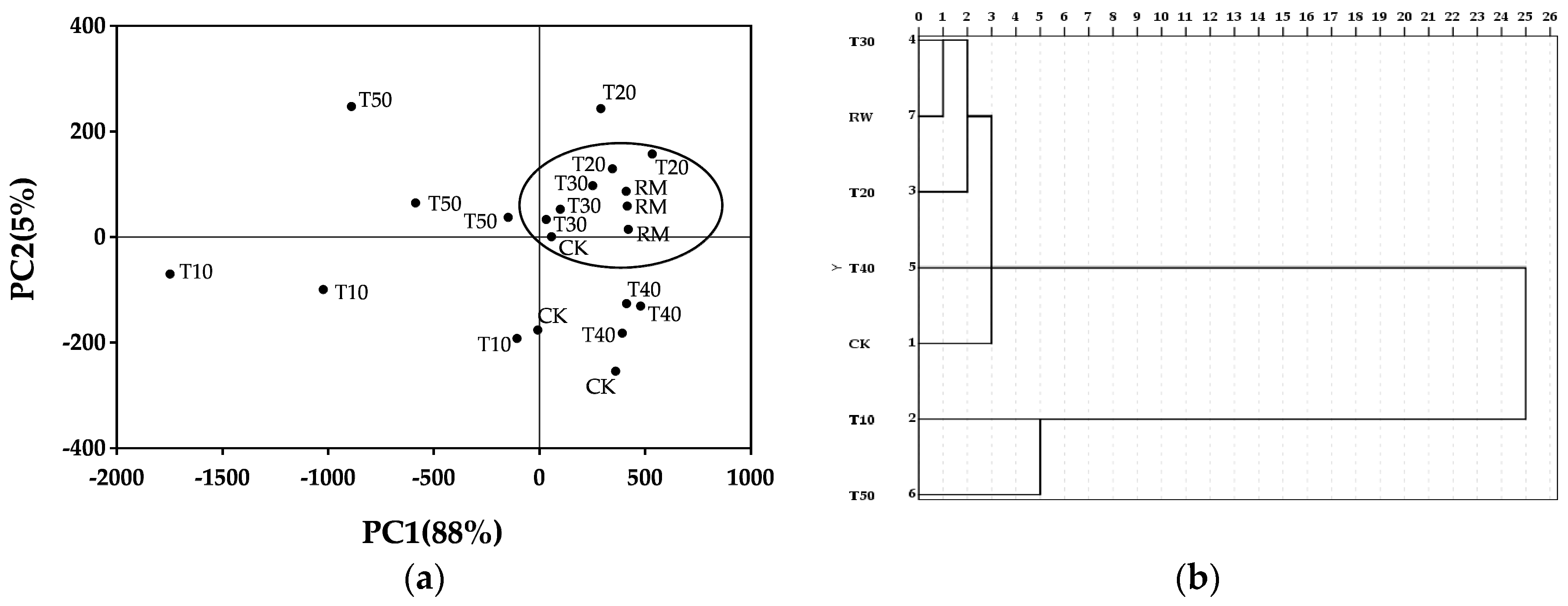
2.2. Analysis with e-Tongue Using PCA and CA
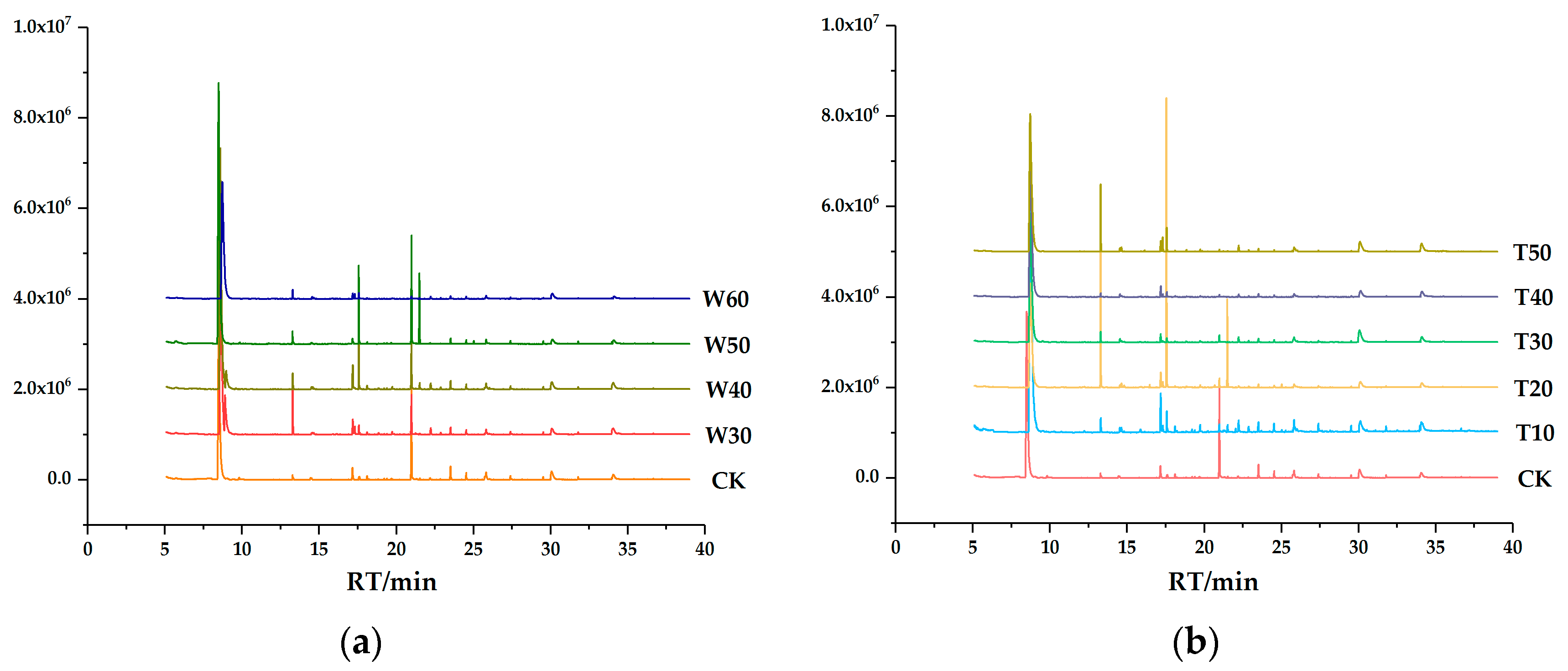
2.3. GC-MS Analysis
3. Discussion
3.1. Sensory Evaluation Analysis
3.2. Principal Component Analysis and Cluster Analysis
3.3. GC-MS
3.3.1. Alkanes
3.3.2. Acids
3.3.3. Ketones
3.3.4. Aldehydes
3.3.5. Alcohols
3.3.6. Sulfur Compounds
4. Materials and Methods
4.1. Materials
4.2. Experiment Design and Methods
4.2.1. Raw Material Collection
4.2.2. Samples Preparation
4.2.3. Sensory Analysis
4.2.4. e-Tongue Analysis
4.2.5. Headspace Extraction of Volatile Compounds
4.2.6. GC-MS Analysis
4.2.7. Qualitation and Semi-Quantitation
Qualitation of Volatile Compounds
Quantitation of Volatile Compounds
4.2.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Food-based Dietary Guidelines. Available online: http://www.fao.org/nutrition/nutrition-education/food-dietary-guidelines/en/ (accessed on 21 January 2016).
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition-a review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Ai, N.S.; Liu, H.L.; Wang, J.; Zhang, X.M.; Zhang, H.J.; Chen, H.T.; Sun, B.G. Triple-channel comparative analysis of volatile flavour composition in raw whole and skim milk via electronic nose, GC-MS and GC-O. Anal. Methods 2015, 7, 4278–4284. [Google Scholar] [CrossRef]
- Pereira, P.C.; Vicente, F. Chapter 13-milk nutritive role and potential benefits in human health. In Nutrients in Dairy and Their Implications for Health and Disease; Watson, R.S., Collier, R.J., Preedy, V.R., Eds.; Academic Press: Salt Lake city, UT, USA, 2018; pp. 161–176. [Google Scholar]
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [CrossRef]
- Atamian, S.; Olabi, A.; Kebbe Baghdadi, O.; Toufeili, I. The characterization of the physicochemical and sensory properties of full-fat, reduced-fat and low-fat bovine, caprine, and ovine Greek yogurt (Labneh). Food Sci. Nutr. 2014, 2, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Badings, H.T.; Neeter, R. Recent advances in the study of aroma compounds of milk and dairy products. Neth. Milk Dairy J. 1980, 34, 9–30. [Google Scholar]
- Kinsella, J.E.; Patton, S.; Dimick, P.S. The flavor potential of milk fat. A review of its chemical nature and biochemical origin. J. Am. Oil Chem. Soc. 1967, 44, 449–454. [Google Scholar] [CrossRef]
- Tamsma, A.; Kurtz, F.E.; Bright, R.S.; Pallansch, M.J. Contribution of milk fat to the flavor of milk. J. Dairy Sci. 1969, 52, 1910–1913. [Google Scholar] [CrossRef]
- Mela, D.J. Sensory preferences for fats: What, who, why? Food Qual. Prefer. 1990, 2, 95–101. [Google Scholar] [CrossRef]
- Richardson-Harman, N.J.; Stevens, R.; Walker, S.; Gamble, J.; Miller, M.; Wong, M.; McPherson, A. Mapping consumer perceptions of creaminess and liking for liquid dairy products. Food Qual. Prefer. 2000, 11, 239–246. [Google Scholar] [CrossRef]
- Drewnowski, A.; Greenwood, M.R.C. Cream and sugar: Human preferences for high-fat foods. Physiol. Behav. 1983, 30, 629–633. [Google Scholar] [CrossRef]
- Mela, D.J. Sensory assessment of fat content in fluid dairy products. Appetite 1988, 10, 37–44. [Google Scholar] [CrossRef]
- Pangborn, R.M.; Bos, K.E.; Stern, J.S. Dietary fat intake and taste responses to fat in milk by under-, normal, and overweight women. Appetite 1985, 6, 25–40. [Google Scholar] [CrossRef]
- Pangborn, R.M.; Dunkley, W.L. Sensory discrimination of fat and solids-not-fat in milk. J. Dairy Sci. 1964, 47, 719–726. [Google Scholar] [CrossRef]
- Pangborn, R.M.; Giovanni, M.E. Dietary intake of sweet foods and of dairy fats and resultant gustatory responses to sugar in lemonade and to fat in milk. Appetite 1984, 5, 317–327. [Google Scholar] [CrossRef]
- Norris, G.H.; Jiang, C.; Ryan, J.; Porter, C.M.; Blesso, C.N. Milk sphingomyelin improves lipid metabolism and alters gut microbiota in high fat diet-fed mice. J. Nutr. Biochem. 2016, 30, 93–101. [Google Scholar] [CrossRef] [PubMed]
- La Berge, A.F. How the ideology of low fat conquered America. J. Hist. Med. Allied Sci. 2008, 63, 139–177. [Google Scholar] [CrossRef]
- Briefel, R.R.; Johnson, C.L. Secular trends in dietary intake in the United States. Annu. Rev. Nutr. 2004, 24, 401–431. [Google Scholar] [CrossRef]
- Patel, H.A.; Singh, H.; Anema, S.G.; Creamer, L.K. Effects of heat and high hydrostatic pressure treatments on disulfide bonding interchanges among the proteins in skim milk. J. Agric. Food Chem. 2006, 54, 3409–3420. [Google Scholar] [CrossRef]
- Villamiel, M.; De Jong, P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. J. Agric. Food Chem. 2000, 48, 472–478. [Google Scholar] [CrossRef]
- Contarini, G.; Povolo, M.; Leardi, R.; Toppino, P.M. Influence of heat treatment on the volatile compounds of milk. J. Agric. Food Chem. 1997, 45, 3171–3177. [Google Scholar] [CrossRef]
- Corredig, M.; Dalgleish, D.G. Effect of different heat treatments on the strong binding interactions between whey proteins and milk fat globules in whole milk. J. Dairy Res. 1996, 63, 441–449. [Google Scholar] [CrossRef]
- Ye, A.; Singh, H.; Taylor, M.W.; Anema, S.G. Interactions of fat globule surface proteins during concentration of whole milk in a pilot-scale multiple-effect evaporator. J. Dairy Res. 2004, 71, 471–479. [Google Scholar] [CrossRef]
- Donato, L.; Guyomarc’H, F.; Amiot, S.; Dalgleish, D.G. Formation of whey protein/κ-casein complexes in heated milk: Preferential reaction of whey protein with κ-casein in the casein micelles. Int. Dairy J. 2007, 17, 1161–1167. [Google Scholar] [CrossRef]
- Nursten, H.E. The flavour of milk and dairy products: I. Milk of different kinds, milk powder, butter and cream. Int. J. Dairy Technol. 2010, 50, 48–56. [Google Scholar] [CrossRef]
- Vazquez-Landaverde, P.A.; Velazquez, G.; Torres, J.A.; Qian, M.C. Quantitative determination of thermally derived off-flavor compounds in milk using solid-phase microextraction and gas chromatography. J. Dairy Sci. 2005, 88, 3764–3772. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- López-Fandiño, R. High pressure-induced changes in milk proteins and possible applications in dairy technology. Int. Dairy J. 2006, 16, 1119–1131. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in classification and quality control of edible oils: A review. Food chem. 2018, 246, 192–201. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable electronic nose based on electrochemical sensors for food quality assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef]
- Röck, F.; Barsan, N.; Weimar, U. Electronic nose: Current status and future trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sensor rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Macías, M.; Agudo, J.; Manso, A.; Orellana, C.; Velasco, H.; Caballero, R. A compact and low cost electronic nose for aroma detection. Sensors 2013, 13, 5528–5541. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Ma, J.K.; Chen, L.; Jiang, L.W.; Xie, J.; Li, P.; He, J. GC-MS Characterization of volatile flavor compounds in stinky Tofu brine by optimization of headspace solid-phase microextraction conditions. Molecules 2018, 23, 3155. [Google Scholar] [CrossRef] [PubMed]
- Fabre, M.; Aubry, V.; Guichard, E. Comparison of different methods: Static and dynamic headspace and solid-phase microextraction for the measurement of interactions between milk proteins and flavor compounds with an application to emulsions. J. Agric. Food Chem. 2002, 50, 1497–1501. [Google Scholar] [CrossRef]
- Sharma, S.K.; Dalgleish, D.G. Interactions between milk serum proteins and synthetic fat globule membrane during heating of homogenized whole milk. J. Agric. Food Chem. 1993, 41, 1407–1412. [Google Scholar] [CrossRef]
- Corredig, M.; Dalgleish, D.G. The mechanisms of the heat-induced interaction of whey proteins with casein micelles in milk. Int. Dairy J. 1999, 9, 233–236. [Google Scholar] [CrossRef]
- Jeurnink, T.J.M.; De Kruif, K.G. Changes in milk on heating: Viscosity measurements. J. Dairy Res. 1993, 60, 139–150. [Google Scholar] [CrossRef]
- Aisala, H.; Sola, J.; Hopia, A.; Linderborg, K.M.; Sandell, M. Odor-contributing volatile compounds of wild edible Nordic mushrooms analyzed with HS–SPME–GC–MS and HS–SPME–GC–O/FID. Food Chem. 2019, 283, 566–578. [Google Scholar] [CrossRef]
- Hruškar, M.; Major, N.; Krpan, M.; Krbavčic’, I.P.; Šaric’, G.; Markovic’, K.; Vahčić, N. Evaluation of milk and dairy products by electronic tongue. Mljekarstvo 2009, 59, 193–200. [Google Scholar]
- Iranmanesh, M.; Ezzatpanah, H.; Akbari-Adergani, B.; Karimi Torshizi, M.A. SPME/GC-MS characterization of volatile compounds of Iranian traditional dried Kashk. Int. J. Food Prop. 2018, 21, 1067–1079. [Google Scholar] [CrossRef]
- Erkaya, T.; ŞENGÜL, M. Comparison of volatile compounds in yoghurts made from cows’, buffaloes’, ewes’ and goats’ milks. Int. J. Dairy Technol. 2011, 64, 240–246. [Google Scholar] [CrossRef]
- Li, Y.H.; Wang, W.J. Short communication: Formation of oxidized flavor compounds in concentrated milk and distillate during milk concentration. J. Dairy Sci. 2016, 99, 9647–9651. [Google Scholar] [CrossRef] [PubMed]
- Buchin, S.; Delague, V.; Duboz, G.; Berdague, J.L.; Beuvier, E.; Pochet, S.; Grappin, R. Influence of pasteurization and fat composition of milk on the volatile compounds and flavor characteristics of a semi-hard cheese. J. Dairy Sci. 1998, 81, 3097–3108. [Google Scholar] [CrossRef]
- Vagenas, G.; Roussis, I.G. Fat-derived volatiles of various products of cows’, ewes’, and goats’ milk. Int. J. Food Prop. 2012, 15, 665–682. [Google Scholar] [CrossRef]
- Toelstede, S.; Hofmann, T. Sensomics mapping and identification of the key bitter metabolites in Gouda cheese. J. Agric. Food Chem. 2008, 56, 2795–2804. [Google Scholar] [CrossRef] [PubMed]
- Sfakianakis, P.; Tzia, C. Flavour profiling by gas chromatography–mass spectrometry and sensory analysis of yoghurt derived from ultrasonicated and homogenised milk. Int. Dairy J. 2017, 75, 120–128. [Google Scholar] [CrossRef]
- Yue, J.; Zheng, Y.; Liu, Z.; Deng, Y.; Jing, Y.; Luo, Y.; Zhao, Y. Characterization of volatile compounds in microfiltered pasteurized milk using solid-phase microextraction and GC× GC-TOFMS. Int. J. Food Prop. 2015, 18, 2193–2212. [Google Scholar] [CrossRef]
- Gioacchini, A.M.; De Santi, M.; Guescini, M.; Brandi, G.; Stocchi, V. Characterization of the volatile organic compounds of Italian ‘Fossa’cheese by solid-phase microextraction gas chromatography/mass spectrometry. Rapid Commun. Mass Sp. 2010, 24, 3405–3412. [Google Scholar] [CrossRef]
- Barbieri, G.; Bolzoni, L.; Careri, M.; Mangia, A.; Parolari, G.; Spagnoli, S.; Virgili, R. Study of the volatile fraction of Parmesan cheese. J. Agric. Food Chem. 1994, 42, 1170–1176. [Google Scholar] [CrossRef]
- Fernández-GarcíA, E.; Carbonell, M.; Gaya, P.; Nuñez, M. Evolution of the volatile components of ewes raw milk Zamorano cheese. Seasonal variation. Int. Dairy J. 2004, 14, 701–711. [Google Scholar] [CrossRef]
- Fernández-GarcíA, E.; Carbonell, M.; Nuñez, M. Volatile fraction and sensory characteristics of Manchego cheese. 1. Comparison of raw and pasteurized milk cheese. J. Dairy Res. 2002, 69, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; Ramírez, R. Formation of the aroma of a raw goat milk cheese during maturation analysed by SPME-GC-MS. Food Chem. 2011, 129, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, T.; Mihara, S.; Nishimura, O.; Kamiya, Y.; Aitoku, A.; Hayashi, J. Chapter 12-Flavor volatiles formed by heated milk. In The analysis and control of less-desirable flavors in foods and beverages; Charalambous, G., Ed.; Academic Press: Salt Lake city, UT, USA, 1980; pp. 241–265. [Google Scholar]
- Al-Attabi, Z.; D’arcy, B.R.; Deeth, H.C. Volatile sulphur compounds in UHT milk. Crit. Rev. Food Sci. Nutr. 2008, 49, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Luigi, M.; Jocelyne, D.; Patrick, E.; Francesco, A. Neutral volatile compounds in the raw milks from different species. J. Dairy Res. 1993, 60, 199–213. [Google Scholar] [CrossRef]
- Toso, B.; Procida, G.; Stefanon, B. Determination of volatile compounds in cows\” milk using headspace GC-MS. J. Dairy Res. 2002, 69, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.; Sidel, J.L. Quantitative Descriptive Analysis: Developments, Applications and the Future. In Proceedings of the Inst Food Technologists, Sensory-Evaluation-Division Symposium on Sensory Science-retrospection of Past Milestones and Sensing the Future at the Meeting of Institute-of-Food-Technologists, Atlanta, GA, USA, 20–24 June 1998; pp. 48–52. [Google Scholar]
- Serhan, M.; Mattar, J.R.; Debs, L. Concentrated yogurt (labneh) made of a mixture of goats’ and cows’ milk: Physicochemical, microbiological and sensory analysis. Small Rumin. Res. 2016, 138, 46–52. [Google Scholar] [CrossRef]
- Bruzantin, F.P.; Daniel, J.L.P.; Da Silva, P.P.M.; Spoto, M.H.F. Physicochemical and sensory characteristics of fat-free goat milk yogurt added to stabilizers and skim milk powder fortification. J. Dairy Sci. 2016, 99, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Alpha, M.O.S. α-Astree User’s Manual; Alpha M.O.S.: Toulouse, France, 2003. [Google Scholar]
- Yang, Y.; Chen, Q.; Shen, C.; Zhang, S.; Gan, Z.; Hu, R.; Zhao, J.H.; Ni, Y.Y. Evaluation of monosodium glutamate, disodium inosinate and guanylate umami taste by an electronic tongue. J. Food Eng. 2013, 116, 627–632. [Google Scholar] [CrossRef]
- Newman, J.; Harbourne, N.; O’Riordan, D.; Jacquier, J.C.; O’Sullivan, M. Comparison of a trained sensory panel and an electronic tongue in the assessment of bitter dairy protein hydrolysates. J. Food Eng. 2014, 128, 127–131. [Google Scholar] [CrossRef]
- Bueno, M.; Resconi, V.C.; Campo, M.M.; Ferreira, V.; Escudero, A. Development of a robust HS-SPME-GC-MS method for the analysis of solid food samples. Analysis of volatile compounds in fresh raw beef of differing lipid oxidation degrees. Food Chem. 2019, 281, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Radovic, B.S.; Careri, M.; Mangia, A.; Musci, M.; Gerboles, M.; Anklam, E. Contribution of dynamic headspace GC–MS analysis of aroma compounds to authenticity testing of honey. Food Chem. 2001, 72, 511–520. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
| Processing Methods | Advantages | Disadvantages |
|---|---|---|
| Thermal treatment | Provide acceptable safety and shelf life, improve the functional properties [20] | May give rise to chemical and physical changes and reduce the content or bioavailability of some nutrients when ultra-high temperature [28] |
| Ultrasonic treatment | Homogenization of milk due to smaller fat globules with a granular surface [28] | Difficult to become a commercial process on its own [21] |
| High pressure | Minimal effects on the sensory and nutritional quality and as a tool for modification of macromolecular constituents [20] | Require a large capital investment [29] |
| No. | Compounds | RT/min d | RI | Concentration (μg/L) | Identification h | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cal. e | Ref. f | CK | W30 | W40 | W50 | W60 | ||||
| Ketones | ||||||||||
| 1 | 2-Nonanone | 14.47 | 1310 | 1366 | 0.57 ± 0.11 | 0.50 ± 0.23 | 0.40 ± 0.07 | 0.47 ± 0.10 | 0.69 ± 0.08 | MS/STD/RI |
| 2 | 2-Undecanone | 19.73 | 1509 | 1599 | 0.41 ± 0.06 | 0.53 ± 0.10 | 0.66 ± 0.11 | 0.43 ± 0.32 | 0.21 ± 0.14 | MS/STD/RI |
| 3 | Acetophenone | 20.87 | 1555 | 1627 | 0.06 ± 0.01 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.07 ± 0.03 | 0.05 ± 0.02 | MS/STD/RI |
| Total | 1.05 ± 0.12 | 1.06 ± 0.24 | 1.10 ± 0.16 | 0.97 ± 0.39 | 0.96 ± 0.22 | |||||
| Acids | ||||||||||
| 1 | Hexanoic acid | 25.74 | 1765 | 1850 | 1.03 ± 0.33 | 0.47 ± 0.35 | 0.54 ± 0.11 | 1.33 ± 0.01 | - g | MS/STD/RI |
| 2 | Octanoic acid | 30.06 | 1969 | 2038 | 7.55 ± 2.53 | 6.48 ± 2.16 | 6.04 ± 0.36 | 14.75 ± 7.97 | 4.48 ± 2.88 | MS/STD/RI |
| 3 | n-Decanoic acid | 34.07 | 2176 | 2246 | 6.60 ± 2.06 | 4.80 ± 0.85 | 3.88 ± 1.13 | 9.94 ± 4.92 | 1.59 ± 0.64 | MS/STD/RI |
| Total | 15.18 ± 3.50 ab | 11.75 ± 1.92 b | 10.46 ± 0.78 b | 26.01 ± 12.76 a | 6.06 ± 3.48 b | |||||
| Aldehydes | ||||||||||
| 1 | Octanal | 11.78 | 1214 | 1292 | - | - | - | 0.18 ± 0.14 | - | MS/STD/RI |
| 2 | Nonanal | 14.55 | 1313 | 1390 | 0.56 ± 0.18 | 0.43 ± 0.07 | 0.46 ± 0.17 | 1.42 ± 0.88 | 0.44 ± 0.11 | MS/STD/RI |
| 3 | Decanal | 17.23 | 1412 | 1472 | - | 1.27 ± 0.81 | 0.18 ± 0.09 | 0.98 ± 0.37 | 1.21 ± 0.73 | MS/STD/RI |
| Total | 0.56 ± 0.18 b | 1.70 ± 0.79 ab | 0.64 ± 0.25 b | 2.58 ± 1.17 a | 1.65 ± 0.61 ab | |||||
| Alcohols | ||||||||||
| 1 | 2-Ethyl-1-hexanol | 17.16 | 1410 | 1484 | 3.93 ± 0.20 | 2.33 ± 0.73 | 3.86 ± 1.78 | 4.23 ± 3.19 | 2.16 ± 0.99 | MS/STD/RI |
| 2 | 1-Octanol | 18.83 | 1474 | 1558 | 0.27 ± 0.04 | 0.24 ± 0.07 | 0.20 ± 0.10 | 0.51 ± 0.33 | 0.10 ± 0.02 | MS/STD/RI |
| Total | 4.20 ± 0.17 | 2.58 ± 0.80 | 4.06 ± 1.88 | 4.73 ± 3.51 | 2.26 ± 1.01 | |||||
| Alkanes | ||||||||||
| 1 | Tetradecane | 14.81 | 1323 | 1400 | - | 0.11 ± 0.00 | - | 0.15 ± 0.01 | 0.17 ± 0.01 | MS/STD/RI |
| Total | - | 0.11 ± 0.00c | - | 0.15 ± 0.01b | 0.17 ± 0.01a | |||||
| Sulfur compounds | ||||||||||
| 1 | Dimethyl sulfone | 26.28 | 1789 | 1895 | 0.10 ± 0.00 | - | - | 0.15 ± 0.10 | - | MS/STD |
| Total | 0.10 ± 0.00 | - | - | 0.15 ± 0.10 | - | |||||
| No. | Compounds | RT/min c | RI | Concentration (μg/L) | Identification g | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal. d | Ref. e | CK | T10 | T20 | T30 | T40 | T50 | ||||
| Ketones | |||||||||||
| 1 | 2-Nonanone | 14.47 | 1310 | 1366 | 0.57 ± 0.11 | 0.80 ± 0.01 | 1.15 ± 0.59 | 0.47 ± 0.10 | 0.90 ± 0.02 | 1.05 ± 0.13 | MS/STD/RI |
| 2 | 2-Undecanone | 19.73 | 1509 | 1599 | 0.41 ± 0.06 | - f | 0.90 ± 0.22 | 0.43 ± 0.32 | 0.44 ± 0.12 | 0.46 ± 0.14 | MS/STD/RI |
| 3 | Acetophenone | 20.87 | 1555 | 1627 | 0.06 ± 0.01 | - | 0.09 ± 0.01 | 0.07 ± 0.03 | 0.03 ± 0.00 | 0.05 ± 0.02 | MS/STD/RI |
| Total | 1.05 ± 0.12 b | 0.80 ± 0.01 b | 2.15 ± 0.82 a | 0.97 ± 0.39 b | 1.38 ± 0.13 ab | 1.55 ± 0.28 ab | |||||
| Acids | |||||||||||
| 1 | Hexanoic acid | 25.74 | 1765 | 1850 | 1.03 ± 0.33 | - | - | 1.33 ± 0.01 | - | - | MS/STD/RI |
| 2 | Octanoic acid | 30.06 | 1969 | 2038 | 7.55 ± 2.53 | 4.06 ± 2.66 | 7.13 ± 2.52 | 14.75 ± 7.97 | 9.19 ± 3.14 | 9.97 ± 2.16 | MS/STD/RI |
| 3 | n-Decanoic acid | 34.07 | 2176 | 2246 | 6.60 ± 2.06 | 3.50 ± 1.98 | 6.26 ± 3.04 | 9.94 ± 4.92 | 7.07 ± 2.12 | 6.90 ± 3.69 | MS/STD/RI |
| Total | 15.18 ± 3.50 ab | 7.56 ± 4.64 b | 13.39 ± 5.35 ab | 26.01 ± 12.76 a | 16.26 ± 5.16 ab | 16.87 ± 5.78 ab | |||||
| Aldehydes | |||||||||||
| 1 | Octanal | 11.78 | 1214 | 1292 | - | - | - | 0.18 ± 0.14 | - | 0.23 ± 0.09 | MS/STD/RI |
| 2 | Nonanal | 14.55 | 1313 | 1390 | 0.56 ± 0.18 | 0.81 ± 0.26 | 1.41 ± 0.63 | 1.42 ± 0.88 | 0.59 ± 0.15 | 1.46 ± 0.33 | MS/STD/RI |
| 3 | Decanal | 17.23 | 1412 | 1472 | - | 1.46 ± 0.29 | 1.86 ± 0.63 | 0.98 ± 0.37 | 2.33 ± 1.55 | 4.39 ± 2.13 | MS/STD/RI |
| Total | 0.56 ± 0.18 b | 2.27 ± 0.03 b | 3.27 ± 1.24 b | 2.58 ± 1.17 b | 2.91 ± 1.70 b | 6.09 ± 2.54 a | |||||
| Alcohols | |||||||||||
| 1 | 2-Ethyl-1-hexanol | 17.16 | 1410 | 1484 | 3.93 ± 0.20 | 3.63 ± 0.34 | 5.92 ± 3.06 | 4.23 ± 3.19 | 2.46 ± 0.14 | 2.17 ± 0.69 | MS/STD/RI |
| 2 | 1-Octanol | 18.83 | 1474 | 1558 | 0.27 ± 0.04 | 0.19 ± 0.03 | 0.40 ± 0.10 | 0.51 ± 0.33 | 0.20 ± 0.01 | 0.47 ± 0.18 | MS/STD/RI |
| Total | 4.20 ± 0.17 | 3.82 ± 0.37 | 6.32 ± 3.14 | 4.73 ± 3.51 | 2.65 ± 0.15 | 2.64 ± 0.87 | |||||
| Alkanes | |||||||||||
| 1 | Tetradecane | 14.81 | 1323 | 1400 | - | 0.34 ± 0.17 | 0.93 ± 0.13 | 0.15 ± 0.01 | 0.19 ± 0.05 | 0.14 ± 0.05 | MS/STD/RI |
| Total | - | 0.34 ± 0.17 b | 0.93 ± 0.13 a | 0.15 ± 0.01 b | 0.19 ± 0.05 b | 0.14 ± 0.05 b | |||||
| Sulfur compounds | |||||||||||
| 1 | Dimethyl sulfone | 26.28 | 1789 | 1895 | 0.10 ± 0.00 | - | - | 0.15 ± 0.10 | - | - | MS/STD |
| Total | 0.10 ± 0.00 | - | - | 0.15 ± 0.10 | - | - | |||||
| No. | Sensory Attributes | Evaluation Method |
|---|---|---|
| 1 | Aftertaste (milk flavor) | After tasting, intensity of residual milk flavor. |
| 2 | Aftertaste (sweetness) | After tasting, intensity of residual sweetness. |
| 3 | Aftertaste (fat-sense) | After tasting, intensity of fat feeling in the mouth. |
| 4 | Aroma | Inherent flavor of milk, the aroma is gentle, scented, natural and without off-flavor. |
| 5 | Sweetness | Intensity of the inherent sweetness for sample. |
| 6 | Off-flavor | Smell should not appear in milk, such as stink and musty. |
| 7 | Sourness | Intensity of sourness was felt in the mouth. |
| 8 | Saltiness | Intensity of salt were felt in the mouth. Slight salt should exist in the optimum sample |
| 9 | Milk flavor | Intensity of inherent milk flavor was felt in the mouth. |
| 10 | Butter | Intensity of greasy feeling in the mouth. |
| 11 | Texture | Whether the sample is a uniform liquid, and whether there is a clot or precipitation. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Tong, L.; Chi, X.; Ai, N.; Cao, Y.; Sun, B. Comparison of Sensory and Electronic Tongue Analysis Combined with HS-SPME-GC-MS in the Evaluation of Skim Milk Processed with Different Preheating Treatments. Molecules 2019, 24, 1650. https://doi.org/10.3390/molecules24091650
Pan M, Tong L, Chi X, Ai N, Cao Y, Sun B. Comparison of Sensory and Electronic Tongue Analysis Combined with HS-SPME-GC-MS in the Evaluation of Skim Milk Processed with Different Preheating Treatments. Molecules. 2019; 24(9):1650. https://doi.org/10.3390/molecules24091650
Chicago/Turabian StylePan, Minghui, Lingjun Tong, Xuelu Chi, Nasi Ai, Yungang Cao, and Baoguo Sun. 2019. "Comparison of Sensory and Electronic Tongue Analysis Combined with HS-SPME-GC-MS in the Evaluation of Skim Milk Processed with Different Preheating Treatments" Molecules 24, no. 9: 1650. https://doi.org/10.3390/molecules24091650
APA StylePan, M., Tong, L., Chi, X., Ai, N., Cao, Y., & Sun, B. (2019). Comparison of Sensory and Electronic Tongue Analysis Combined with HS-SPME-GC-MS in the Evaluation of Skim Milk Processed with Different Preheating Treatments. Molecules, 24(9), 1650. https://doi.org/10.3390/molecules24091650







