Advances in Rosin-Based Chemicals: The Latest Recipes, Applications and Future Trends
Abstract
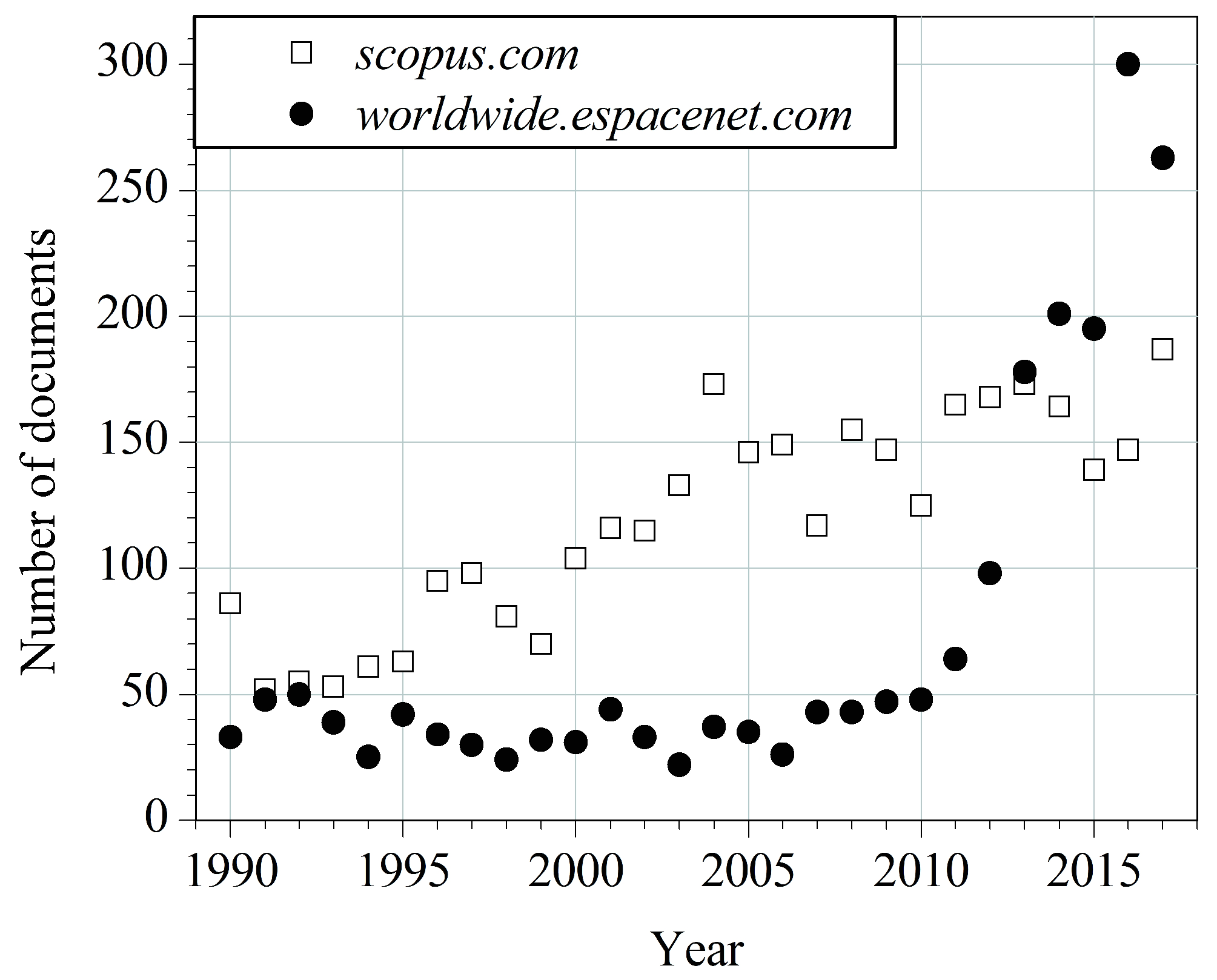
1. Introduction
2. Basic Information about Rosin
3. Rosin-based Chemicals
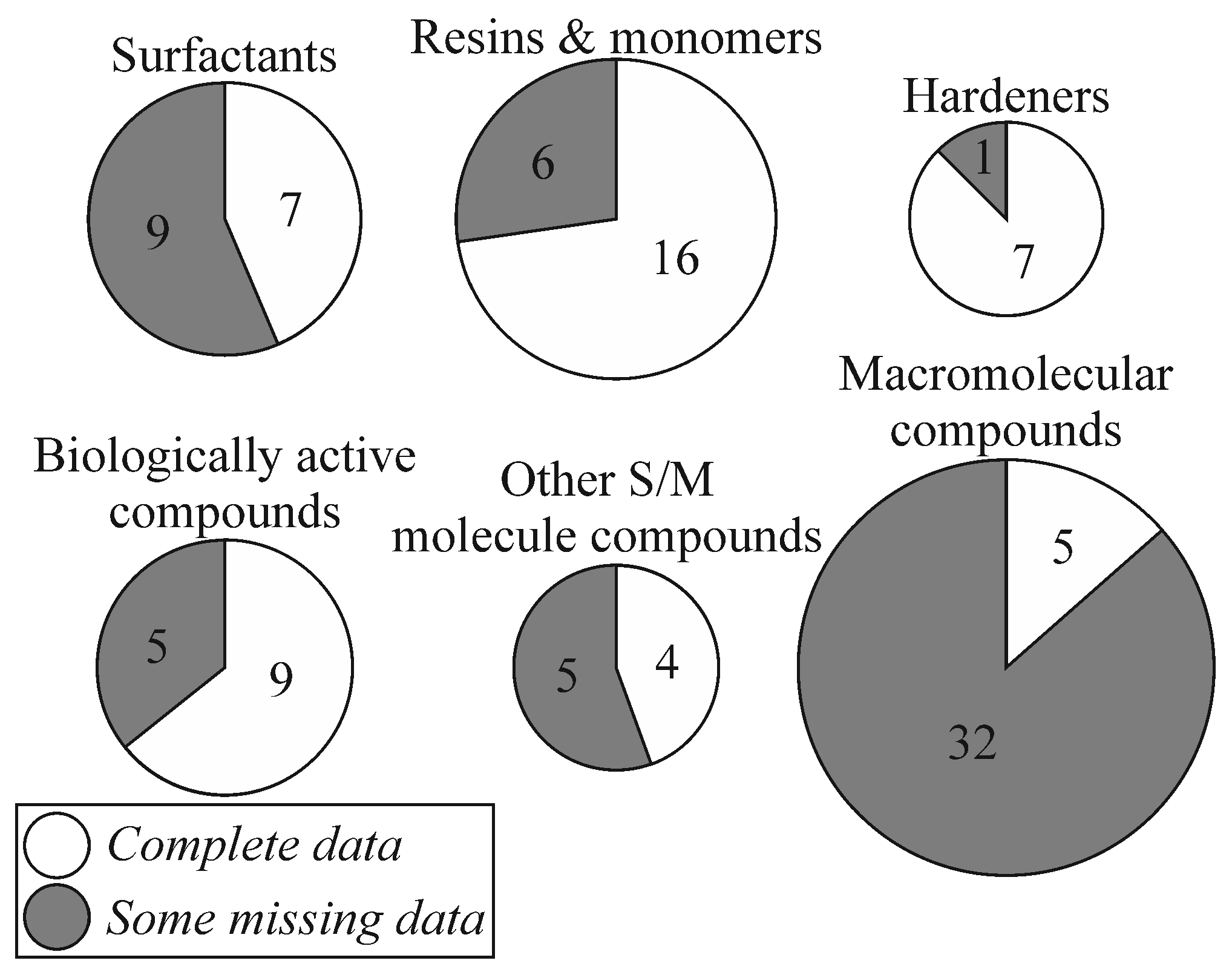
3.1. General Comments on the Whole Review
3.2. Small and Medium Molecule Compounds
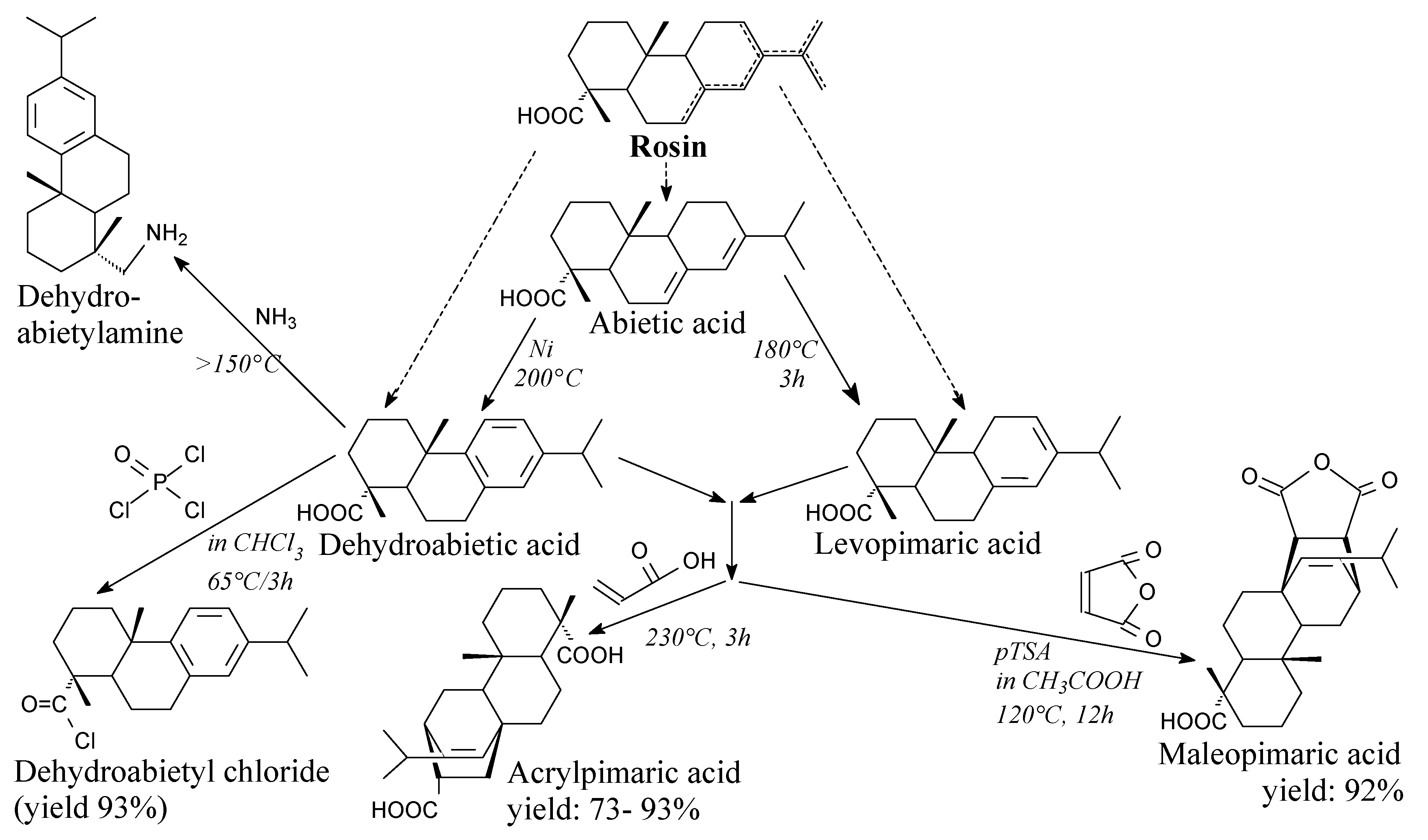
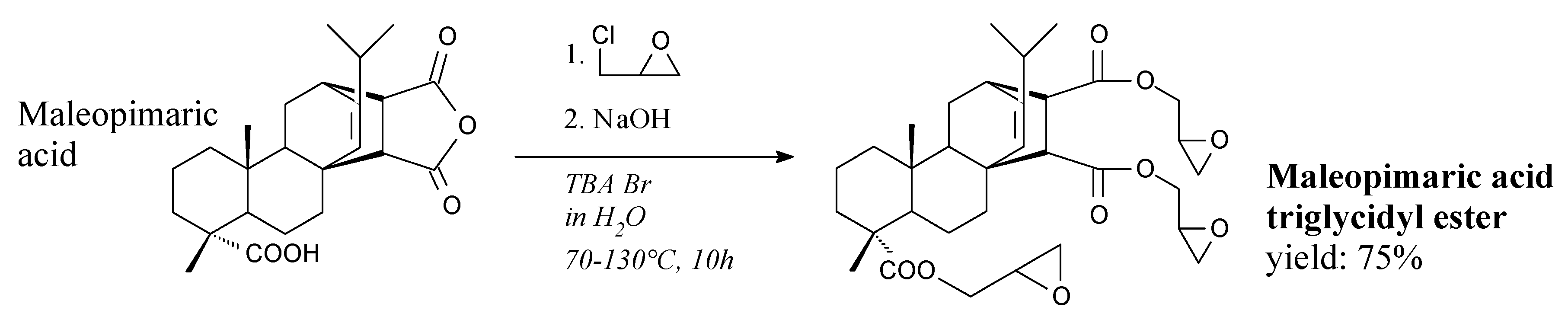
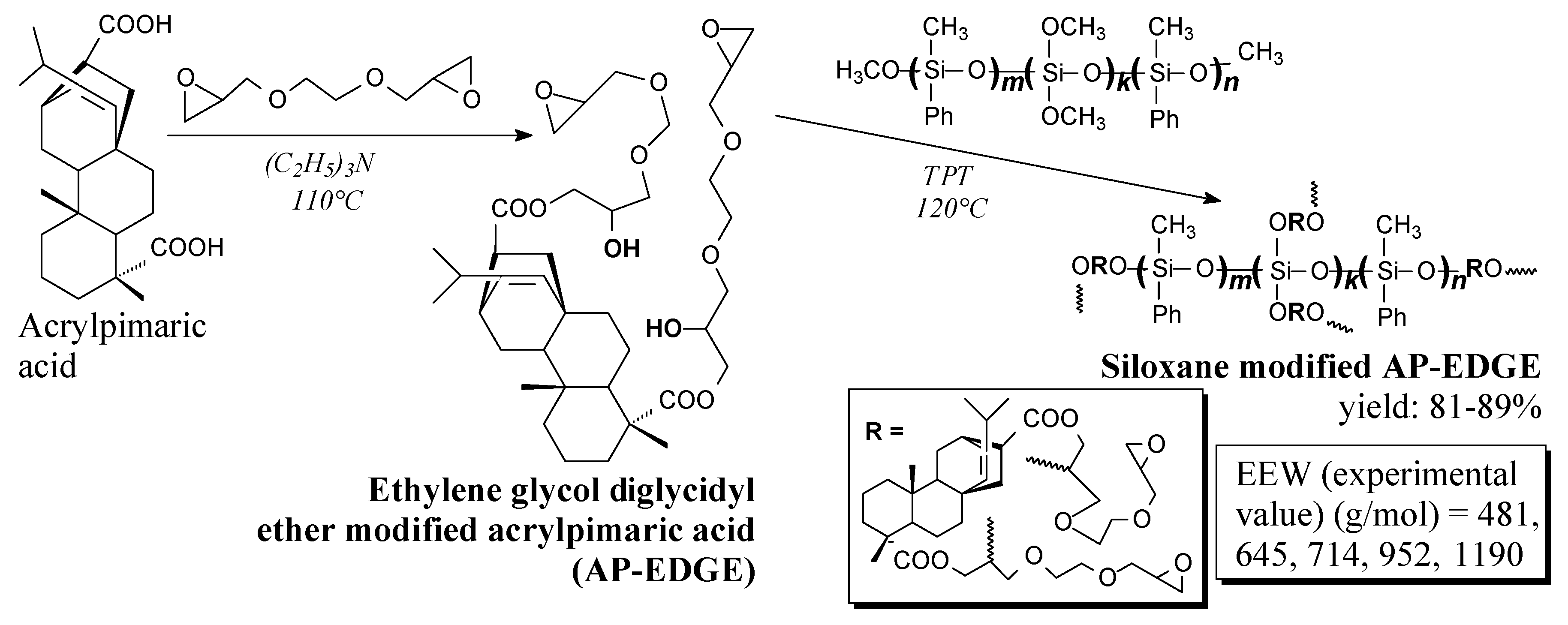
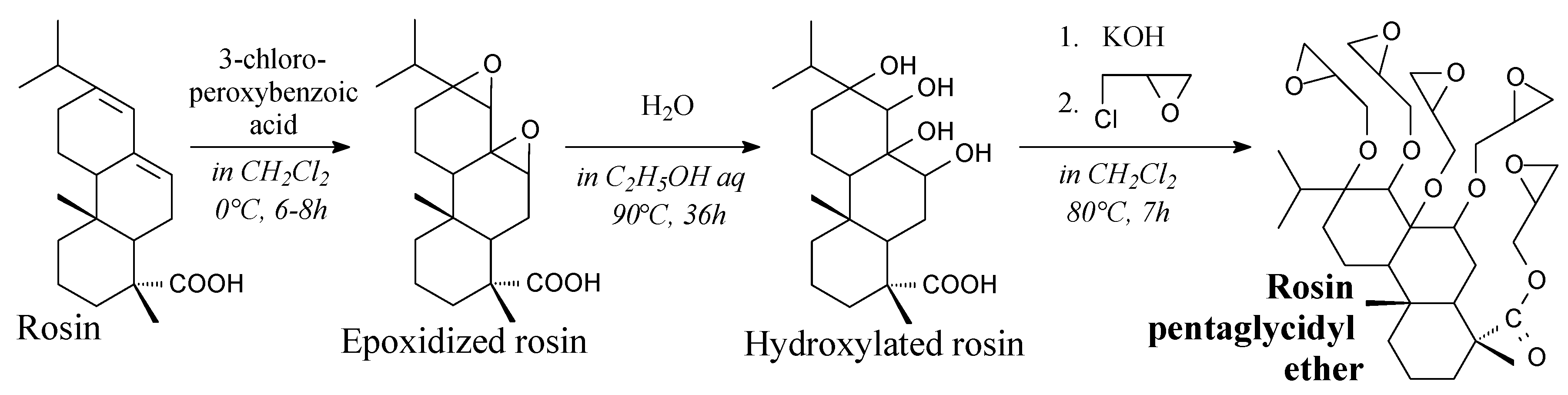
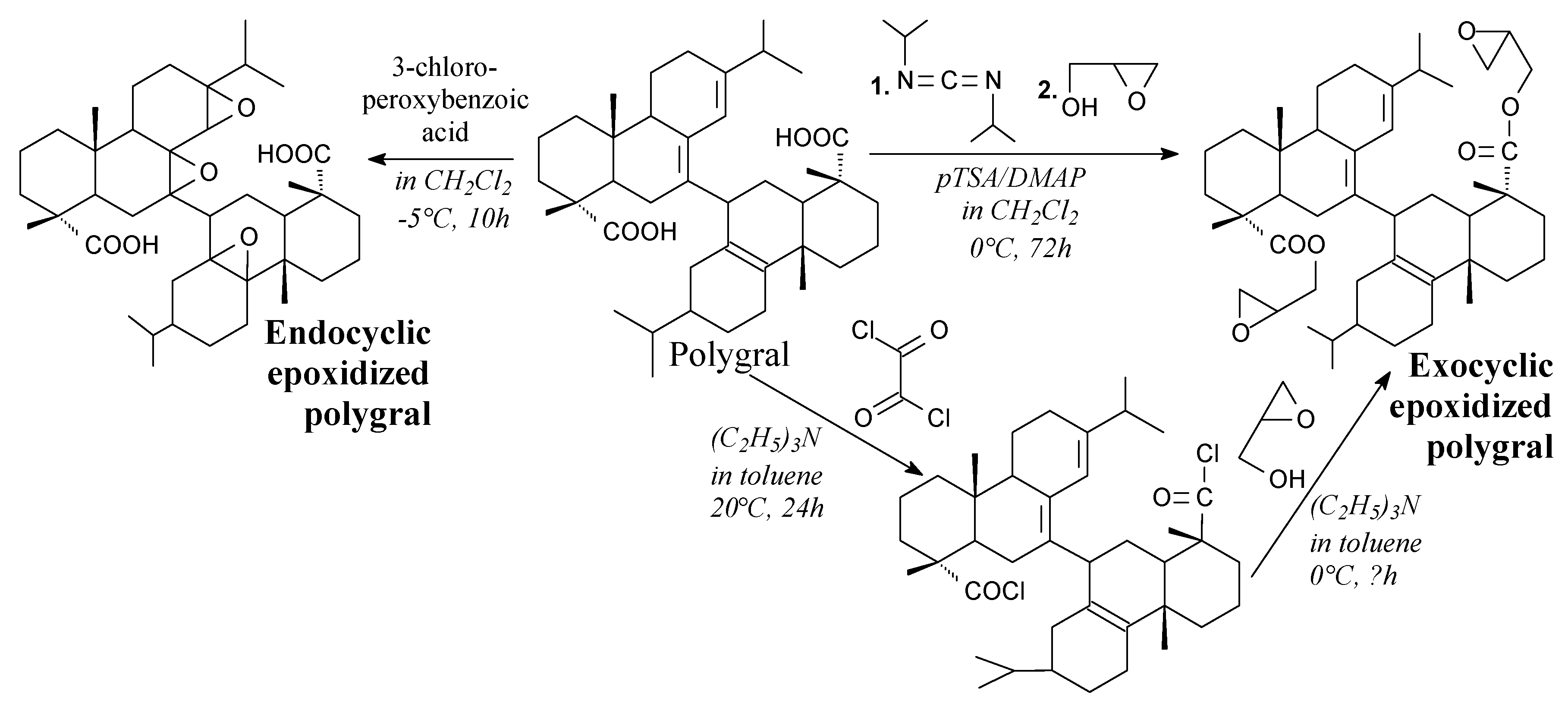
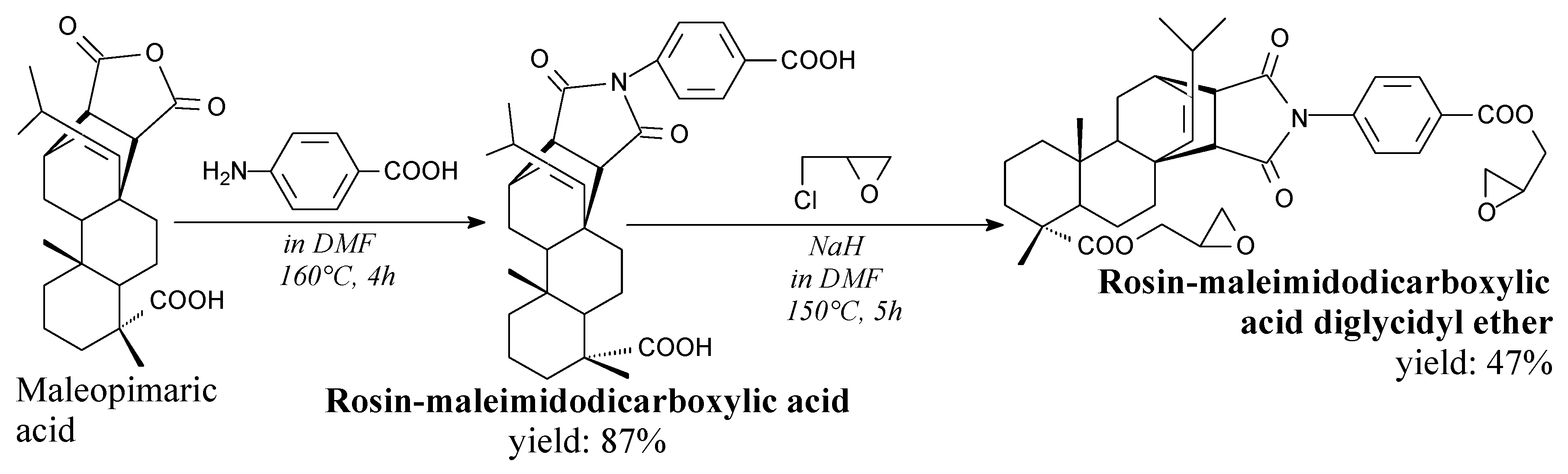
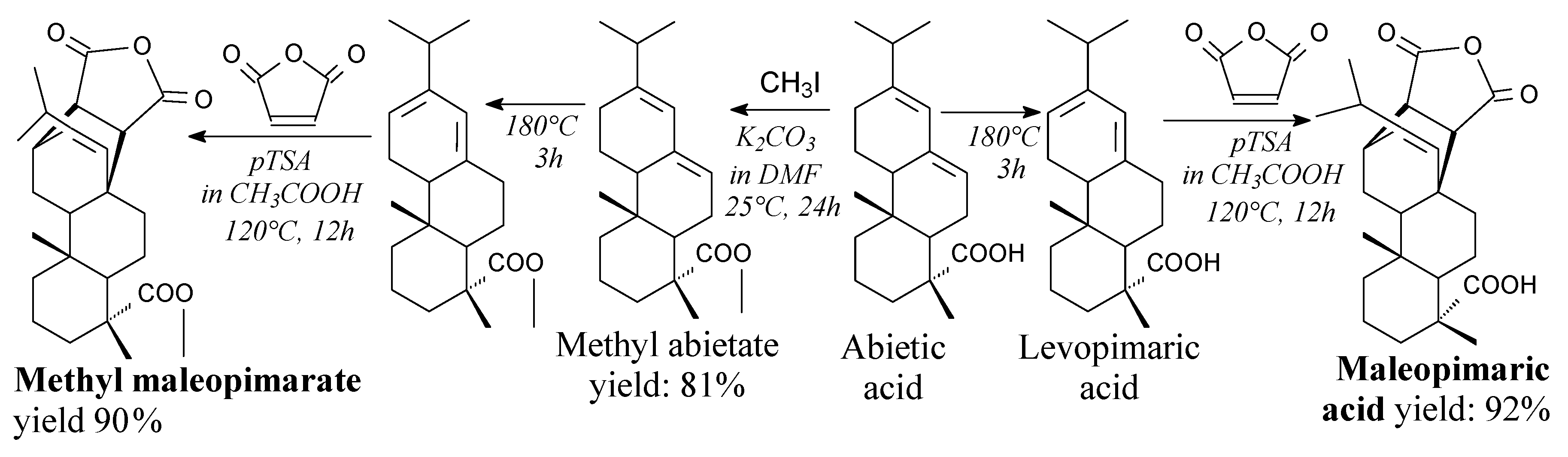
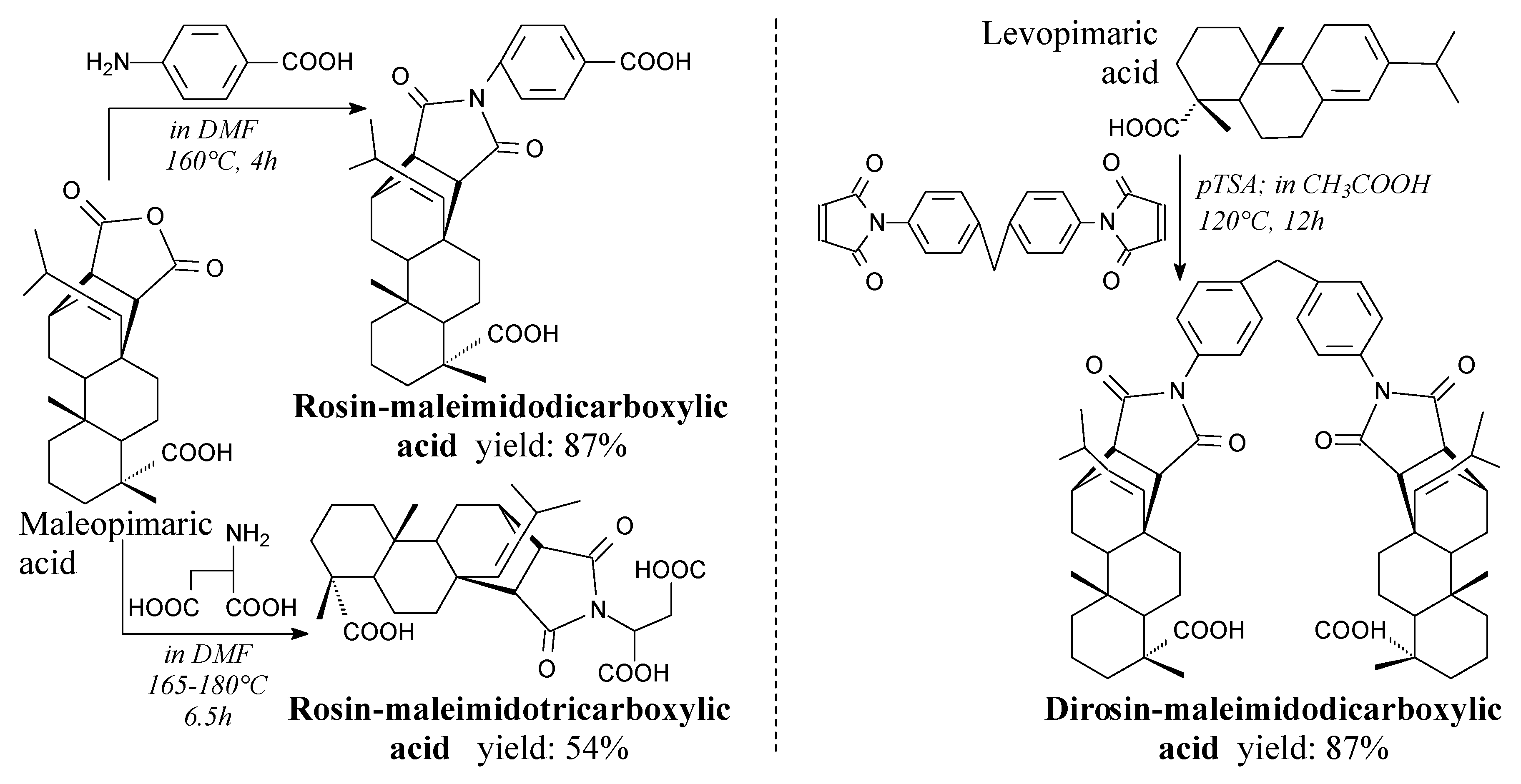
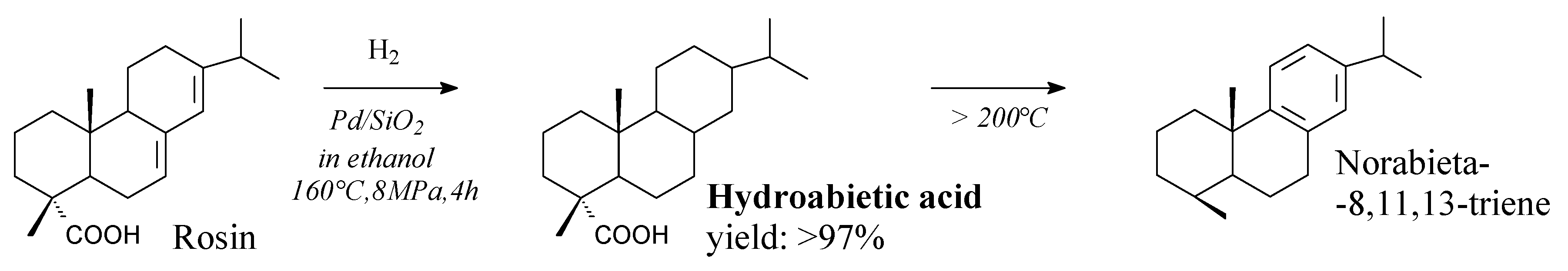
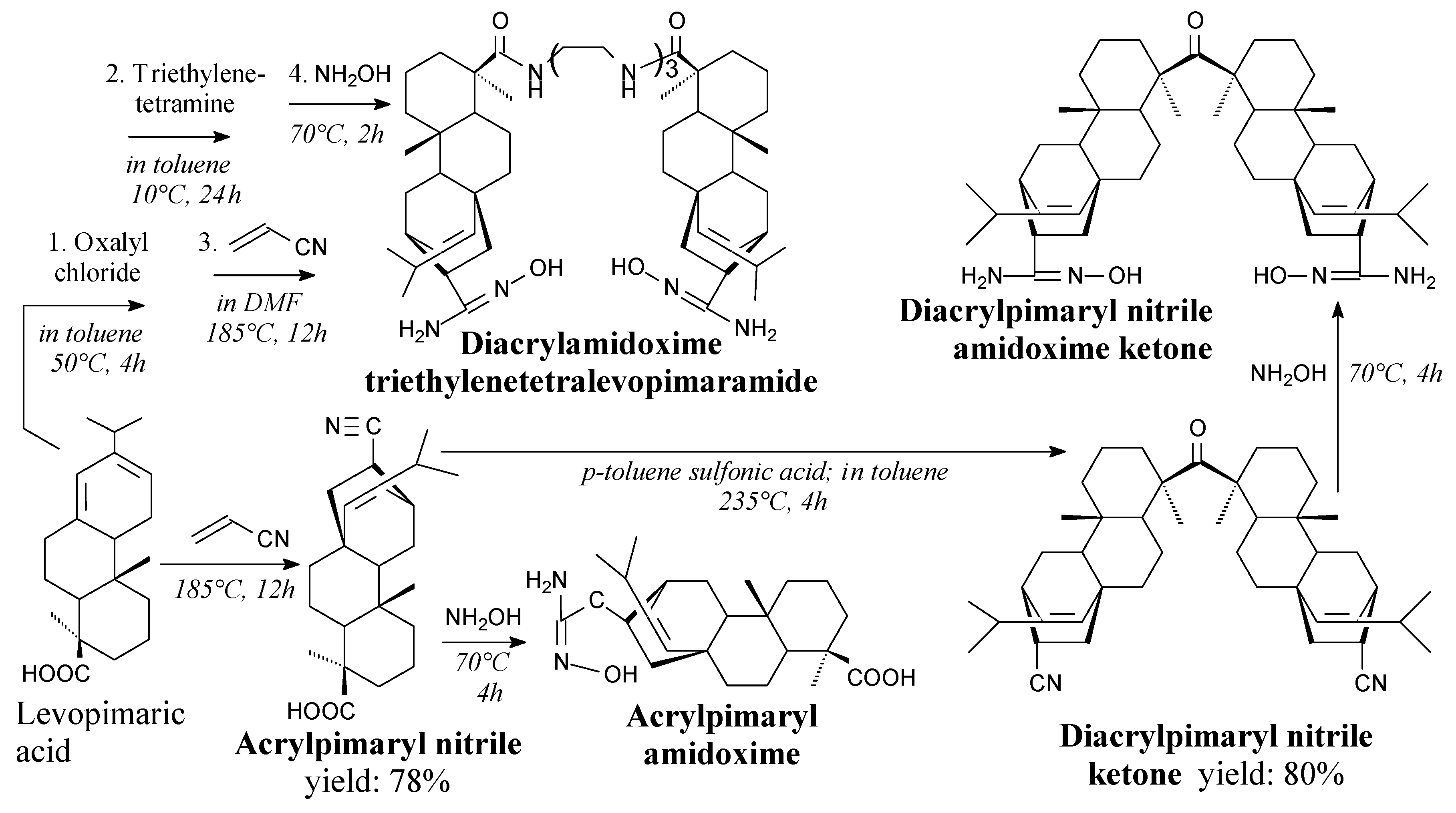
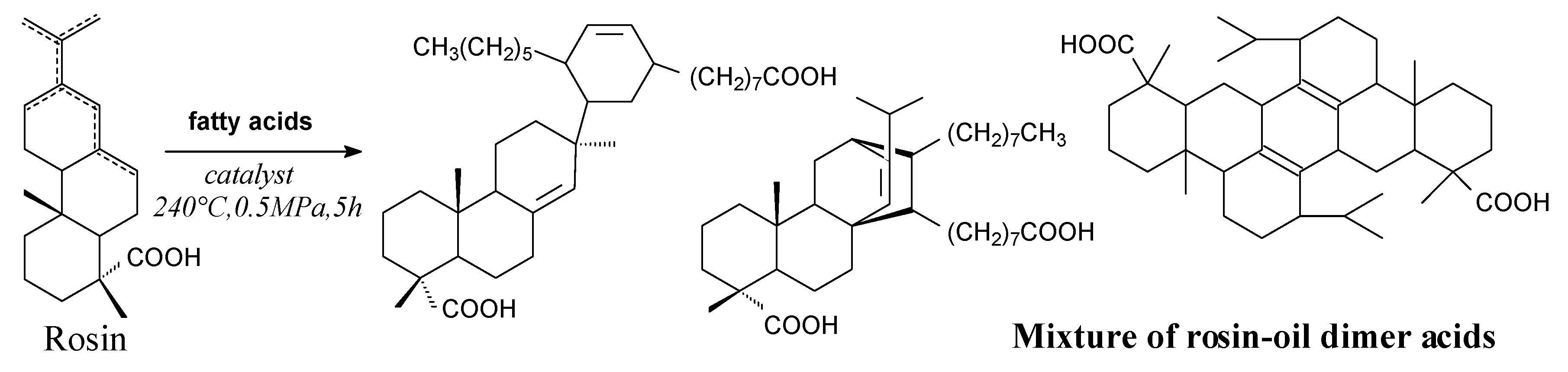
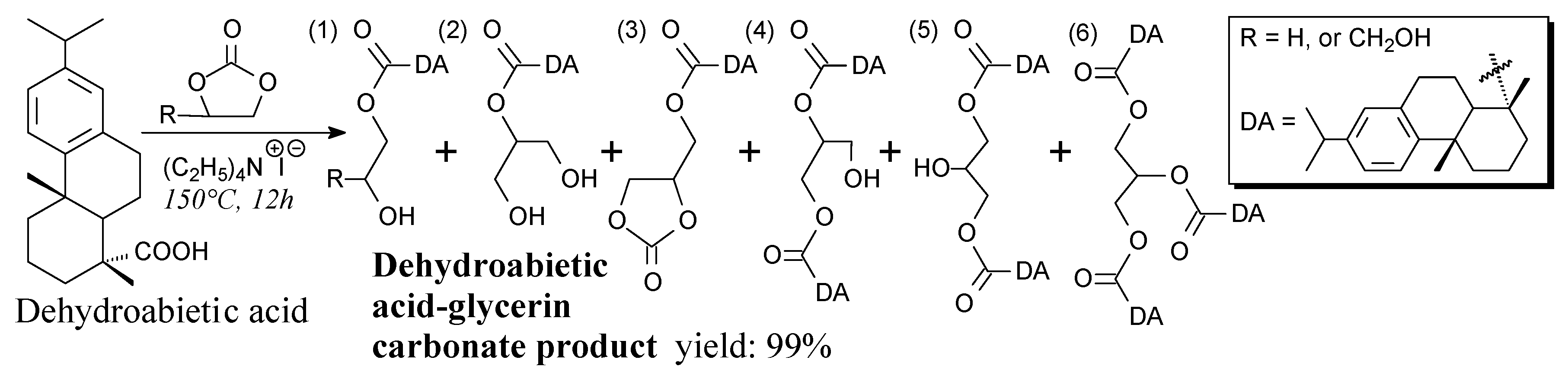
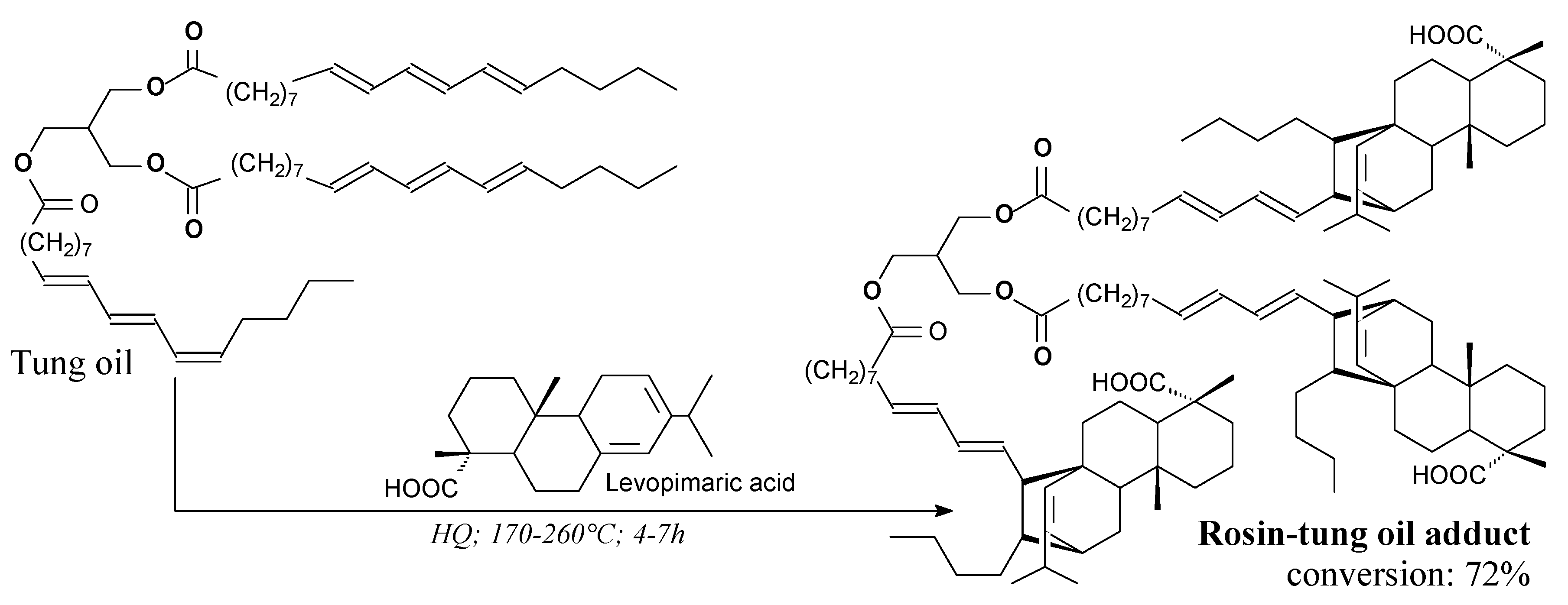
3.2.1. Intermediates
3.2.2. Resins and Monomers
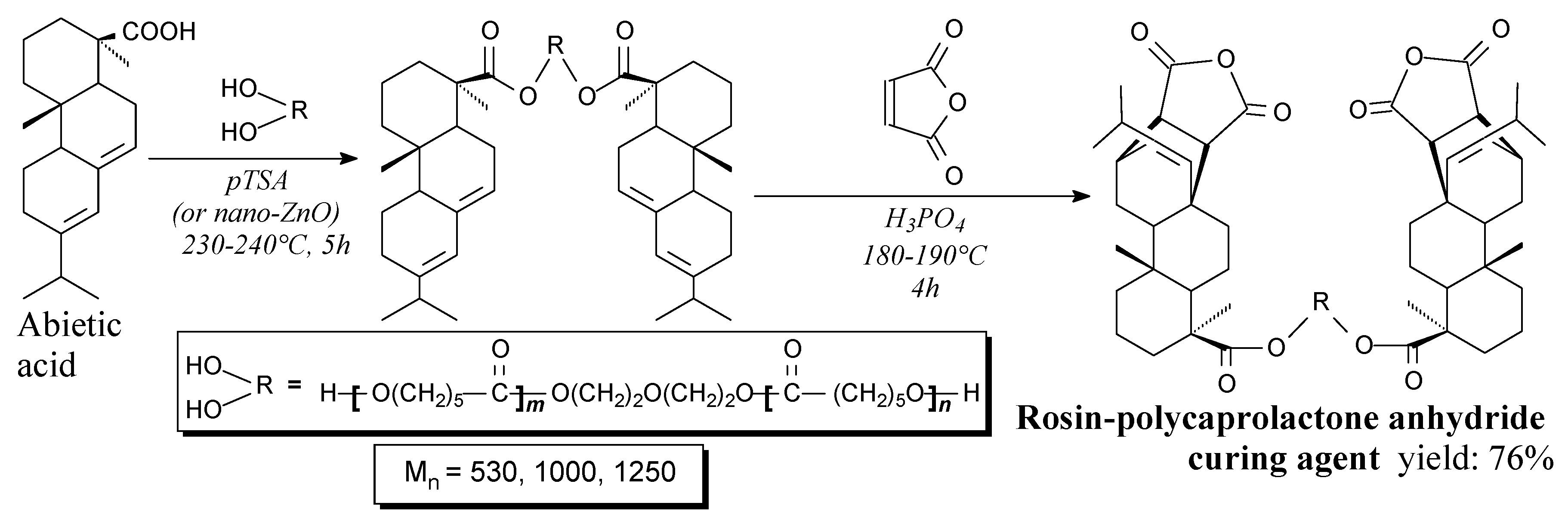
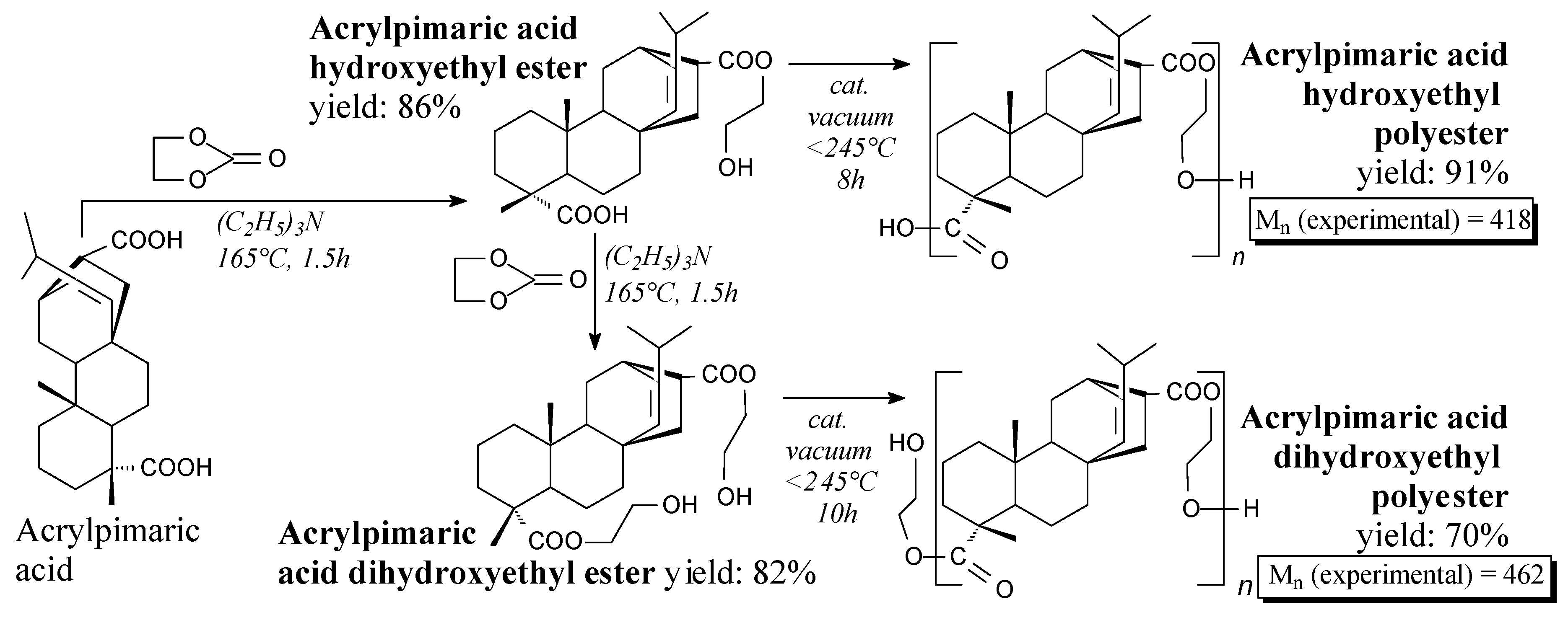
3.2.3. Hardeners
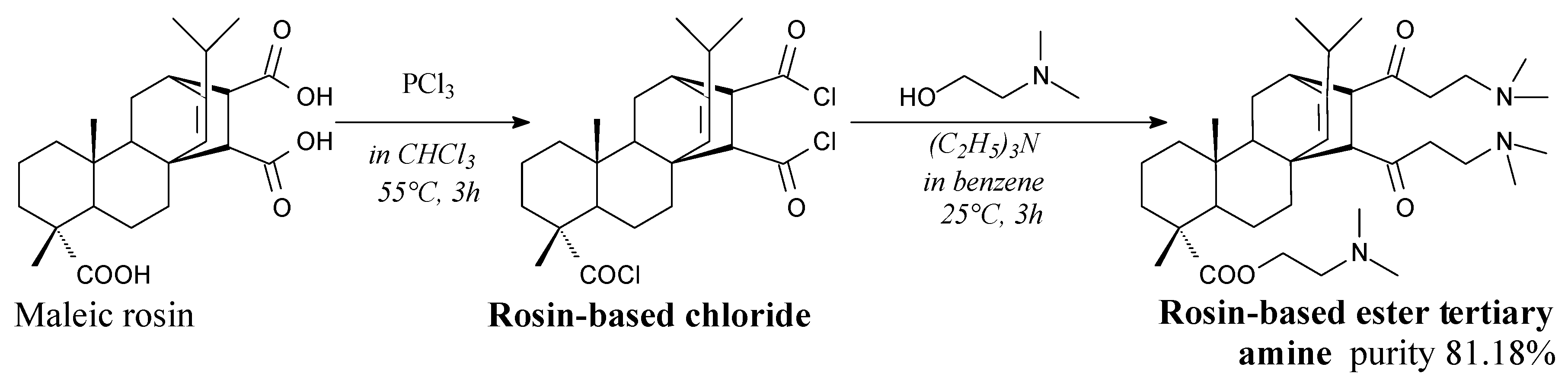
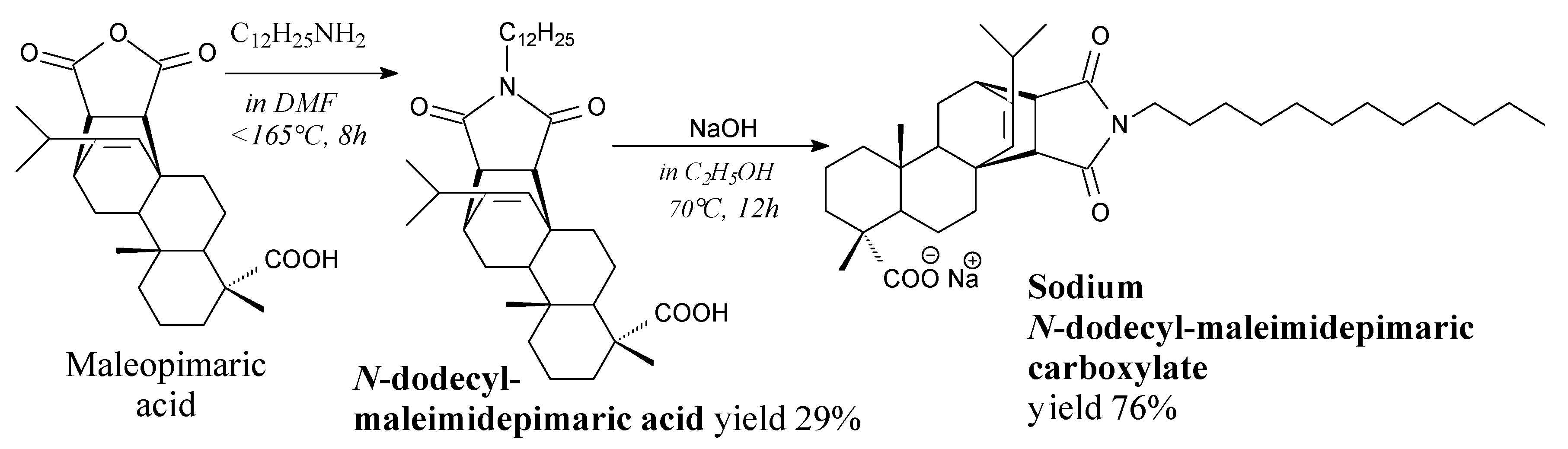
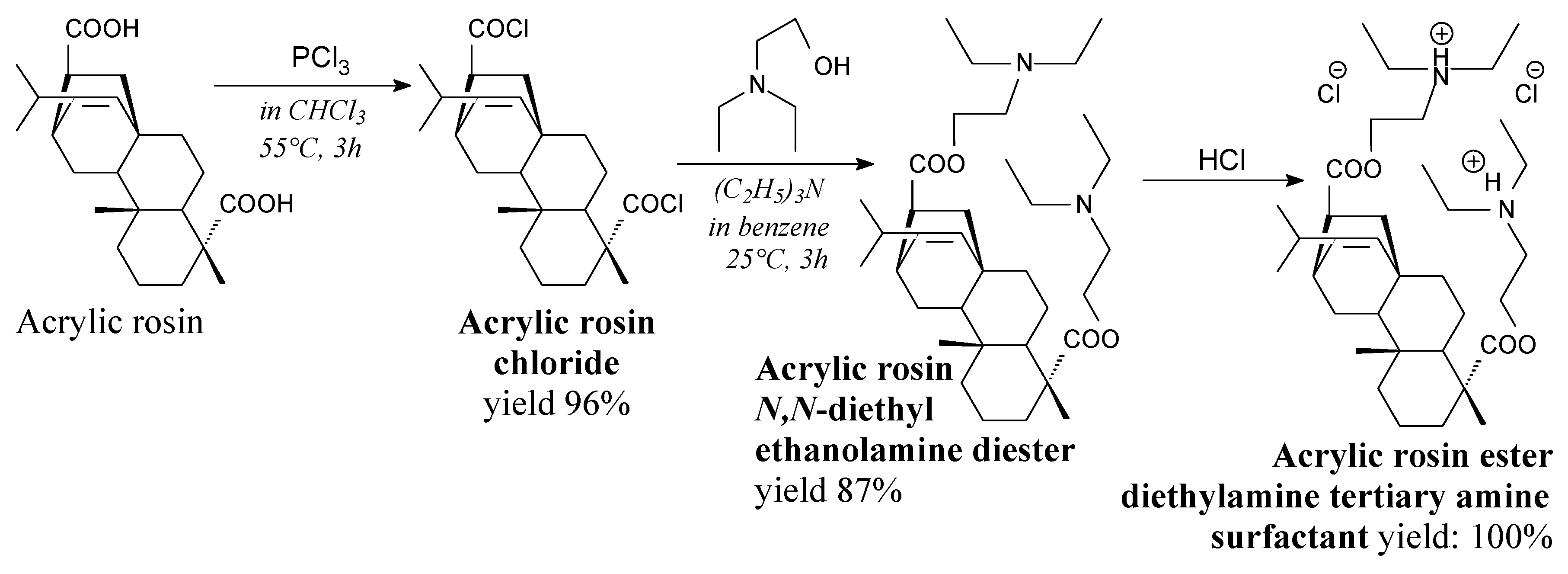
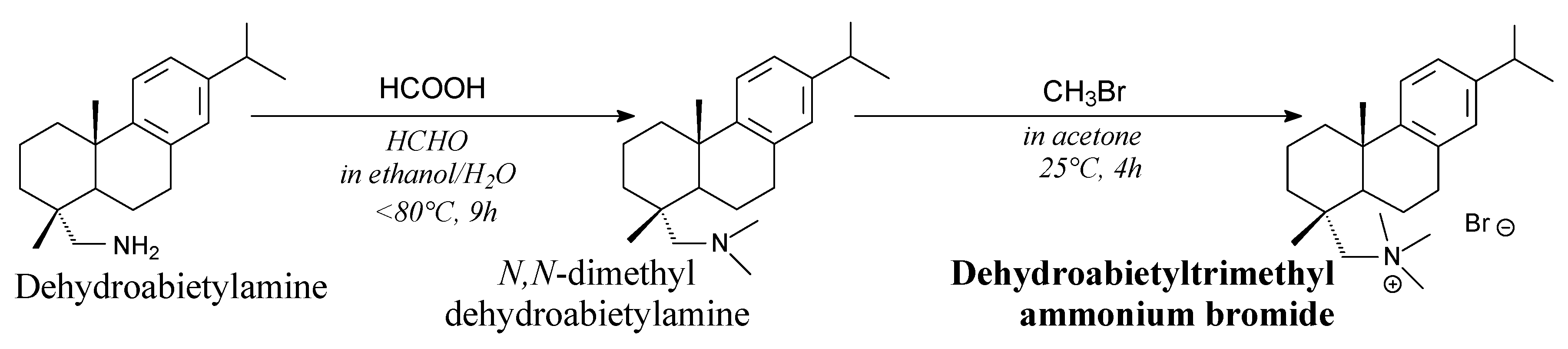
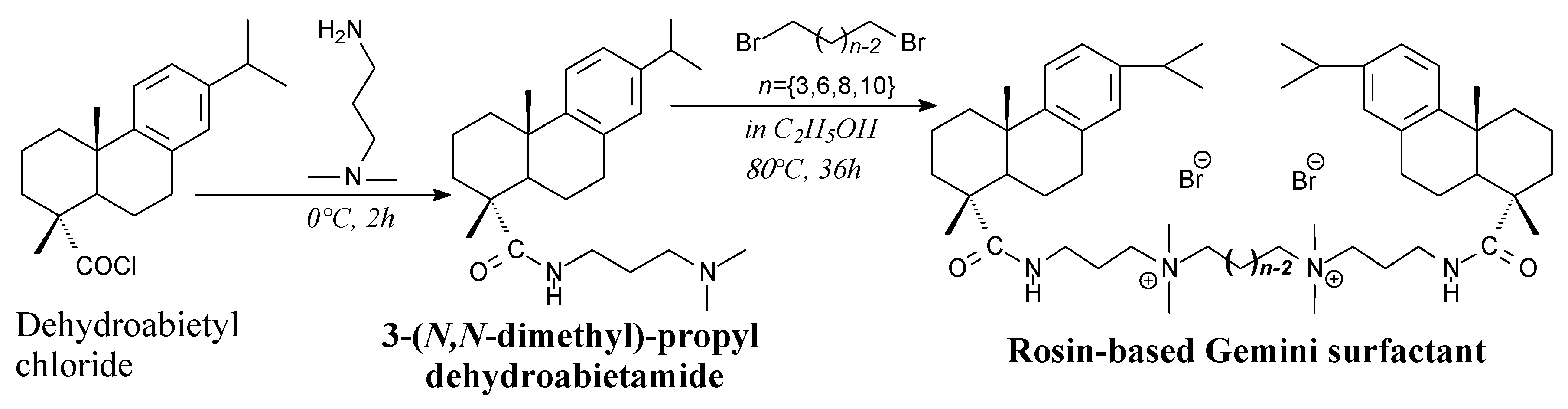
3.2.4. Surfactants
3.2.5. Biologically Active Compounds
3.2.6. Other Small/Medium Molecule Products
3.3. Macromolecular Compounds
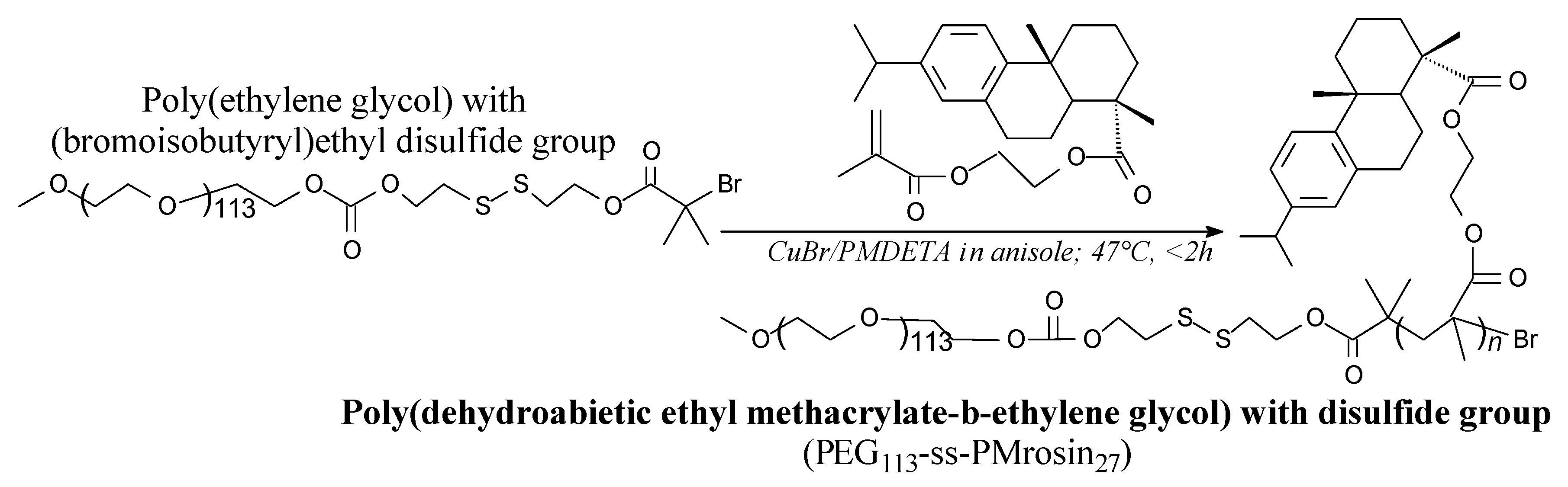
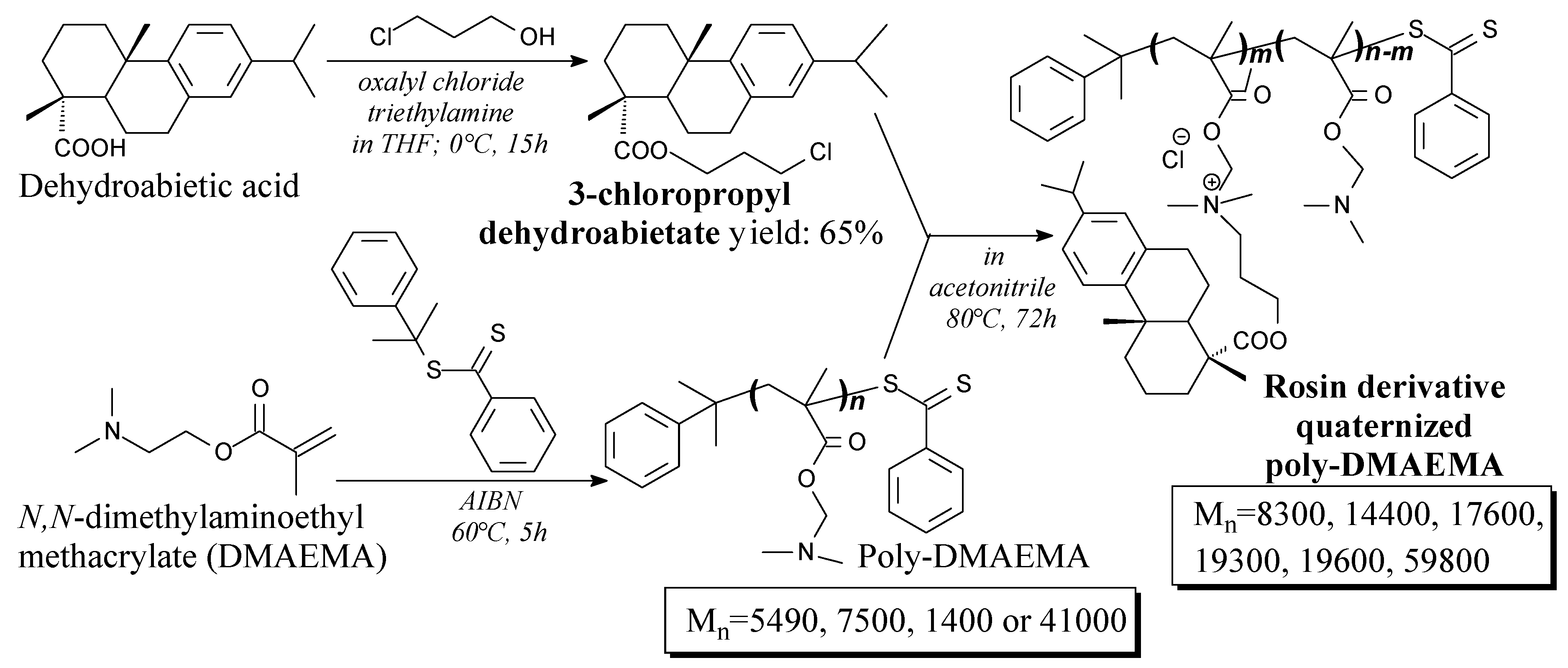
3.3.1. Polymers for Biomedical Applications
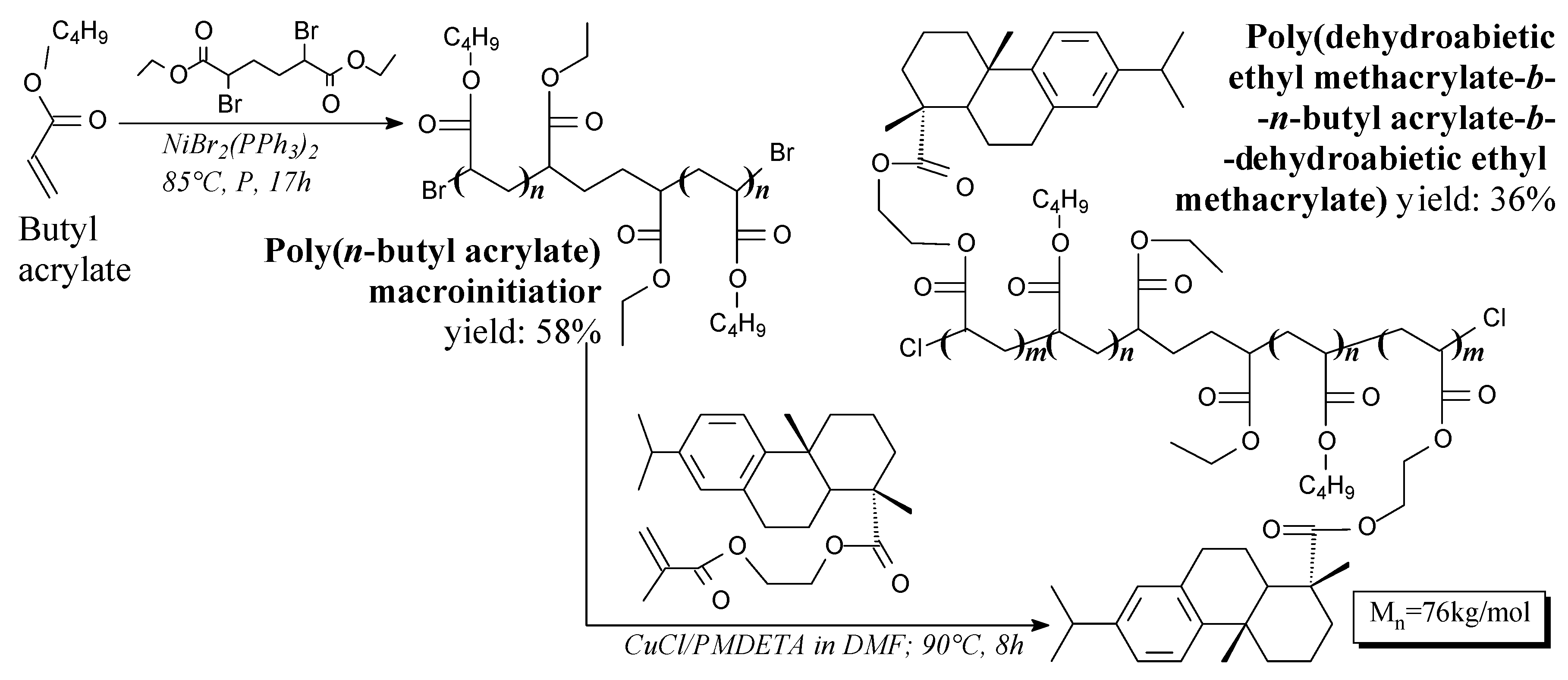
3.3.2. Elastomers
3.3.3. Coatings and Adhesives
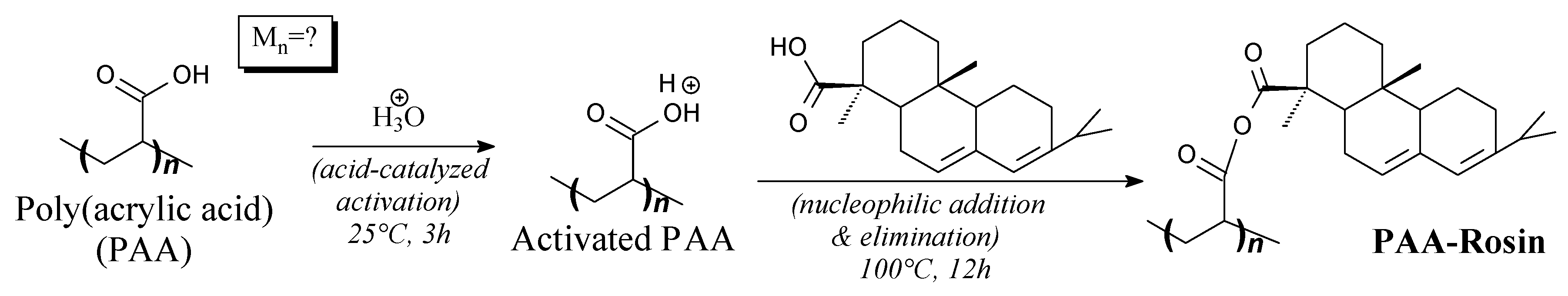
3.3.4. Surfactants
3.3.5. Sorbents
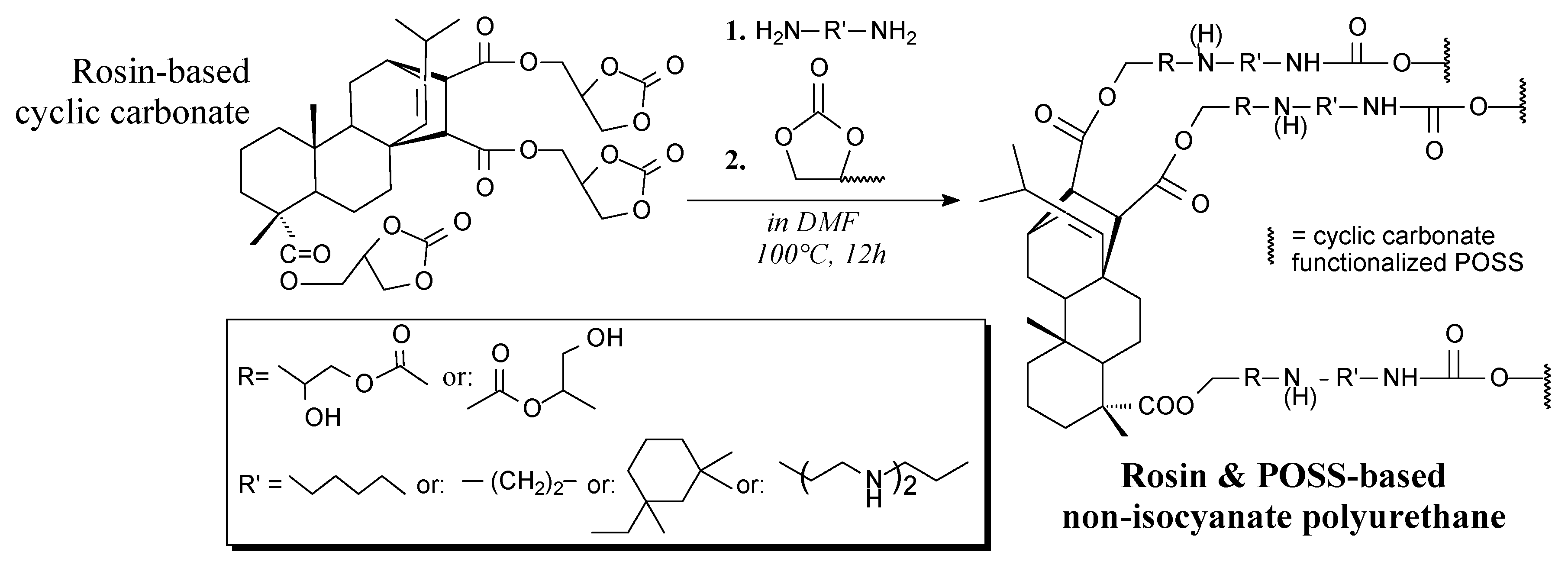
3.3.6. Organosilicons
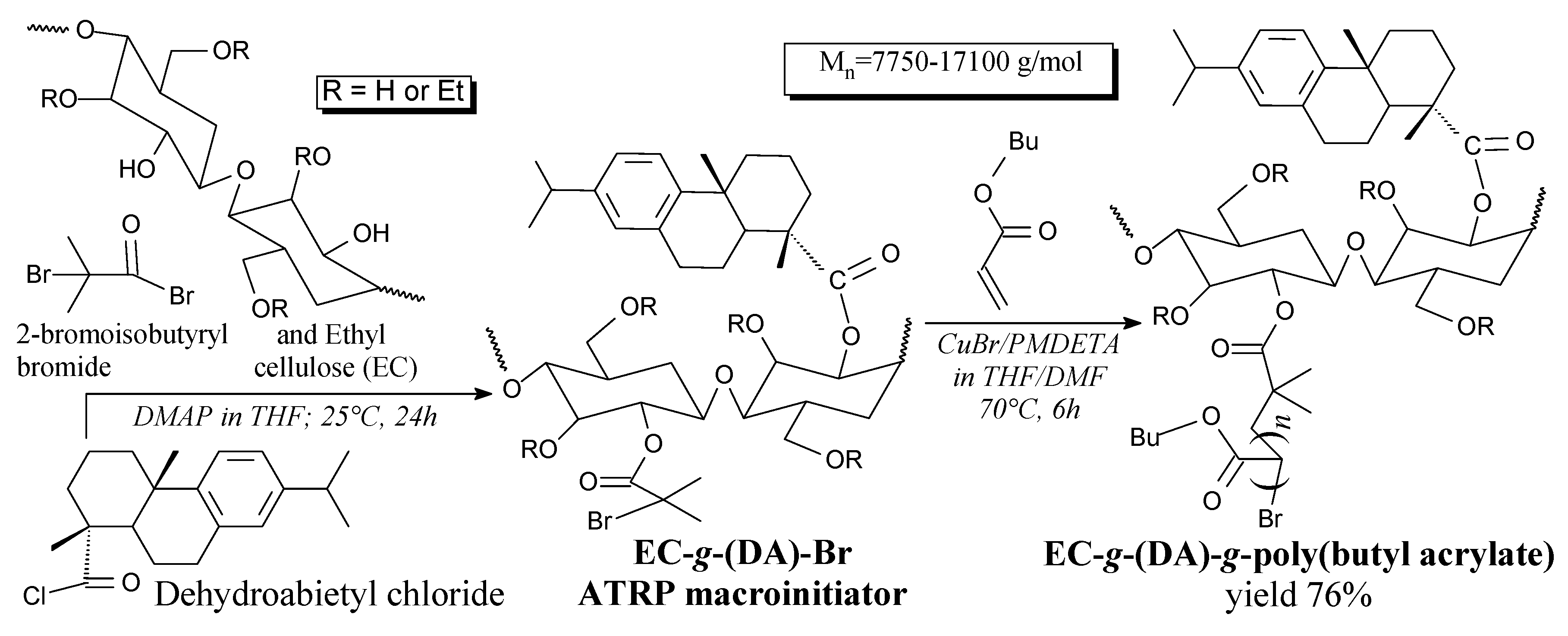
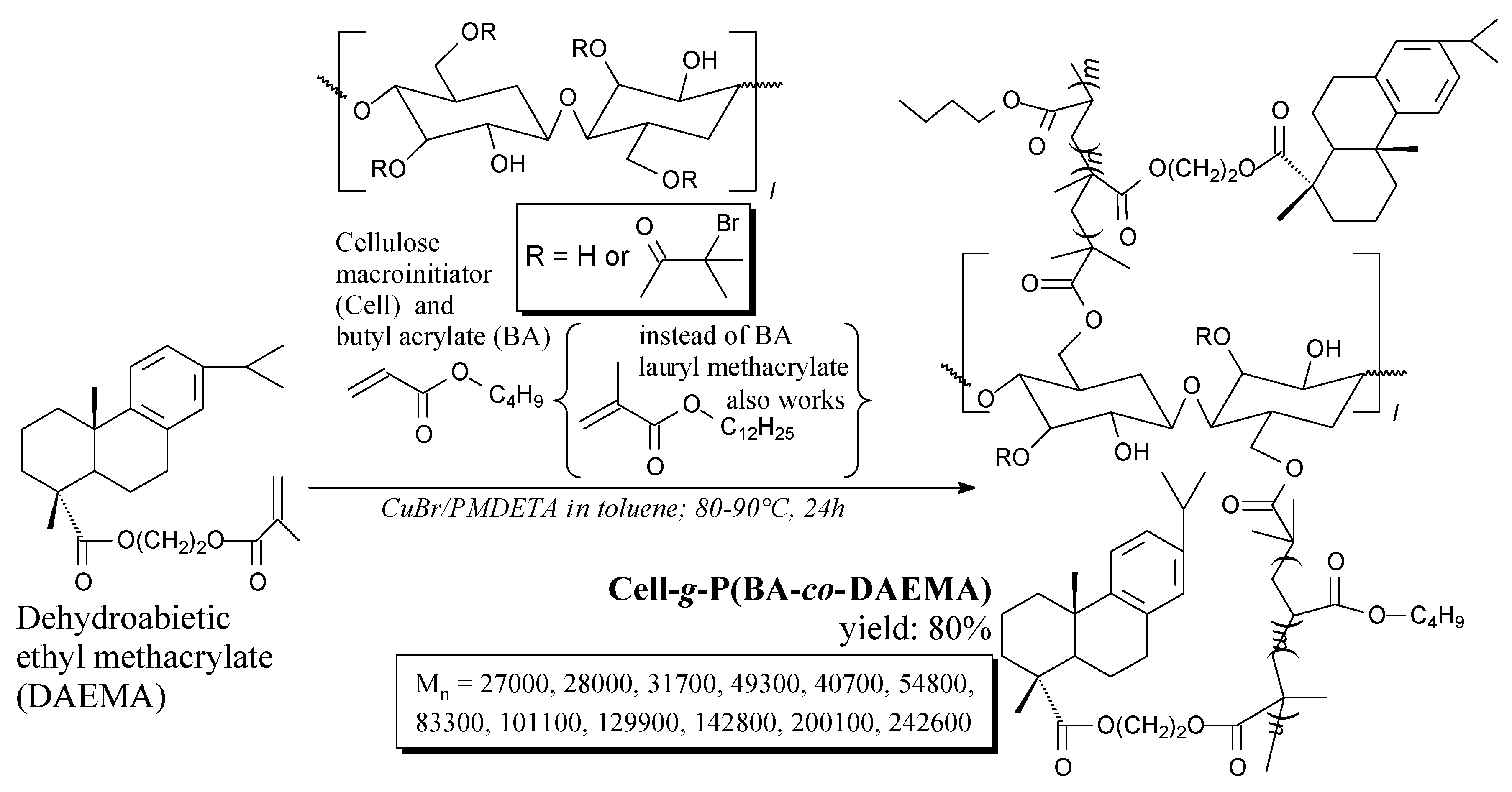
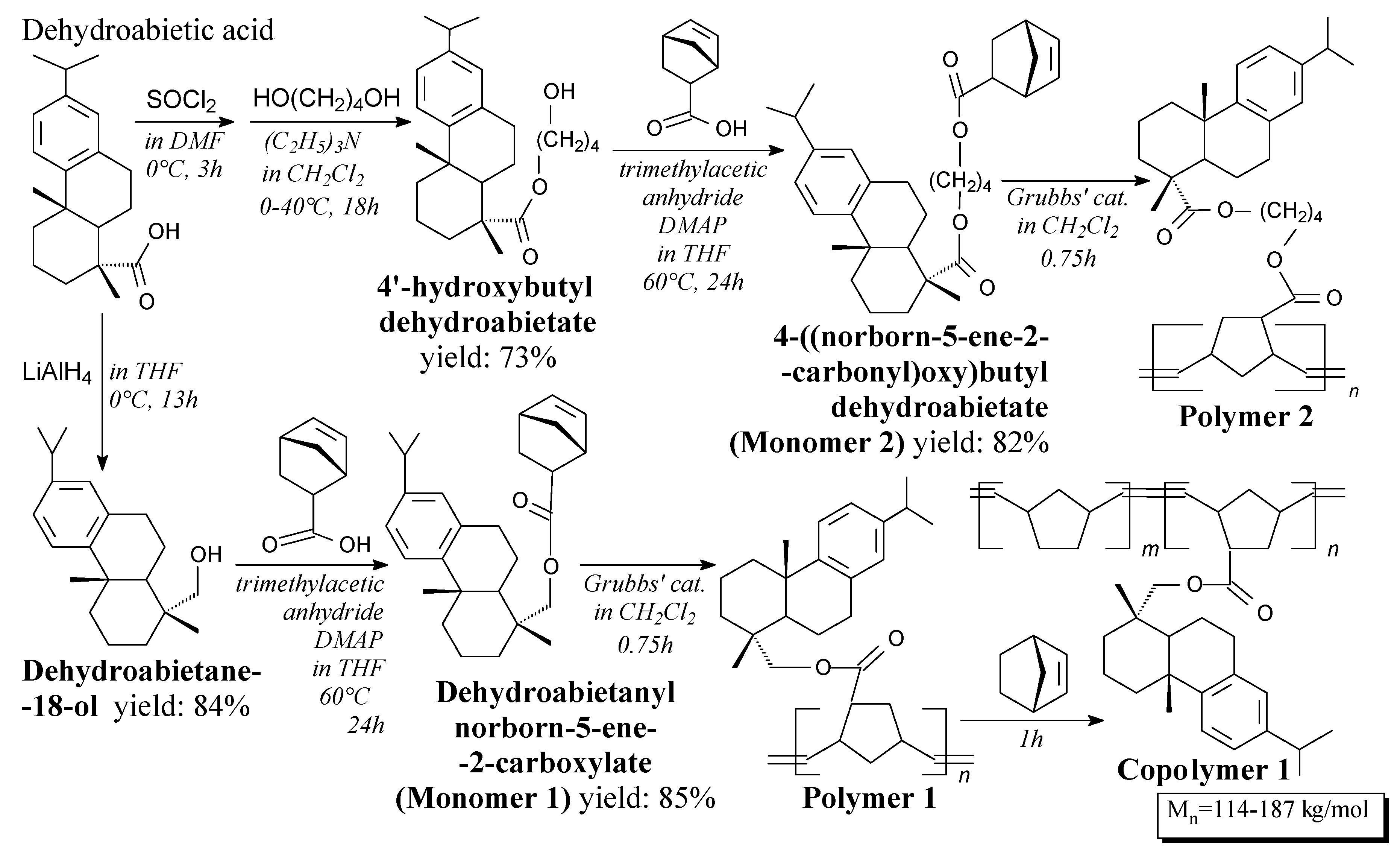
3.3.7. Polysaccharides
3.3.8. Other Materials
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AA | adipic acid; |
| AIBA | 2,2′-azobis(2-methylpropionamidine) dihydrochloride; |
| AIBN | 2,2′-azobisisobutyronitrile; |
| ATRP | atom transfer radical polymerization; |
| BA | butyl acrylate; |
| Bu | butyl group; |
| Cat. | catalyst; |
| DA | dehydroabietic acid; |
| DBU | 1,8-diazabicyclo[5.4.0]undec-7-ene; |
| DCC | N,N′-dicyclohexylcarbodiimide; |
| DDPD | dehydroabietyl phosphate diester; |
| DMAP | 4-dimethylaminopyridine; |
| DMF | dimethylformamide; |
| DMSO | dimethyl sulfoxide; |
| EC | ethyl cellulose; |
| EEW | epoxy equivalent weight; |
| Et | ethyl group; |
| HDMA Cl | hexadecyltrimethylammonium chloride; |
| HDTMAB | hexadecyl trimethyl ammonium bromide; |
| HPI | hydrophilic polyester intermediate; |
| HPLC | high-performance liquid chromatography; |
| HQ | hydroquinone; |
| IPA | isophtalic acid; |
| JCR | Journal Citation Reports; |
| m.p. | melting point; |
| Me | methyl group; |
| MEK | methyl ethyl ketone; |
| MPA | maleopimaric acid; |
| NMP | N-methyl pyrrolidone; |
| NMR | nuclear magnetic resonance; |
| NPG | neopentyl glycol; |
| PAA | poly(acrylic acid); |
| PEG | poly(ethylene glycol); |
| Ph | phenyl group; |
| PMDETA | pentamethyldiethylenetriamine; |
| PPh3 | triphenylphosphine; |
| PSA | pressure sensitive adhesives; |
| pTSA | p-toluene sulfonic acid; |
| RAFT | reversible addition-fragmentation chain-transfer polymerization; |
| ROMP | ring-opening metathesis polymerization; |
| UV | ultraviolet; |
| TBA Br | tetrabutylammonium bromide; |
| TEBAC | benzyltriethylammonium chloride; |
| THF | tetrahydrofuran; |
| TMP | trimethylolpropane; |
| TPT | tetraisopropyl titanate; |
| TRL | technology readiness level; |
References
- Silvestre, A.J.D.; Gandini, A. Chapter 4-Rosin: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 67–88. ISBN 978-0-08-045316-3. [Google Scholar]
- Ma, S.; Li, T.; Liu, X.; Zhu, J. Research progress on bio-based thermosetting resins. Polym. Int. 2016, 65, 164–173. [Google Scholar] [CrossRef]
- Ding, C.; Matharu, A.S. Recent developments on biobased curing agents: A review of their preparation and use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent development of biobased epoxy resins: A review. Polym. Plast. Technol. 2018, 57, 133–155. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of petroleum and biobased epoxy resins: A review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, L.; Tang, C. Sustainable elastomers from renewable biomass. Acc. Chem. Res. 2017, 50, 1762–1773. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103. [Google Scholar] [CrossRef]
- Zia, K.M.; Noreen, A.; Zuber, M.; Tabasum, S.; Mujahid, M. Recent developments and future prospects on bio-based polyesters derived from renewable resources: A review. Int. J. Biol. Macromol. 2016, 82, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Parthiban, A. Monomers and polymers derived from renewable or partially renewable resources. In Synthesis and Applications of Copolymers; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 101–124. ISBN 978-1-118-86016-8. [Google Scholar]
- Narayanaperumal, S.; Rivera, D.G.; Silva, R.C.; Paixão, M.W. Terpene-derived bifunctional thioureas in asymmetric organocatalysis. ChemCatChem 2013, 5, 2756–2773. [Google Scholar] [CrossRef]
- Yadav, B.K.; Gidwani, B.; Vyas, A. Rosin: Recent advances and potential applications in novel drug delivery system. J. Bioact. Compat. Polym. 2016, 31, 111–126. [Google Scholar] [CrossRef]
- Lu, Y.J.; Xu, R.S.; Zhao, Z.D.; Zhang, P.H.; Wang, M.X. Recent progress on derivation and chemical modification of rosin acids. Adv. Mater. Res. 2013, 785–786, 1111–1116. [Google Scholar] [CrossRef]
- Robinson, V.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Alan Andersen, F. Amended safety assessment of tall oil acid, sodium tallate, potassium tallate, and ammonium tallate. Int. J. Toxicol. 2009, 28, 252S–258S. [Google Scholar] [CrossRef]
- Illing, H.P.A.; Malmfors, T.; Rodenburg, L. Skin sensitization and possible groupings for ‘read across’ for rosin based substances. Regul. Toxicol. Pharm. 2009, 54, 234–241. [Google Scholar] [CrossRef]
- Botham, P.A.; Lees, D.; Illing, H.P.A.; Malmfors, T. On the skin sensitisation potential of rosin and oxidised rosin. Regul. Toxicol. Pharm. 2008, 52, 257–263. [Google Scholar] [CrossRef]
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit. Rev. Environ. Sci. Technol. 2019, 1–39. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.-P. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2014, 114, 1082–1115. [Google Scholar] [CrossRef]
- Yadav, S.K.; Schmalbach, K.M.; Kinaci, E.; Stanzione, J.F.; Palmese, G.R. Recent advances in plant-based vinyl ester resins and reactive diluents. Eur. Polym. J. 2018, 98, 199–215. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, J. Chapter 19: Bio-based epoxies and composites as environmentally friendly alterinative materials. In Thermosets, 2nd ed.; Elsevier: Amsterdam, Holland, 2018; ISBN 978-0-08-101021-1. [Google Scholar]
- Global Rosin Market-World Rosin Market Size, Trends, Analysis and Segment Forecasts to 2022-Rosin Industry Research, Outlook, Application, Product, Share, Growth, Key Opportunities, Dynamics, Analysis, Rosin Report-Grand View Research, Inc. Available online: https://www.grandviewresearch.com/industry-analysis/rosin-market (accessed on 29 May 2018).
- La Colophane et Son Avenir Dans le Domaine du Sudoe. Available online: https://docplayer.net/21677781-Chemical-division-what-is-rosin-rosin-rosin-is-a-solid-form-of-natural-solid-form-of-natural-resin-obtained-from-conifers-and-mainly-pine-trees.html (accessed on 7 January 2019).
- Chen, G.F. Developments in the field of rosin chemistry and its implications in coatings. Prog. Org. Coat. 1992, 20, 139–167. [Google Scholar] [CrossRef]
- Mckeon, L.; Regan, F.; Burns, B.; Leonard, R. Determination of resin acid composition in rosin samples using cyclodextrin-modified capillary electrophoresis. J. Sep. Sci. 2014, 37, 2791–2796. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Li, S.; Li, J.; Liang, T. Micro-distribution and fixation of a rosin-based micronized-copper preservative in poplar wood. Int. Biodeter. Biodegr. 2013, 83, 63–70. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Li, S.; Li, J. The combined effects of copper sulfate and rosin sizing agent treatment on some physical and mechanical properties of poplar wood. Constr. Build. Mater. 2013, 40, 33–39. [Google Scholar] [CrossRef]
- Hien, N.T.T.; Li, J.; Li, S. Effects of water-borne rosin on the fixation and decay resistance of copper-based preservative treated wood. Bioresources 2012, 7, 3573–3584. [Google Scholar]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Ruisi, F. Nanocomposites based on esterified colophony and halloysite clay nanotubes as consolidants for waterlogged archaeological woods. Cellulose 2017, 24, 3367–3376. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A.; Malucelli, G. Thermal shielding performances of nano-structured intumescent coatings containing organo-modified layered double hydroxides. Prog. Org. Coat. 2015, 78, 504–510. [Google Scholar] [CrossRef]
- Gaillard, Y.; Girard, M.; Monge, G.; Burr, A.; Ceretti, E.D.; Felder, E. Superplastic behavior of rosin/beeswax blends at room temperature. J. Appl. Polym. Sci. 2013, 128, 2713–2719. [Google Scholar] [CrossRef]
- Bellotti, N.; del Amo, B.; Romagnoli, R. Quaternary ammonium “tannate” for antifouling coatings. Ind. Eng. Chem. Res. 2012, 51, 16626–16632. [Google Scholar] [CrossRef]
- Pinori, E.; Berglin, M.; Brive, L.M.; Hulander, M.; Dahlström, M.; Elwing, H. Multi-seasonal barnacle (Balanus improvisus) protection achieved by trace amounts of a macrocyclic lactone (ivermectin) included in rosin-based coatings. Biofouling 2011, 27, 941–953. [Google Scholar] [CrossRef]
- Gutierrez, J.; Tercjak, A. Natural gum rosin thin films nanopatterned by poly(styrene)-block-poly(4-vinylpiridine) block copolymer. RSC Adv. 2014, 4, 32024–32030. [Google Scholar] [CrossRef]
- Narayanan, M.; Loganathan, S.; Valapa, R.B.; Thomas, S.; Varghese, T.O. UV protective poly(lactic acid)/rosin films for sustainable packaging. Int. J. Biol. Macromol. 2017, 99, 37–45. [Google Scholar] [CrossRef]
- Yousaf, B.; Liu, G.; Abbas, Q.; Wang, R.; Ullah, H.; Mian, M.M.; Amina; Rashid, A. Enhanced removal of hexavalent chromium from aqueous media using a highly stable and magnetically separable rosin-biochar-coated TiO2@C nanocomposite. RSC Adv. 2018, 8, 25983–25996. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Zhang, G.; Qian, P.Y. Environmentally friendly antifouling coatings based on biodegradable polymer and natural antifoulant. ACS Sustain. Chem. Eng. 2017, 5, 6304–6309. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Wang, K.; Li, J.; Zhang, S.; Xia, C.; Shi, S.Q.; Cai, L. Improvement of water resistance, dimensional stability, and mechanical properties of poplar wood by rosin impregnation. Eur. J. Wood Prod. 2016, 74, 177–184. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, B.-R.; Oh, E.-S. Application of a bio-derivative, rosin, as a binder additive for lithium titanium oxide electrodes in lithium-ion batteries. J. Power Sources 2015, 273, 608–612. [Google Scholar] [CrossRef]
- Makhutov, N.A.; Ushakov, B.N.; Vasil’ev, I.E. Strength assessment and defect detection in welded pipeline seams by means of brittle tensosensitive coatings. Russ. Eng. Res. 2011, 31, 123–127. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, J.; Zhang, D.; Sun, H.; Yin, L.; Ma, L.; Chen, J.; Ma, D.; Cheng, H.-M.; Ren, W. Rosin-enabled ultraclean and damage-free transfer of graphene for large-area flexible organic light-emitting diodes. Nat. Commun. 2017, 8, 14560. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Bian, P.; Xue, Y.; Qian, X.; Yu, H.; Chen, W.; Hu, X.; Wang, P.; Wu, D.; Duan, Q.; et al. Combination of microsized mineral particles and rosin as a basis for converting cellulosic fibers into “sticky” superhydrophobic paper. Carbohydr. Polym. 2017, 174, 95–102. [Google Scholar] [CrossRef]
- Singh, V.; Joshi, S.; Malviya, T. Carboxymethyl cellulose-rosin gum hybrid nanoparticles: An efficient drug carrier. Int. J. Biol. Macromol. 2018, 112, 390–398. [Google Scholar] [CrossRef]
- Varshosaz, J.; Javanmard, S.H.; Soghrati, S.; Behdadfar, B. Magnetic chondroitin targeted nanoparticles for dual targeting of montelukast in prevention of in-stent restenosis. RSC Adv. 2016, 6, 12337–12347. [Google Scholar] [CrossRef]
- Pomin, S.P.; Lima, I.A.d.; Pezarini, R.R.; Cavalcanti, O.A. Evaluation of rosin gum and Eudragit® RS PO as a functional film coating material. AAPS PharmSciTech 2017, 18, 2854–2861. [Google Scholar] [CrossRef]
- Moustafa, H.; El Kissi, N.; Abou-Kandil, A.I.; Abdel-Aziz, M.S.; Dufresne, A. PLA/PBAT bionanocomposites with antimicrobial natural rosin for green packaging. ACS Appl. Mater. Interfaces 2017, 9, 20132–20141. [Google Scholar] [CrossRef]
- Nirmala, R.; Woo-il, B.; Navamathavan, R.; Kim, H.Y.; Park, S.-J. Preparation and characterizations of rosin based thin films and fibers. J. Nanosci. Nanotechnol. 2015, 15, 4653–4659. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, R.; Woo-il, B.; Navamathavan, R.; Kalpana, D.; Lee, Y.S.; Kim, H.Y. Influence of antimicrobial additives on the formation of rosin nanofibers via electrospinning. Colloid Surf. B 2013, 104, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, R.; Baek, W.; Navamathavan, R.; Kim, T.W.; Kalpana, D.; Park, M.; Kim, H.Y.; Park, S.-J. Bactericidal efficacy of electrospun rosin/poly(ε-caprolactone) nanofibers. Macromol. Res. 2014, 22, 139–145. [Google Scholar] [CrossRef]
- Nirmala, R.; Kalpana, D.; Navamathavan, R.; Park, M.; Kim, H.Y.; Park, S.-J. Antimicrobial activity of electrospun polyurethane nanofibers containing composite materials. Korean J. Chem. Eng. 2014, 31, 855–860. [Google Scholar] [CrossRef]
- Bayer, I.S.; Tiwari, M.K.; Megaridis, C.M. Biocompatible poly(vinylidene fluoride)/cyanoacrylate composite coatings with tunable hydrophobicity and bonding strength. Appl. Phys. Lett. 2008, 93, 173902. [Google Scholar] [CrossRef]
- Sharma, L.; Singh, C. Composite film developed from the blends of sesame protein isolate and gum rosin and their properties thereof. Polym. Compos. 2018, 39, 1480–1487. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.K.; Lee, D.-S.; Yoo, J.-E.; Shin, M.-S.; Yamabe, N.; Kim, S.-N.; Lee, S.; Kim, K.H.; Lee, H.-J.; et al. Abietic acid isolated from pine resin (Resina Pini) enhances angiogenesis in HUVECs and accelerates cutaneous wound healing in mice. J. Ethnopharmacol. 2017, 203, 279–287. [Google Scholar] [CrossRef]
- Sakamoto, K.; Suzuki, Y.; Yamamura, H.; Ohya, S.; Muraki, K.; Imaizumi, Y. Molecular mechanisms underlying pimaric acid-induced modulation of voltage-gated K+ channels. J. Pharmacol. Sci. 2017, 133, 223–231. [Google Scholar] [CrossRef]
- Kim, N.-H.; Son, Y.; Jeong, S.-O.; Hur, J.M.; Bang, H.S.; Lee, K.-N.; Kim, E.-C.; Chung, H.-T.; Pae, H.-O. Tetrahydroabietic acid, a reduced abietic acid, inhibits the production of inflammatory mediators in raw264.7 macrophages activated with lipopolysaccharide. J. Clin. Biochem. Nutr. 2010, 46, 119–125. [Google Scholar] [CrossRef][Green Version]
- Filippov, L.O.; Foucaud, Y.; Filippova, I.V.; Badawi, M. New reagent formulations for selective flotation of scheelite from a skarn ore with complex calcium minerals gangue. Miner. Eng. 2018, 123, 85–94. [Google Scholar] [CrossRef]
- Peng, H.; Shan, X.; Kang, J.; Ling, X.; Wang, D. Influence of rotary disk configurations on droplets characteristics in molten slag granulation for waste heat recovery. Appl. Therm. Eng. 2018, 135, 269–279. [Google Scholar] [CrossRef]
- Huang, F.; Li, H.; Yi, Z.; Wang, Z.; Xie, Y. The rheological properties of self-compacting concrete containing superplasticizer and air-entraining agent. Constr. Build. Mater. 2018, 166, 833–838. [Google Scholar] [CrossRef]
- Patel, V.K.; Rawat, N. Physico-mechanical properties of sustainable Sagwan-teak wood flour/polyester composites with/without gum rosin. Sustain. Mater. Technol. 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Ge, S.; Yang, J.; Chang, R.; Liang, C.; Xiong, L.; Zhao, M.; Li, M.; Sun, Q. Synthesis and study the properties of StNPs/gum nanoparticles for salvianolic acid B-oral delivery system. Food Chem. 2017, 229, 111–119. [Google Scholar] [CrossRef]
- Domene-López, D.; Guillén, M.M.; Martin-Gullon, I.; García-Quesada, J.C.; Montalbán, M.G. Study of the behavior of biodegradable starch/polyvinyl alcohol/rosin blends. Carbohydr. Polym. 2018, 202, 299–305. [Google Scholar] [CrossRef]
- Kato, H.; Nakamura, A.; Matsubara, K. Dynamics and role of rosin acid molecules for preparation of well-dispersed CaCO3 colloidal suspensions. J. Nanopart. Res. 2012, 14, 950. [Google Scholar] [CrossRef]
- Sifontes, Á.B.; Gutierrez, B.; Mónaco, A.; Yanez, A.; Díaz, Y.; Méndez, F.J.; Llovera, L.; Cañizales, E.; Brito, J.L. Preparation of functionalized porous nano-γ-Al2O3 powders employing colophony extract. Biotechnol. Rep. 2014, 4, 21–29. [Google Scholar] [CrossRef]
- Huang, X.; Qian, X.; Li, J.; Lou, S.; Shen, J. Starch/rosin complexes for improving the interaction of mineral filler particles with cellulosic fibers. Carbohydr. Polym. 2015, 117, 78–82. [Google Scholar] [CrossRef]
- Soltes, J.; Zinkel, D.F. Chemistry of rosin. In Naval Stores: Production, Chemistry, Utilization; Pulp Chemicals Association: New York, NY, USA, 1989; ISBN 978-0-685-30903-2. [Google Scholar]
- Nakamura, Y.; Sakai, Y.; Imamura, K.; Ito, K.; Fujii, S.; Urahama, Y. Effects of the compatibility of a polyacrylic block copolymer/tackifier blend on the phase structure and tack of a pressure-sensitive adhesive. J. Appl. Polym. Sci. 2012, 123, 2883–2893. [Google Scholar] [CrossRef]
- Zhang, M.; Luo, Z.; Zhang, J.; Chen, S.; Zhou, Y. Effects of a novel phosphorus–nitrogen flame retardant on rosin-based rigid polyurethane foams. Polym. Degrad. Stab. 2015, 120, 427–434. [Google Scholar] [CrossRef]
- Ding, K.; John, A.; Shin, J.; Lee, Y.; Quinn, T.; Tolman, W.B.; Hillmyer, M.A. High-performance pressure-sensitive adhesives from renewable triblock copolymers. Biomacromolecules 2015, 16, 2537–2539. [Google Scholar] [CrossRef]
- Ahn, B.K.; Sung, J.; Kim, N.; Kraft, S.; Sun, X.S. UV-curable pressure-sensitive adhesives derived from functionalized soybean oils and rosin ester. Polym. Int. 2013, 62, 1293–1301. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Jiménez-López, M.; Aldas, M.; López, J. Combined effect of linseed oil and gum rosin as natural additives for PVC. Ind. Crop Prod. 2017, 99, 196–204. [Google Scholar] [CrossRef]
- Guo, P.; Danish, M.; Du, P.; Kong, Z.; Guan, R. Viscoelastic and adhesive properties of polystyrene-hydrogenated (3,4-polyisoprene and 1,4-polyisoprene)-polystyrene and polymethyl methacrylate-polybutyl acrylate-polymethyl methacrylate-based HMPSA. J. Adhes. Sci. Technol. 2014, 28, 417–433. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Kim, Y.-W.; Shin, J. Preparation and characterization of a renewable pressure-sensitive adhesive system derived from ε-decalactone, l-lactide, epoxidized soybean oil, and rosin ester. ACS Sustain. Chem. Eng. 2015, 3, 2309–2320. [Google Scholar] [CrossRef]
- Zhang, K.; Shang, S.; Sun, P.; Shen, M.; Wang, D. Hydrogenated rosin ester latexes/waterborne polyacrylate blends for pressure-sensitive adhesives. J. Appl. Polym. Sci. 2016, 133, 42965. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, G.; Zhou, Y.; Hu, L.; Feng, G. Thermal performances and fire behaviors of rosin-based rigid polyurethane foam nanocomposites. J. Therm. Anal. Calorim. 2015, 119, 411–424. [Google Scholar] [CrossRef]
- Gao, L.; Zheng, G.; Zhou, Y.; Hu, L.; Feng, G.; Zhang, M. Synergistic effect of expandable graphite, diethyl ethylphosphonate and organically-modified layered double hydroxide on flame retardancy and fire behavior of polyisocyanurate-polyurethane foam nanocomposite. Polym. Degrad. Stab. 2014, 101, 92–101. [Google Scholar] [CrossRef]
- Lee, H.J.; Choi, I.; Kim, K.; Kim, H.S.; Choi, W.M.; Oh, E.S. Performance of various rosin-derivatives as binder additives for lithium titanium oxide anodes. J. Electroanal. Chem. 2016, 782, 241–244. [Google Scholar] [CrossRef]
- Moyano, M.A.; París, R.; Martín-Martínez, J.M. Assessment of the compatibility in hot melts by using different thermoanalytical methods. Ethylene/n-butyl acrylate (EBA) hot melts containing tackifiers of different nature. J. Therm. Anal. Calorim. 2017, 129, 1495–1503. [Google Scholar] [CrossRef]
- Moyano, M.A.; París, R.; Martín-Martínez, J.M. Changes in compatibility, tack and viscoelastic properties of ethylene n-butyl acrylate (EBA) copolymer-pentaerythritol rosin ester blend by adding microcrystalline wax, Fischer-Tropsch wax and mixture of waxes. Int. J. Adhes. Adhes. 2016, 65, 47–53. [Google Scholar] [CrossRef]
- Yamamura, K.; Shitajima, K.; Fujii, S.; Nakamura, Y.; Hamada, Y.; Hagiwara, S.; Kishi, H.; Urahama, Y.; Sasaki, M. Temperature dependence of tack and pulse NMR analysis of polystyrene block copolymer/tackifier system. J. Adhes. Sci. Technol. 2013, 27, 2727–2740. [Google Scholar] [CrossRef]
- Chu, H.-H.; Chiang, W.-L.; Chuang, K.S. Viscoelastic and adhesive properties of PMMA-b-PtBA with tackifier. Int. J. Adhes. Adhes. 2012, 38, 89–94. [Google Scholar] [CrossRef]
- Atta, A.M.; Al-Lohedan, H.A. Influence of nonionic rosin surfactants on surface activity of silica particles and stability of oil in water emulsions. J. Surfactant. Deterg. 2014, 17, 1043–1053. [Google Scholar] [CrossRef]
- Liu, X.; Xin, W.; Zhang, J. Rosin-based acid anhydrides as alternatives to petrochemical curing agents. Green Chem. 2009, 11, 1018–1025. [Google Scholar] [CrossRef]
- Liu, X.Q.; Huang, W.; Jiang, Y.H.; Zhu, J.; Zhang, C.Z. Preparation of a bio-based epoxy with comparable properties to those of petroleum-based counterparts. Express Polym. Lett. 2012, 6, 293–298. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J. High-performance biobased epoxy derived from rosin. Polym. Int. 2010, 59, 607–609. [Google Scholar] [CrossRef]
- Li, P.; Wang, T.; Lei, F.; Tang, P.; Tan, X.; Liu, Z.; Shen, L. Rosin-based molecularly imprinted polymers as the stationary phase in high-performance liquid chromatography for selective separation of berberine hydrochloride. Polym. Int. 2014, 63, 1699–1706. [Google Scholar] [CrossRef]
- Yu, C.L.; Zhang, F.A.; Gong, Q.H. Preparation of maleic rosin-based macromonomer and copolymerization with styrene and methyl methacrylate. Adv. Mater. Res. 2011, 236–238, 728–731. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Li, J.; Lei, F.; Su, X.; Liu, M.; Li, P.; Tan, X. Synthesis and characterization of molecularly imprinted polymers with modified rosin as a cross-linker and selective SPE-HPLC detection of basic orange II in foods. Anal. Methods 2014, 6, 6397–6406. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, X.; Zhang, R.; Zhu, J.; Jiang, Y. Synthesis and properties of full bio-based thermosetting resins from rosin acid and soybean oil: The role of rosin acid derivatives. Green Chem. 2013, 15, 1300–1310. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Z.; Bi, L.; Chen, Y.; Wang, J.; Xu, S. Synthesis of a multifunctional hard monomer from rosin: the relationship of allyl structure in maleopimarate and UV-curing property. Sci. Rep. 2018, 8, 2399. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Liu, Y.; Chen, Y.; Wang, C.; Tang, C.; Chu, F. Synthesis and characterization of a novel rosin-based monomer: Free-radical polymerization and epoxy curing. Green Mater. 2013, 1, 105–113. [Google Scholar] [CrossRef]
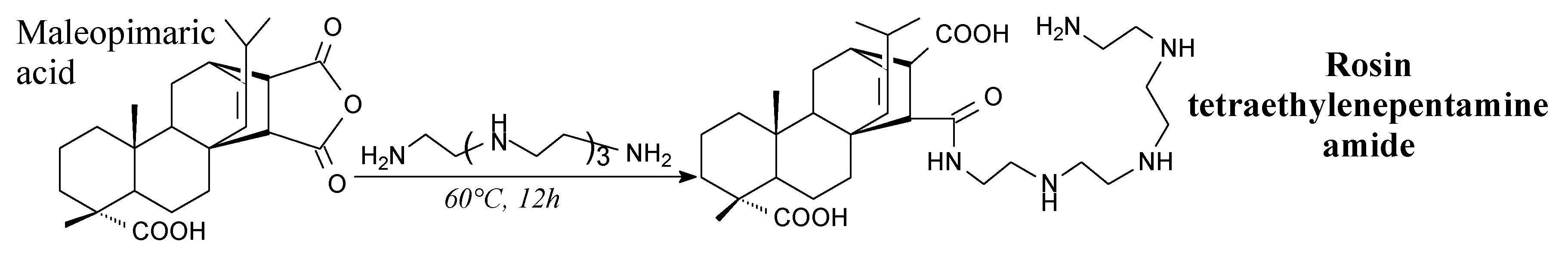
- Si, H.; Liu, H.; Shang, S.; Song, J.; Liao, S.; Wang, D.; Song, Z. Maleopimaric acid-modified two-component waterborne polyurethane for coating applications. J. Appl. Polym. Sci. 2016, 133, 43292. [Google Scholar] [CrossRef]
- Li, R.; Zhang, P.; Liu, T.; Muhunthan, B.; Xin, J.; Zhang, J. Use of hempseed-oil-derived polyacid and rosin-derived anhydride acid as cocuring agents for epoxy materials. ACS Sustain. Chem. Eng. 2018, 6, 4016–4025. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Wei, J.; Tong, J.; Yi, X. Curing kinetics and mechanical properties of bio-based composite using rosin-sourced anhydrides as curing agent for hot-melt prepreg. Sci. China Technol. Sci. 2017, 60, 1318–1331. [Google Scholar] [CrossRef]
- Liu, X.; Xin, W.; Zhang, J. Rosin-derived imide-diacids as epoxy curing agents for enhanced performance. Bioresour. Technol. 2010, 101, 2520–2524. [Google Scholar] [CrossRef]
- Mustata, F.; Tudorachi, N.; Bicu, I. Thermosetting resins obtained via sequential photo and thermal crosslinking of epoxy resins. Curing kinetics, thermal properties and morphology. Compos. Part B 2013, 55, 470–478. [Google Scholar] [CrossRef]
- Li, S.; Zou, T.; Liu, X.; Tao, M. Synthesis and characterization of benzoxazine monomers from rosin and their thermal polymerization. Des. Monomers Polym. 2014, 17, 40–46. [Google Scholar] [CrossRef][Green Version]
- Wang, H.; Liu, X.; Liu, B.; Zhang, J.; Xian, M. Synthesis of rosin-based flexible anhydride-type curing agents and properties of the cured epoxy. Polym. Int. 2009, 58, 1435–1441. [Google Scholar] [CrossRef]
- Jaswal, S.; Gaur, B. Structure-property correlation study of bio-based multifunctional vinyl ester resin in presence of methacrylated lignin model compounds. Polym. Sci. Ser. B 2015, 57, 417–433. [Google Scholar] [CrossRef]
- Qin, J.; Liu, H.; Zhang, P.; Wolcott, M.; Zhang, J. Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym. Int. 2014, 63, 760–765. [Google Scholar] [CrossRef]
- Zhai, Z.; Yan, X.; Song, Z.; Shang, S.; Rao, X. Annular and threadlike wormlike micelles formed by a bio-based surfactant containing an extremely large hydrophobic group. Soft Matter 2018, 14, 499–507. [Google Scholar] [CrossRef]
- Lin, H.; Yang, M.; Tian, C.; Han, C.; Song, J.; Duan, J.; Jiang, J. Design of diversified self-assembly systems based on a natural rosin-based tertiary amine for doxorubicin delivery and excellent emulsification. Colloid Surf. B 2018, 165, 191–198. [Google Scholar] [CrossRef]
- Zhai, Z.; Yan, X.; Xu, J.; Song, Z.; Shang, S.; Rao, X. Phase behavior and aggregation in a catanionic system dominated by an anionic surfactant containing a large rigid group. Chem. Eur. J. 2018, 24, 9033–9040. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Shang, T.; Zhu, J. Synthesis, characterization and application of a dispersant based on rosin for coal-water slurry. Int. J. Min. Sci. Technol. 2014, 24, 695–699. [Google Scholar] [CrossRef]
- Atta, M.A.; El-Mahdy, A.G.; Dyab, K.F.A.; Allohedan, A.H. Application of highly surface active cationic surfactants based on rosin as corrosion inhibitor for tubing steel during acidization of petroleum oil and gas wells. Int. J. Electrochem. Sci. 2013, 8, 9629–9643. [Google Scholar]
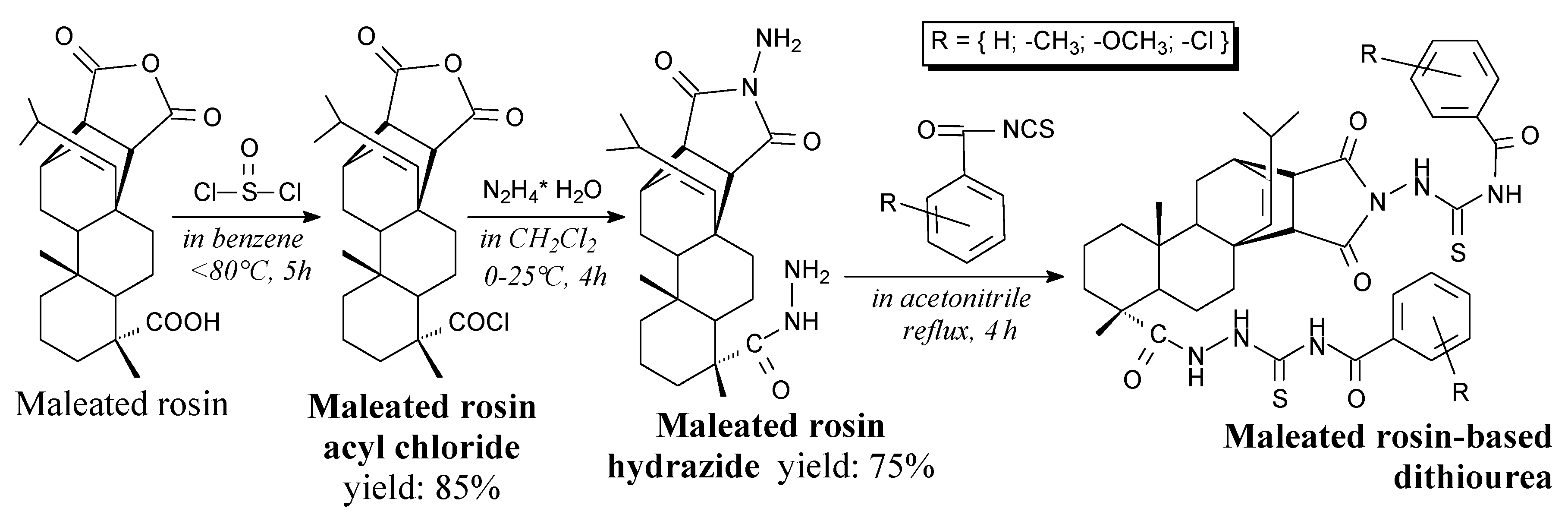
- Lin, G.-S.; Dong, S.-Q.; Duan, W.-G.; Cen, B.; Xu, X.-T.; Yang, Z.-Q. Synthesis and biological activities of maleated rosin-based dithiourea compounds. Holzforschung 2013, 68, 549–554. [Google Scholar] [CrossRef]
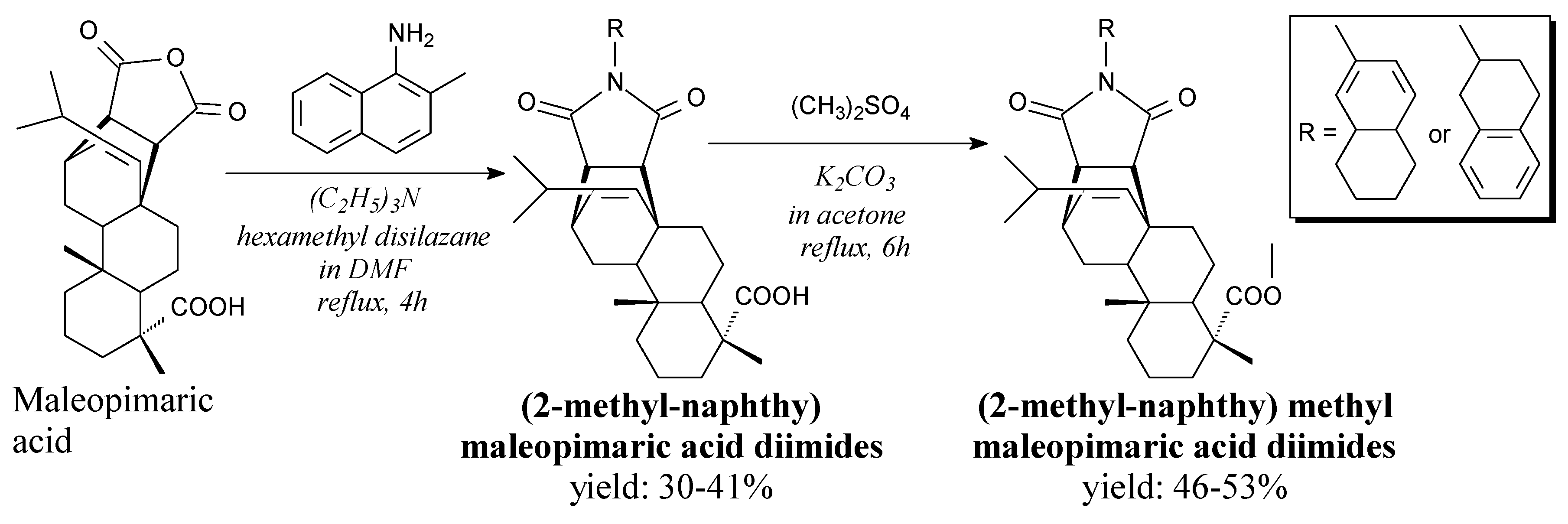
- Yao, G.; Ye, M.; Huang, R.; Li, Y.; Zhu, Y.; Pan, Y.; Liao, Z.-X.; Wang, H. Synthesis and antitumor activity evaluation of maleopimaric acid N-aryl imide atropisomers. Bioorg. Med. Chem. Lett. 2013, 23, 6755–6758. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Zhou, G. Synthesis of rosin-based imidoamine-type curing agents and curing behavior with epoxy resin. Polym. Int. 2011, 60, 557–563. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, W.; Song, J.; Zhang, Y.; Jiang, X.; Wang, R. Anticancer effects of a novel class rosin-derivatives with different mechanisms. Bioorg. Med. Chem. Lett. 2013, 23, 3868–3872. [Google Scholar] [CrossRef]
- Xu, X.; Song, Z.; Shang, S.; Cui, S.; Rao, X. Synthesis and properties of novel rosin-based water-borne polyurethane. Polym. Int. 2011, 60, 1521–1526. [Google Scholar] [CrossRef]
- Si, H.; Liu, H.; Shang, S.; Song, J.; Liao, S.; Wang, D.; Song, Z. Preparation and properties of maleopimaric acid-based polyester polyol dispersion for two-component waterborne polyurethane coating. Prog. Org. Coat. 2016, 90, 309–316. [Google Scholar] [CrossRef]
- Xu, X.; Shang, S.; Song, Z.; Cui, S.; Wang, H.; Wang, D. Preparation and characterization of rosin-based waterborne polyurethane from maleopimaric acid polyester polyol. Bioresources 2011, 6, 2460–2470. [Google Scholar]
- Liu, H.; Cui, S.; Shang, S.; Wang, D.; Song, J. Properties of rosin-based waterborne polyurethanes/cellulose nanocrystals composites. Carbohydr. Polym. 2013, 96, 510–515. [Google Scholar] [CrossRef]
- Wu, Q.; Yao, G.; Zhang, Y.; Wang, H.; Yang, L.; Zhu, Y.; Pan, Y. In situ synthesis of rosin derived chiral derivatizing agents for 31P NMR assays of amine and alcohol enantiomers. Chem. Res. Chin. Univ. 2013, 29, 894–899. [Google Scholar] [CrossRef]
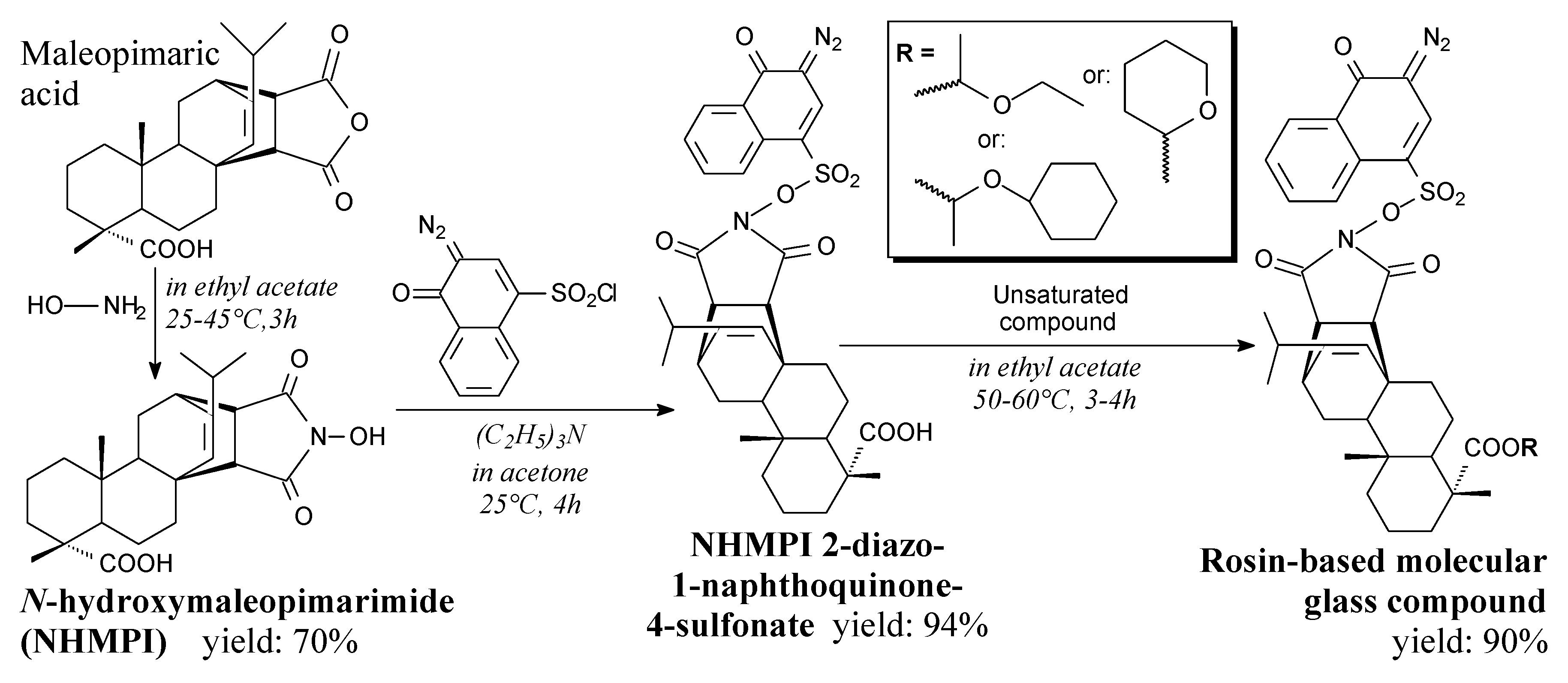
- Yu, J.; Xu, N.; Liu, Z.; Wang, L. Novel one-component positive-tone chemically amplified i-line molecular glass photoresists. ACS Appl. Mater. Interfaces 2012, 4, 2591–2596. [Google Scholar] [CrossRef]
- Tan, W.-X.; Lin, Z.-T.; Bu, H.-T.; Tian, Y.; Jiang, G.-B. Nano-micelles based on a rosin derivative as potent sorbents and sinking agents with high absorption capabilities for the removal of metal ions. RSC Adv. 2012, 2, 7279–7289. [Google Scholar] [CrossRef]
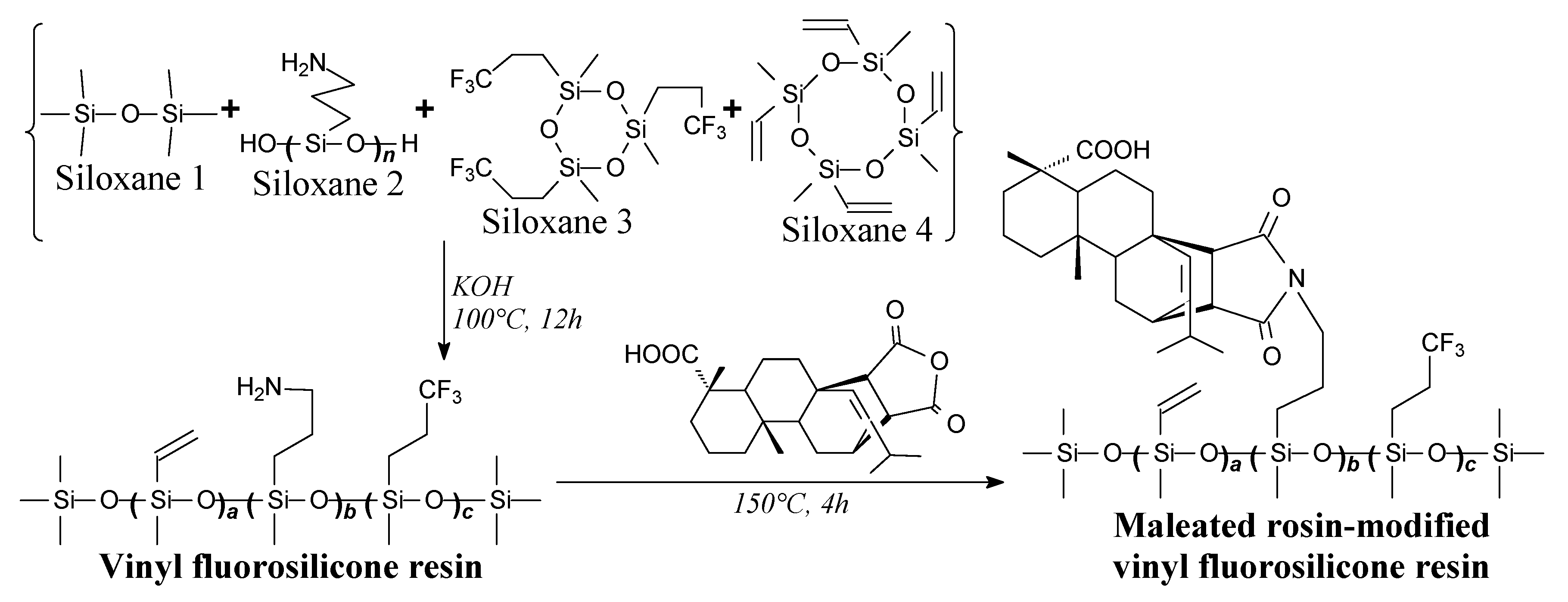
- Xu, T.; Liu, H.; Song, J.; Shang, S.; Song, Z.; Zou, K.; Yang, C. Synthesis and characterization of maleated rosin-modified fluorosilicone resin and its fluorosilicone rubber. J. Appl. Polym. Sci. 2015, 132, 41888. [Google Scholar] [CrossRef]
- Ha, Y.B.; Jin, M.Y.; Oh, S.S.; Ryu, D.H. Synthesis of an environmentally friendly phenol-free resin for printing ink. Bull. Korean Chem. Soc. 2012, 33, 3413–3416. [Google Scholar] [CrossRef]
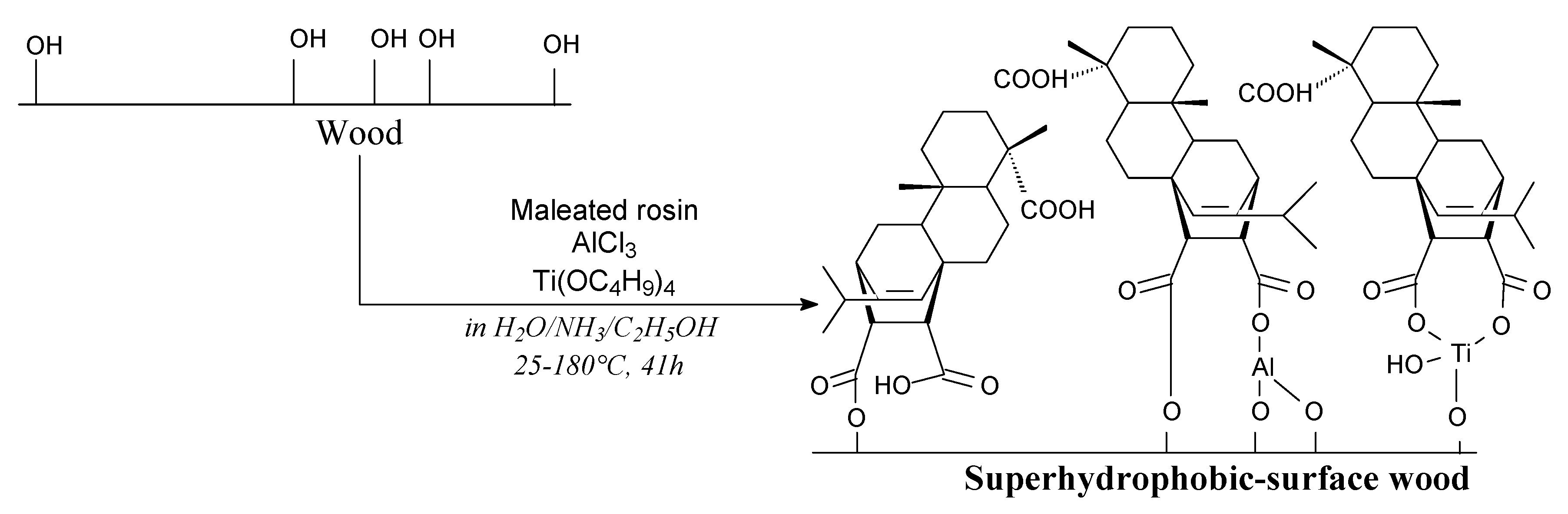
- Yang, M.; Chen, X.; Li, J.; Lin, H.; Zhang, S.; Han, C. Preparation of wood with better water-resistance properties by a one-step impregnation of maleic rosin. J. Adhes. Sci. Technol. 2018, 1–13. [Google Scholar] [CrossRef]
- Yang, M.; Chen, X.; Lin, H.; Han, C.; Zhang, S. A simple fabrication of superhydrophobic wood surface by natural rosin based compound via impregnation at room temperature. Eur. J. Wood Prod. 2018, 76, 1417–1425. [Google Scholar] [CrossRef]
- Li, H.; Lin, R.; He, J.; Long, H.; Liao, W.; Chen, Q. Effect of pretreatment on the enzymatic synthesis of rosin acid starch. New J. Chem. 2016, 40, 2856–2862. [Google Scholar] [CrossRef]
- Tudorachi, N.; Mustaţă, F.; Bicu, I. Thermal decomposition of some Diels–Alder adducts of resin acids: study of kinetics process. J. Anal. Appl. Pyrol. 2012, 98, 106–114. [Google Scholar] [CrossRef]
- Deng, L.; Ha, C.; Sun, C.; Zhou, B.; Yu, J.; Shen, M.; Mo, J. Properties of bio-based epoxy resins from rosin with different flexible chains. Ind. Eng. Chem. Res. 2013, 52, 13233–13240. [Google Scholar] [CrossRef]
- Mandal, M.; Borgohain, P.; Begum, P.; Deka, R.C.; Maji, T.K. Property enhancement and DFT study of wood polymer composites using rosin derivatives as co-monomers. New J. Chem. 2018, 42, 2260–2269. [Google Scholar] [CrossRef]
- Yu, C.; Wang, X.; Chen, C.; Zhang, F. Preparation of polystyrene microspheres using rosin–acrylic acid diester as a cross-linking agent. Ind. Eng. Chem. Res. 2014, 53, 2244–2250. [Google Scholar] [CrossRef]
- Yan, X.; Zhai, Z.; Song, Z.; Shang, S.; Rao, X. Synthesis and properties of polyester-based polymeric surfactants from diterpenic rosin. Ind. Crop. Prod. 2017, 108, 371–378. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Jiang, Y.; Ma, S.; Zhu, J. Bio-based shape memory epoxy resin synthesized from rosin acid. Iran. Polym. J. 2016, 25, 957–965. [Google Scholar] [CrossRef]
- Deng, L.; Shen, M.; Yu, J.; Wu, K.; Ha, C. Preparation, characterization, and flame retardancy of novel rosin-based siloxane epoxy resins. Ind. Eng. Chem. Res. 2012, 51, 8178–8184. [Google Scholar] [CrossRef]
- Lin, H.X.; Yang, M.S.; Li, J.; Chen, X.Y.; Jiang, J.X.; Han, C.R. A novel bola-type rosin-based functional surfactant and its synergistic effect with natural surfactant saponin. J. Surfactant. Deterg. 2017, 20, 1205–1212. [Google Scholar] [CrossRef]
- Gao, Y.; Li, L.; Chen, H.; Li, J.; Song, Z.; Shang, S.; Song, J.; Wang, Z.; Xiao, G. High value-added application of rosin as a potential renewable source for the synthesis of acrylopimaric acid-based botanical herbicides. Ind. Crop. Prod. 2015, 78, 131–140. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Song, Z.; Song, J.; Shang, S.; Xiao, G.; Wang, Z.; Rao, X. Turning renewable resources into value-added products: Development of rosin-based insecticide candidates. Ind. Crop Prod. 2015, 76, 660–671. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Li, S.; Wang, J.; Liu, D. Synthesis of a rosin amide and its inhibition of wood decay fungi. Adv. Mater. Res. 2010, 113–116, 2232–2236. [Google Scholar] [CrossRef]
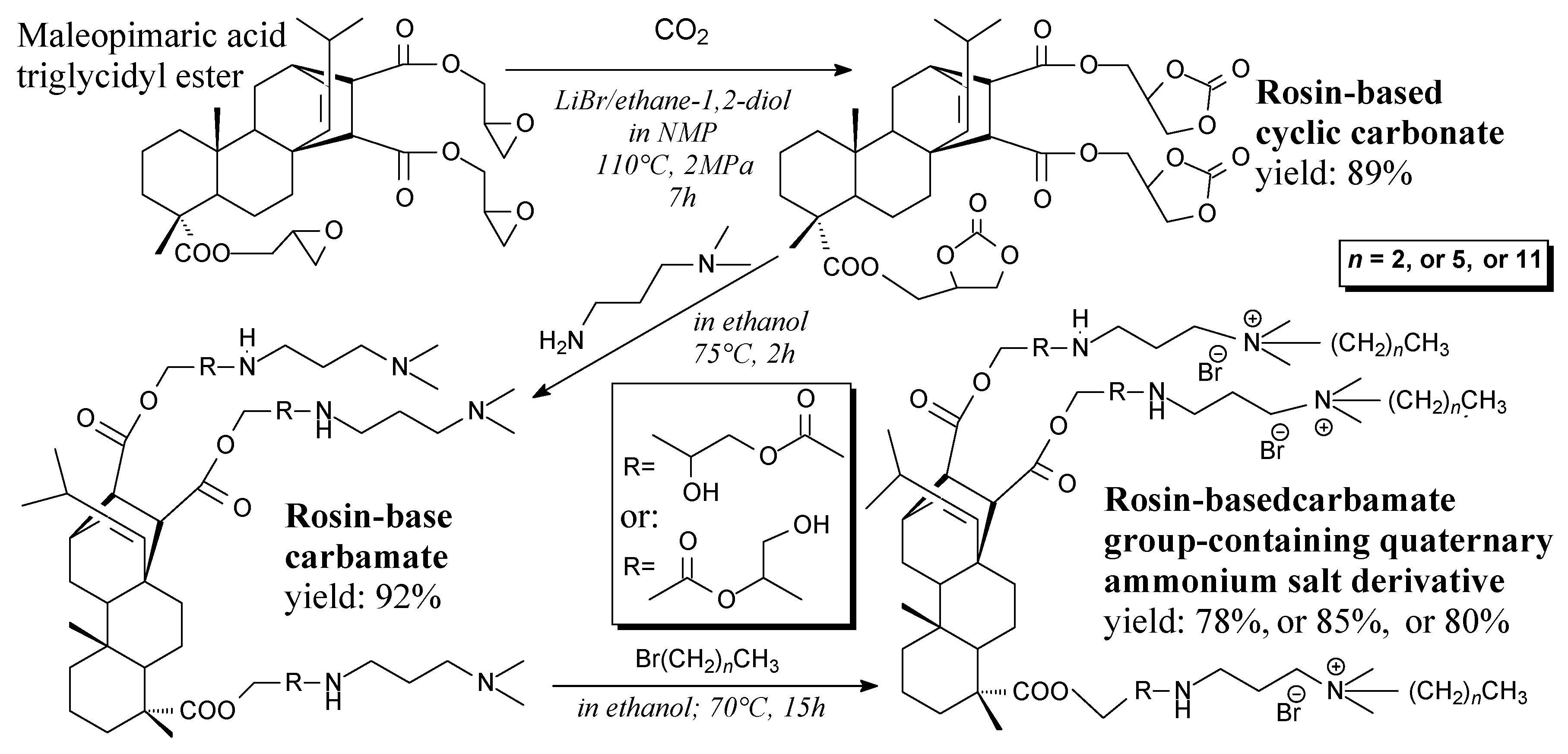
- Liu, G.; Chen, C.; Wu, G.; Kong, Z. Preparation and antimicrobial activity of rosin-based carbamate group-containing quaternary ammonium salt derivatives. Bioresources 2013, 8, 4218–4226. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Tian, B.; Liang, T.; Jin, Y. Synthesis and characterization of a rosin gemini surfactant. Mater. Sci. Forum 2011, 685, 285–290. [Google Scholar] [CrossRef]
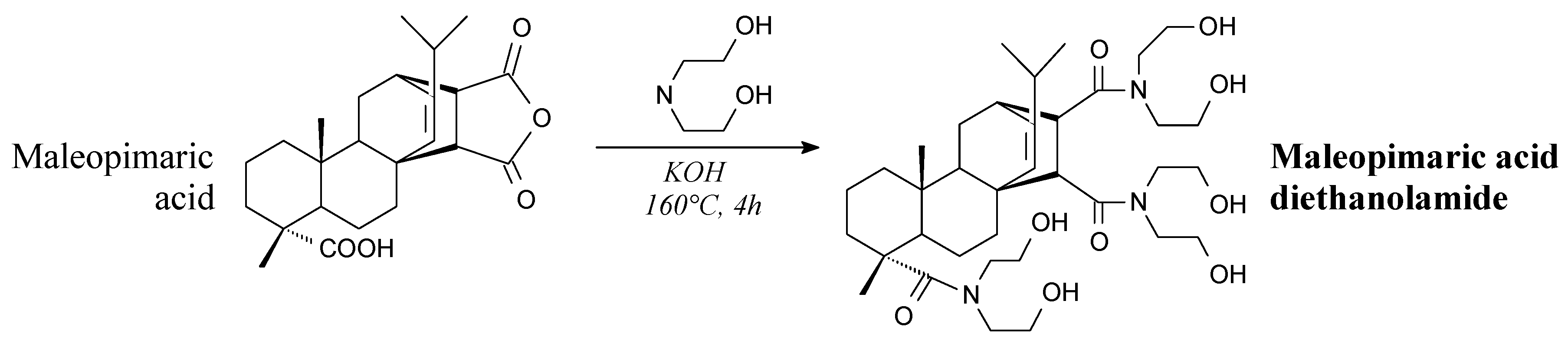
- Liang, T.; Li, S.; Li, S.; Zhang, L. Synthesis of N-(3-rosin acyloxy-2-hydroxyl) propyl-N,N diethanolamine and its anti-fungal activity. Adv. Mater. Res. 2011, 280, 124–127. [Google Scholar] [CrossRef]
- Jin, Y.; Li, S.; Liang, T.; Chen, Z. Synthesis of quaternary ammonium salt from rosin and its inhibition to some wood decay fungi. Mater. Sci. Forum 2011, 685, 291–297. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Li, S.; Chen, Z.; Tian, B.; Wang, D. Synthesis and characterization of bisN-(3-rosin acyloxy-2-hydroxyl) propyl-N,N dimethylamine. Adv. Mater. Res. 2010, 113–116, 2197–2200. [Google Scholar] [CrossRef]
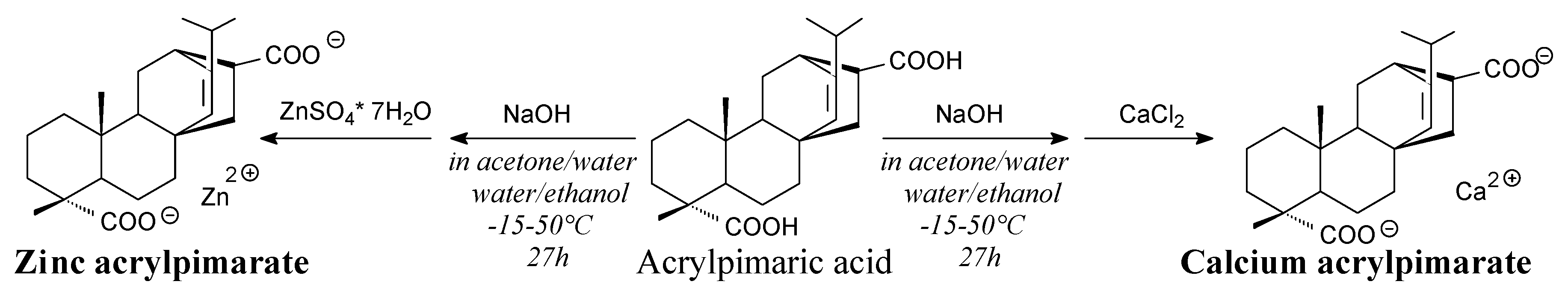
- Li, M.; Zhang, J.; Huang, K.; Li, S.; Jiang, J.; Xia, J. Mixed calcium and zinc salts of dicarboxylic acids derived from rosin and dipentene: preparation and thermal stabilization for PVC. RSC Adv. 2014, 4, 63576–63585. [Google Scholar] [CrossRef]
- Mustata, F.; Bicu, I. A novel route for synthesizing esters and polyesters from the Diels–Alder adduct of levopimaric acid and acrylic acid. Eur. Polym. J. 2010, 46, 1316–1327. [Google Scholar] [CrossRef]
- Mustata, F.R.; Tudorachi, N.; Bicu, I. Biobased epoxy matrix from diglycidyl ether of bisphenol A and epoxidized corn oil, cross-linked with Diels–Alder adduct of levopimaric acid with acrylic acid. Ind. Eng. Chem. Res. 2013, 52, 17099–17110. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.X.; Chen, X.Y.; Zhu, J.R.; Yang, M.S.; Yang, J.; Han, C.R. Self-assembled structures and excellent surface properties of a novel anionic phosphate diester surfactant derived from natural rosin acids. J. Colloid Interface Sci. 2017, 486, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wang, S. Synthesis and antimicrobial activities of novel 1H-dibenzo[a,c]carbazoles from dehydroabietic acid. Eur. J. Med. Chem. 2010, 45, 4692–4696. [Google Scholar] [CrossRef]
- Lu, C.; Yu, J.; Wang, C.; Wang, J.; Chu, F. Fabrication of UV-absorbent cellulose-rosin based thermoplastic elastomer via “graft from” ATRP. Carbohydr. Polym. 2018, 188, 128–135. [Google Scholar] [CrossRef]
- Li, W.; Xie, D.; Song, B.; Feng, L.; Pei, X.; Cui, Z. Synthesis and characterization of ordered mesoporous silica using rosin-based gemini surfactants. J. Mater. Sci. 2018, 53, 2434–2442. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Shen, M.; Shang, S.; Song, J.; Jiang, J.; Song, Z. Soybean oil-based thermoset reinforced with rosin-based monomer. Iran. Polym. J. 2018, 27, 405–411. [Google Scholar] [CrossRef]
- Zheng, Y.; Yao, K.; Lee, J.; Chandler, D.; Wang, J.; Wang, C.; Chu, F.; Tang, C. Well-defined renewable polymers derived from gum rosin. Macromolecules 2010, 43, 5922–5924. [Google Scholar] [CrossRef]
- Ding, W.; Wang, S.; Yao, K.; Ganewatta, M.S.; Tang, C.; Robertson, M.L. Physical Behavior of Triblock Copolymer Thermoplastic Elastomers Containing Sustainable Rosin-Derived Polymethacrylate End Blocks. ACS Sustain. Chem. Eng. 2017, 5, 11470–11480. [Google Scholar] [CrossRef]
- Yao, K.; Wang, J.; Zhang, W.; Lee, J.S.; Wang, C.; Chu, F.; He, X.; Tang, C. Degradable rosin-ester-caprolactone graft copolymers. Biomacromolecules 2011, 12, 2171–2177. [Google Scholar] [CrossRef]
- Yu, J.; Liu, Y.P.; Wang, C.P.; Chen, Y.; Wang, J.F.; Chu, F.X. Preparation and characterization of novel naturally renewable resin acid based monomer. Adv. Mater. Res. 2013, 712–715, 139–146. [Google Scholar] [CrossRef]
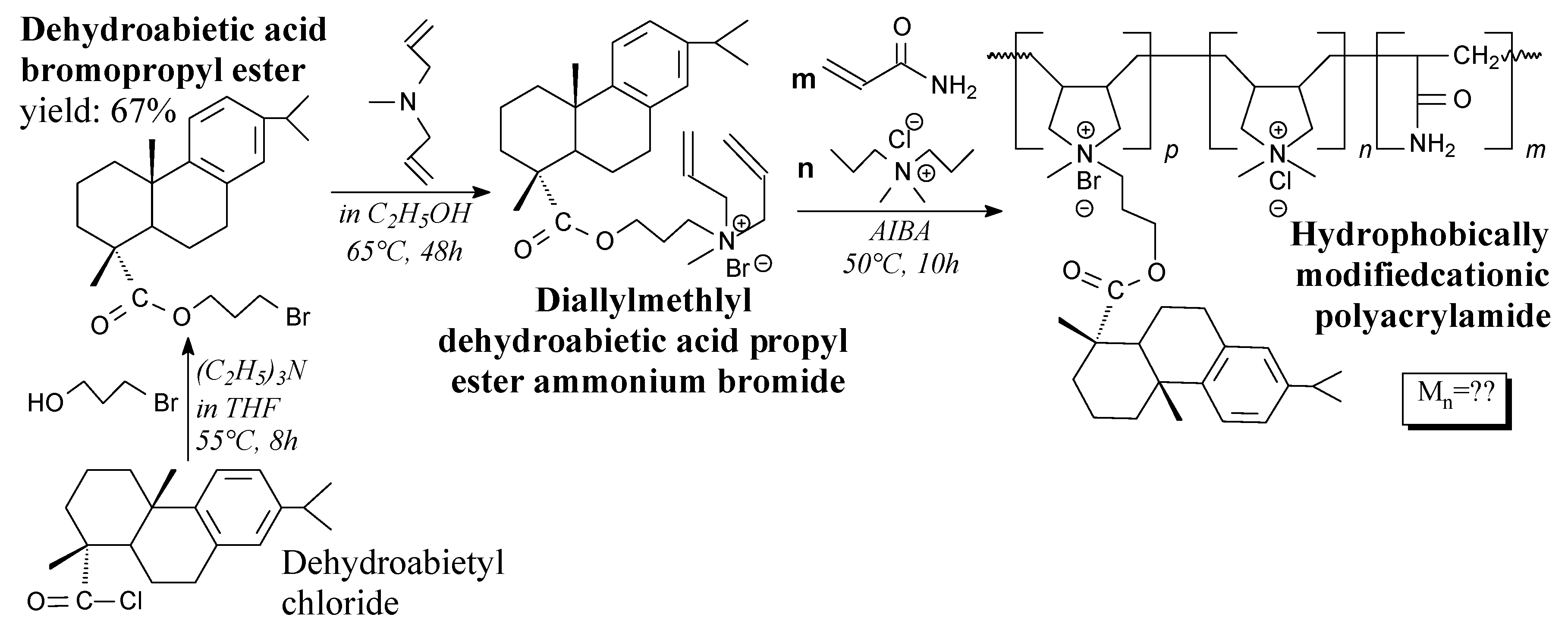
- Zhang, H.; Jiang, J.; Shang, S.; Song, Z.; Song, J. Novel, rosin-based, hydrophobically modified cationic polyacrylamide for kaolin suspension flocculation. J. Appl. Polym. Sci. 2018, 135, 46637. [Google Scholar] [CrossRef]
- Gu, W.; Qiao, C.; Wang, S.-F.; Hao, Y.; Miao, T.-T. Synthesis and biological evaluation of novel N-substituted 1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2014, 24, 328–331. [Google Scholar] [CrossRef] [PubMed]
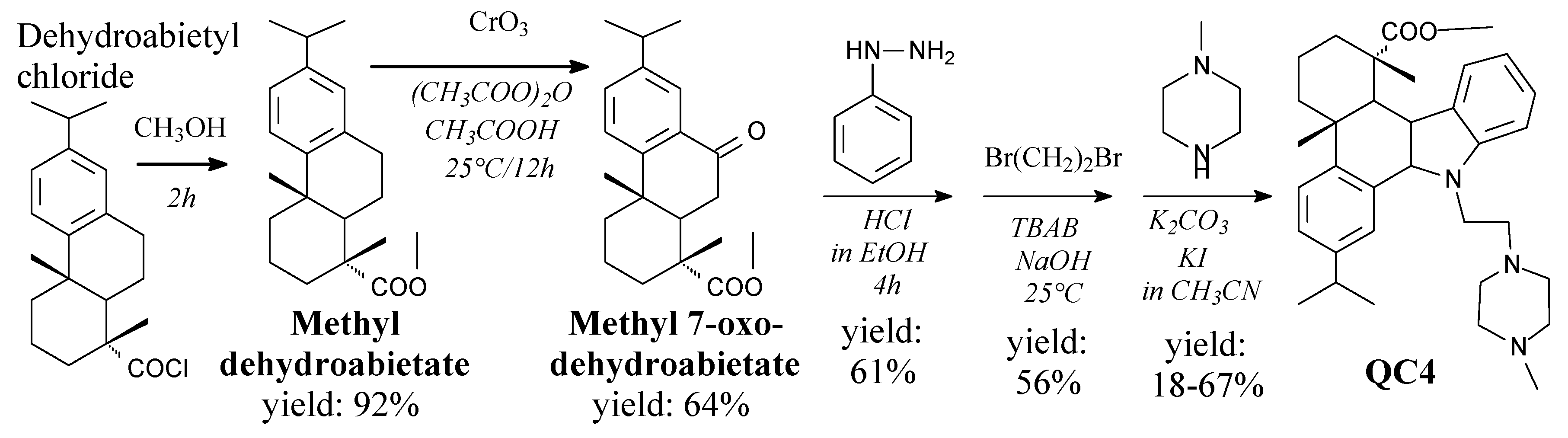
- Luo, D.; Ni, Q.; Ji, A.; Gu, W.; Wu, J.; Jiang, C. Dehydroabietic acid derivative QC4 induces gastric cancer cell death via oncosis and apoptosis. BioMed Res. Int. 2016, 2016, 2581061. [Google Scholar] [CrossRef]
- Gowda, R.; Madhunapantula, S.V.; Kuzu, O.F.; Sharma, A.; Robertson, G.P. Targeting multiple key signaling pathways in melanoma using leelamine. Mol. Cancer Ther. 2014, 13, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Gowda, R.; Sharma, A.; Robertson, G.P. Leelamine mediates cancer cell death through inhibition of intracellular cholesterol transport. Mol. Cancer Ther. 2014, 13, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Gowda, R.; Madhunapantula, S.V.; Sharma, A.; Kuzu, O.F.; Robertson, G.P. Nanolipolee-007, a novel nanoparticle-based drug containing leelamine for the treatment of melanoma. Mol. Cancer Ther. 2014, 13, 2328–2340. [Google Scholar] [CrossRef]
- Singh, K.B.; Ji, X.; Singh, S.V. Therapeutic potential of leelamine, a novel inhibitor of androgen receptor and castration-resistant prostate cancer. Mol. Cancer Ther. 2018, 17, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Liu, X.; Zhu, J.; Zhang, C.; Guo, J. Synthesis, characterization of a rosin-based epoxy monomer and its comparison with a petroleum-based counterpart. J. Macromol. Sci. A 2013, 50, 321–329. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, R.; Li, T.; Zhu, P.; Zhuang, Q. Novel Fully Biobased Benzoxazines from Rosin: Synthesis and Properties. ACS Sustainable Chem. Eng. 2017, 5, 10682–10692. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.X.; Zhao, Z.D.; Wang, Z.; Fan, G. Synthesis of ordered porous SiO2 with pores on the border between the micropore and mesopore regions using rosin-based quaternary ammonium salt. RSC Adv. 2015, 5, 11223–11228. [Google Scholar] [CrossRef]
- Song, F.; Wang, P.; Chen, S.; Wang, Z.; Fan, G. Ordered lamellar supermicroporous titania templating by rosin-derived quaternary ammonium salt. PLoS ONE 2017, 12, e0180178. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Dai, J.; Jiang, Y.; Liu, X.; Zhu, J. Synthesis of multifunctional monomers from rosin for the properties enhancement of soybean-oil based thermosets. Sci. China Technol. Sci. 2017, 60, 1332–1338. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, M.; Huang, X.; Zhang, H.; Shang, S.; Song, J. Synthesis and performance of a thermosetting resin: Acrylated epoxidized soybean oil curing with a rosin-based acrylamide. J. Appl. Polym. Sci. 2017, 134, 44545. [Google Scholar] [CrossRef]
- Huo, L.; Wang, D.; Liu, H.; Jia, P.; Gao, J. Cytoxicity, dynamic and thermal properties of bio-based rosin-epoxy resin/ castor oil polyurethane/ carbon nanotubes bio-nanocomposites. J. Biomater. Sci. Polym. Ed. 2016, 27, 1100–1114. [Google Scholar] [CrossRef] [PubMed]
- El-Ghazawy, R.A.; El-Saeed, A.M.; Al-Shafey, H.I.; Abdul-Raheim, A.-R.M.; El-Sockary, M.A. Rosin based epoxy coating: Synthesis, identification and characterization. Eur. Polym. J. 2015, 69, 403–415. [Google Scholar] [CrossRef]
- Brocas, A.-L.; Llevot, A.; Mantzaridis, C.; Cendejas, G.; Auvergne, R.; Caillol, S.; Carlotti, S.; Cramail, H. Epoxidized rosin acids as co-precursors for epoxy resins. Des. Monomers Polym. 2014, 17, 301–310. [Google Scholar] [CrossRef]
- Mantzaridis, C.; Brocas, A.-L.; Llevot, A.; Cendejas, G.; Auvergne, R.; Caillol, S.; Carlotti, S.; Cramail, H. Rosin acid oligomers as precursors of DGEBA-free epoxy resins. Green Chem. 2013, 15, 3091–3098. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, K.; Chen, X.; Wang, J.; Wang, Z.; Ploehn, H.J.; Wang, C.; Chu, F.; Tang, C. Sustainable thermoplastic elastomers derived from renewable cellulose, rosin and fatty acids. Polym. Chem. 2014, 5, 3170–3181. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, L.; Wang, Z.; Rahman, M.A.; Huang, Y.; Zhu, T.; Wang, R.; Cheng, J.; Wang, C.; Chu, F.; et al. Photoinduced metal-free atom transfer radical polymerization of biomass-based monomers. Macromolecules 2016, 49, 7709–7717. [Google Scholar] [CrossRef]
- An, S.Y.; Hong, S.H.; Tang, C.; Oh, J.K. Rosin-based block copolymer intracellular delivery nanocarriers with reduction-responsive sheddable coronas for cancer therapy. Polym. Chem. 2016, 7, 4751–4760. [Google Scholar] [CrossRef]
- Wang, J.; Yao, K.; Korich, A.L.; Li, S.; Ma, S.; Ploehn, H.J.; Iovine, P.M.; Wang, C.; Chu, F.; Tang, C. Combining renewable gum rosin and lignin: Towards hydrophobic polymer composites by controlled polymerization. J. Polym. Sci. A 2011, 49, 3728–3738. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Zheng, Y.; Yao, K.; Tang, C. Renewable rosin acid-degradable caprolactone block copolymers by atom transfer radical polymerization and ring-opening polymerization. Macromolecules 2010, 43, 8747–8754. [Google Scholar] [CrossRef]
- Wang, J.-F.; Lin, M.-T.; Wang, C.-P.; Chu, F.-X. Study on the synthesis, characterization, and kinetic of bulk polymerization of disproportionated rosin (β-acryloxyl ethyl) ester. J. Appl. Polym. Sci. 2009, 113, 3757–3765. [Google Scholar] [CrossRef]
- Duan, W.; Chen, C.; Jiang, L.; Li, G.H. Preparation and characterization of the graft copolymer of chitosan with poly[rosin-(2-acryloyloxy)ethyl ester]. Carbohydr. Polym. 2008, 73, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Nie, J.; He, Y. From rosin to high adhesive polyurethane acrylate: Synthesis and properties. Int. J. Adhes. Adhes. 2016, 66, 99–103. [Google Scholar] [CrossRef]
- Do, H.-S.; Park, J.-H.; Kim, H.-J. Synthesis and characteristics of photoactive-hydrogenated rosin epoxy methacrylate for pressure sensitive adhesives. J. Appl. Polym. Sci. 2009, 111, 1172–1176. [Google Scholar] [CrossRef]
- Yan, X.; Zhai, Z.; Song, Z.; Shang, S.; Rao, X. Synthesis of comb-like polymeric surfactants with a tricyclic rigid core and their use as dispersants in pymetrozine water suspension concentrates. RSC Adv. 2017, 7, 55741–55747. [Google Scholar] [CrossRef]
- Do, H.-S.; Park, J.-H.; Kim, H.-J. UV-curing behavior and adhesion performance of polymeric photoinitiators blended with hydrogenated rosin epoxy methacrylate for UV-crosslinkable acrylic pressure sensitive adhesives. Eur. Polym. J. 2008, 44, 3871–3882. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Z.; Chen, Y.; Wang, J.; Xu, S.; Gu, Y. Synthesis of allyl acrylpimarate by microwave irradiation and phase-transfer catalytic reaction and its UV-curing reactions as a new monomer. Prog. Org. Coat. 2017, 109, 9–21. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Jiang, Y.; Ma, S.; Zhu, J. Synthesis of epoxy curing agents containing different ring structures and properties investigation of the cured resins. J. Appl. Polym. Sci. 2016, 133, 44219. [Google Scholar] [CrossRef]
- Qin, J.; Chen, X.; Yu, J.; Wang, Y.; Tian, Y.; Wu, S. Nonisothermal crystallization kinetics of isotactic polypropylene containing nucleating agent and dispersant. J. Appl. Polym. Sci. 2010, 117, 1047–1054. [Google Scholar] [CrossRef]
- Wang, J.; Dou, Q. Crystallization behavior and optical and mechanical properties of isotactic polypropylene nucleated with rosin-based nucleating agents. Polym. Int. 2008, 57, 233–239. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, M.; Zhao, Z.; Chen, Y.; Xu, S.; Wang, J.; Bi, L. Facile synthesis of allyl resinate monomer in an aqueous solution under microwave irradiation. J. Chem. Sci. 2015, 127, 1183–1190. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Z.; Gu, Y.; Chen, Y.; Bi, L. Synthesis of rosin allyl ester and its UV-curing characteristics. Polym. J. 2011, 43, 869–873. [Google Scholar] [CrossRef]
- Wang, H.; Liu, B.; Liu, X.; Zhang, J.; Xian, M. Synthesis of biobased epoxy and curing agents using rosin and the study of cure reactions. Green Chem. 2008, 10, 1190–1196. [Google Scholar] [CrossRef]
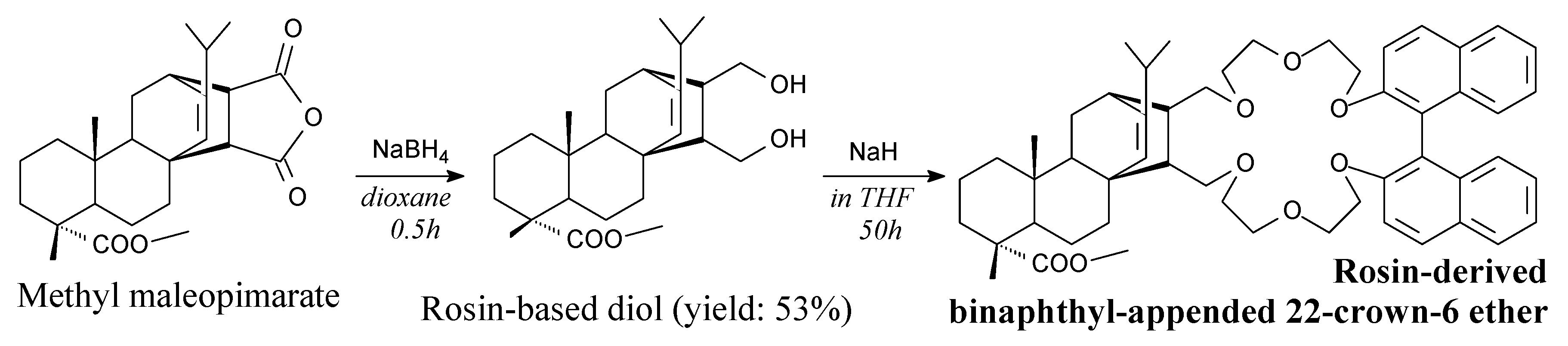
- Wang, H.; He, C.; Pan, Y.; Yao, G.; Wu, Q.; Deng, H. Synthesis and amines enantiomeric recognition ability of binaphthyl-appended 22-crown-6 ethers derived from rosin acid. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 177–183. [Google Scholar] [CrossRef]
- Mustata, F.R.; Tudorachi, N. Epoxy resins cross-linked with rosin adduct derivatives. Cross-linking and thermal behaviors. Ind. Eng. Chem. Res. 2010, 49, 12414–12422. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Xiong, Z.; Liu, X.; Na, H.; Zhang, R.; Zhu, J. Highly recoverable rosin-based shape memory polyurethanes. J. Mater. Chem. A 2013, 1, 3263–3267. [Google Scholar] [CrossRef]
- Pietrzak, K.; Kirpluks, M.; Cabulis, U.; Ryszkowska, J. Effect of the addition of tall oil-based polyols on the thermal and mechanical properties of ureaurethane elastomers. Polym. Degrad. Stab. 2014, 108, 201–211. [Google Scholar] [CrossRef]
- Zhan, S.H.; Jiang, X.H.; Li, J.; Meng, Z.; Chen, L.L.; Han, C.R. Controlled synthesis of hydroxyapatite using a novel natural rosin-based surfactant. Nano 2017, 12, 1750098. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, Y.; Li, S.; Nguyen, T.T.H. Synthesis, characterization, and bioactivity of rosin quaternary ammonium salt derivatives. Bioresources 2013, 8, 735–742. [Google Scholar] [CrossRef]
- Li, J.; Gao, Y.; Shang, S.; Rao, X.; Song, J.; Wang, Z. Synthesis and quantitative structure–activity relationship (QSAR) studies of novel rosin-based diamide insecticides. RSC Adv. 2014, 4, 58190–58199. [Google Scholar] [CrossRef]
- Atta, A.; El-Mahdy, G.; Al-Lohedan, H.; Al-Hussain, S.; Atta, A.M.; El-Mahdy, G.A.; Al-Lohedan, H.A.; Al-Hussain, S.A. Synthesis of environmentally friendly highly dispersed magnetite nanoparticles based on rosin cationic surfactants as thin film coatings of steel. Int. J. Mol. Sci. 2014, 15, 6974–6989. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; El-Saeed, A.M.; El-Mahdy, G.M.; Al-Lohedan, H.A. Application of magnetite nano-hybrid epoxy as protective marine coatings for steel. RSC Adv. 2015, 5, 101923–101931. [Google Scholar] [CrossRef]
- Ishtikhar, M.; Rahisuddin; Khan, M.V.; Khan, R.H. Anti-aggregation property of thymoquinone induced by copper-nanoparticles: A biophysical approach. Int. J. Biol. Macromol. 2016, 93, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Ishtikhar, M.; Chandel, T.I.; Ahmad, A.; Ali, M.S.; Al-lohadan, H.A.; Atta, A.M.; Khan, R.H. Rosin surfactant QRMAE can be utilized as an amorphous aggregate inducer: a case study of mammalian serum albumin. PLoS ONE 2015, 10, e0139027. [Google Scholar] [CrossRef] [PubMed]
- Ishtikhar, M.; Usmani, S.S.; Gull, N.; Badr, G.; Mahmoud, M.H.; Khan, R.H. Inhibitory effect of copper nanoparticles on rosin modified surfactant induced aggregation of lysozyme. Int. J. Biol. Macromol. 2015, 78, 379–388. [Google Scholar] [CrossRef]
- Wang, H.; Nguyen, T.T.H.; Li, S.; Liang, T.; Zhang, Y.; Li, J. Quantitative structure–activity relationship of antifungal activity of rosin derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 347–354. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Tian, B.; Liang, T.; Jin, Y. Synthesis of a rosin gemini surfactant and its properties. Environ. Eng. Sci. 2011, 29, 606–610. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, Z.D.; He, L.Z.; Bi, L.W.; Chen, Y.X. Fabrication of mesoporous ZrO2 by using rosin-based quaternary ammonium salt. Adv. Mater. Res. 2011, 239–242, 3257–3261. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Y.; Zhong, Y.; Peng, J. Synthesis and thermodynamic properties of rosin-based cationic gemini surfactants. J. Surfactant. Deterg. 2014, 17, 453–458. [Google Scholar] [CrossRef]
- Liu, G.; Wu, G.; Chen, J.; Kong, Z. Synthesis, modification and properties of rosin-based non-isocyanate polyurethanes coatings. Prog. Org. Coat. 2016, 101, 461–467. [Google Scholar] [CrossRef]
- Wang, H.; Tian, X.; Yang, D.; Pan, Y.; Wu, Q.; He, C. Synthesis and enantiomeric recognition ability of 22-crown-6 ethers derived from rosin acid and BINOL. Tetrahedron Asymmetry 2011, 22, 381–386. [Google Scholar] [CrossRef]
- Nong, W.J.; Chen, X.P.; Liang, J.Z.; Wang, L.L.; Tong, Z.F.; Huang, K.L.; Wu, R.; Xie, Q.R.; Jia, Y.H.; Li, K.X. Isolation and characterization of abietic acid. Adv. Mater. Res. 2014, 887–888, 551–556. [Google Scholar] [CrossRef]
- Zhang, G.; Jiang, C.; Wang, Z.; Chen, W.; Gu, W.; Ding, Y. Dehydroabietic acid derivative QC2 induces oncosis in hepatocellular carcinoma cells. Biomed Res. Int. 2014, 2014, 682197. [Google Scholar] [CrossRef]
- Li, J.; Song, J.; Shang, S.-B.; Rao, X.-P.; Gao, Y.-Q. Syntheses and antibacterial activity of Schiff bases from 16-isopropyl-5, 9-dimethyltetracyclo [10.2.2.01, 10.04, 9] hexadec-15-ene-5, 14-dicarboxylic acid. Nat. Prod. Res. 2013, 27, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rao, X.; Shang, S.; Gao, Y.; Song, J. Synthesis and antibacterial activity of amide derivatives from acrylopimaric acid. Bioresources 2012, 7, 1961–1971. [Google Scholar] [CrossRef]
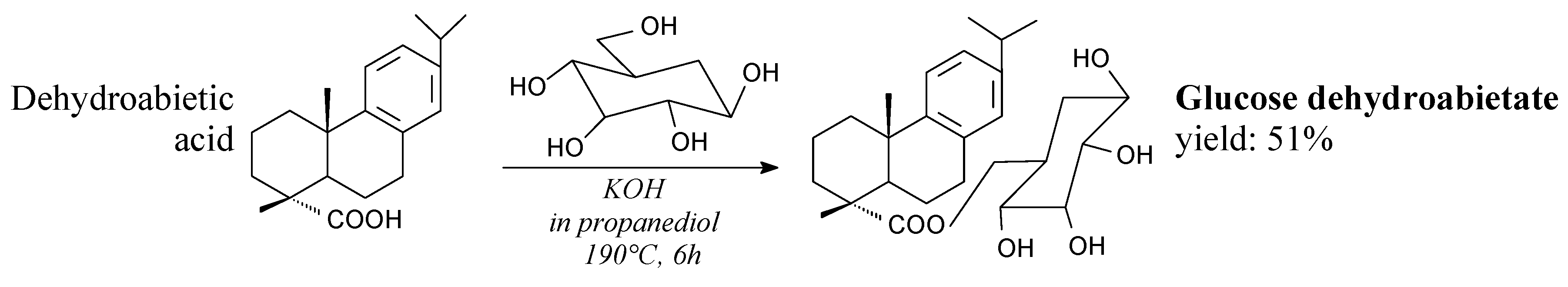
- Shi, Z.; Wang, Y.; Nie, Y.; Yao, X. Synthesis and characterization of glucose dehydroabietate. Adv. Mater. Res. 2012, 396–398, 1260–1264. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Liu, S.; Xie, C.; Yu, S. The selective hydrogenation of rosin to hydroabietic content using Pd/SBA-15 as catalysts. Res. Chem. Intermed. 2017, 43, 1211–1221. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Chen, X.; Wei, X.; Liang, J.; Li, W. Intrinsic kinetics study of rosin hydrogenation on a nickel catalyst supported on spent equilibrium catalyst. RSC Adv. 2017, 7, 25780–25788. [Google Scholar] [CrossRef]
- Wang, M.M.; Liu, S.W.; Li, L.; Yu, S.T.; Xie, C.X.; Song, Z.Q. Hydrogenation of rosin over PVP-stabilized Pd nanoparticles in aqueous/organic biphasic system. Res. Chem. Intermed. 2016, 42, 6181–6190. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, D.; Wei, X.; Liang, J.; Chen, X.; Wang, L. Green catalytic conversion of hydrogenated rosin to glycerol esters using subcritical CO2 in water and the associated kinetics. J. Supercrit. Fluid. 2017, 125, 12–21. [Google Scholar] [CrossRef]
- Bernas, A.; Salmi, T.; Murzin, D.Y.; Mikkola, J.-P.; Rintola, M. Catalytic transformation of abietic acid to hydrocarbons. Top. Catal. 2012, 55, 673–679. [Google Scholar] [CrossRef]
- Wang, L.; Huang, C.; Chen, J.; Wei, X.; Chen, X.; Liang, J. Catalyst-free biodiesel production from industrial rosin residue (dark-grade rosin) using supercritical methanol. Waste Biomass Valori. 2018, 9, 1191–1198. [Google Scholar] [CrossRef]
- Kulikov, A.B.; Onishchenko, M.I.; Maksimov, A.L.; Lysenko, S.V.; Karakhanov, E.A. Hydroconversion of rosin acids in the presence of Pt-containing Al–HMS mesoporous aluminosilicate. Pet. Chem. 2016, 56, 717–723. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Deng, Y.; Zhou, D.; Wang, L. A novel nickel catalyst derived from layered double hydroxides (LDHs) supported on fluid catalytic cracking catalyst residue (FC3R) for rosin hydrogenation. Chem. Eng. J. 2015, 269, 434–443. [Google Scholar] [CrossRef]
- Tong, D.S.; Zheng, Y.M.; Yu, W.H.; Wu, L.M.; Zhou, C.H. Catalytic cracking of rosin over acid-activated montmorillonite catalysts. Appl. Clay Sci. 2014, 100, 123–128. [Google Scholar] [CrossRef]
- Bicu, I.; Mustata, F. Polymers from a levopimaric acid–acrylonitrile Diels–Alder adduct: Synthesis and characterization. J. Polym. Sci. A 2005, 43, 6308–6322. [Google Scholar] [CrossRef]
- Atta, A.; Al-Lohedan, H.; Al-Hussain, S.; Atta, A.M.; Al-Lohedan, H.A.; Al-Hussain, S.A. Functionalization of magnetite nanoparticles as oil spill collector. Int. J. Mol. Sci. 2015, 16, 6911–6931. [Google Scholar] [CrossRef]
- Atta, A.M.; Akl, Z.F. Removal of thorium from water using modified magnetite nanoparticles capped with rosin amidoxime. Mater. Chem. Phys. 2015, 163, 253–261. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.; Zhang, J.; Yang, X.; Zhang, Y.; Li, S.; Song, J.; Huang, K.; Xia, J. Preparation of a new liquid thermal stabilizer from rosin and fatty acid and study of the properties of the stabilized PVC. Polym. Degrad. Stab. 2014, 109, 129–136. [Google Scholar] [CrossRef]
- Chen, P.; Zeng, X.; Li, H.; Liu, X.; Liu, D.; Li, X. Preparation and characterization of polyacrylate/polymerized rosin composite emulsions by seeded semicontinuous emulsion polymerization. J. Appl. Polym. Sci. 2012, 124, 4694–4701. [Google Scholar] [CrossRef]
- Chen, P.; Zeng, X.; Li, H.; Liu, X.; Liu, D.; Li, X. Effect of polymerized rosin on polymer microstructure and adhesive properties in tackified acrylate emulsions. Polym. Plast. Technol. Eng. 2012, 51, 122–127. [Google Scholar] [CrossRef]
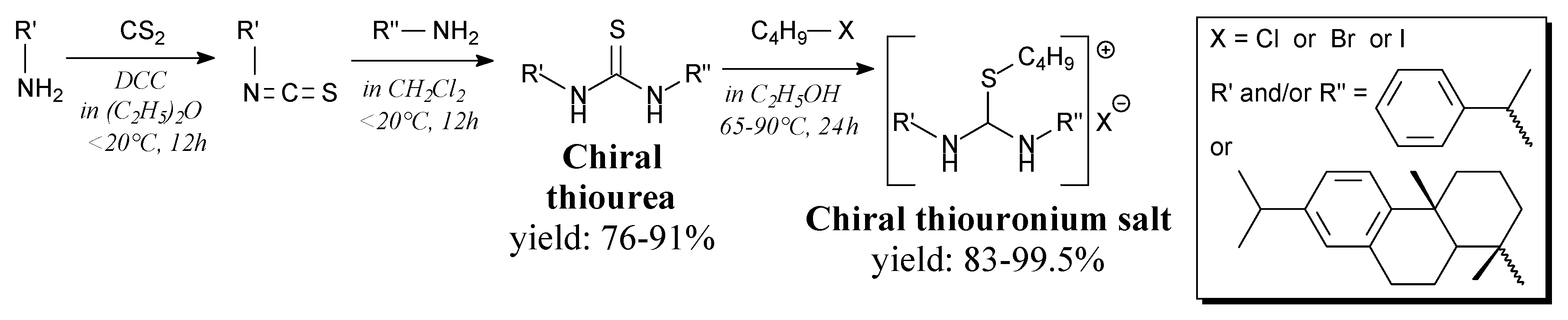
- Foreiter, M.B.; Gunaratne, H.Q.N.; Nockemann, P.; Seddon, K.R.; Stevenson, P.J.; Wassell, D.F. Chiral thiouronium salts: Synthesis, characterisation and application in NMR enantio-discrimination of chiral oxoanions. New J. Chem. 2013, 37, 515–533. [Google Scholar] [CrossRef]
- Guo, X.-T.; Sha, F.; Wu, X.-Y. Highly enantioselective Michael addition of α,α-disubstituted aldehydes to nitroolefins. Res. Chem. Intermed. 2016, 42, 6373–6380. [Google Scholar] [CrossRef]
- Reddy, B.V.S.; Swain, M.; Reddy, S.M.; Yadav, J.S. Enantioselective Michael addition of 2-hydroxy-1,4-naphthoquinone and 1,3-dicarbonyls to β-nitroalkenes catalyzed by a novel bifunctional rosin-indane amine thiourea catalyst. RSC Adv. 2013, 3, 8756–8765. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Jiang, X.; Yan, W.; Wang, R. Highly enantioslective synthesis of multisubstituted polyfunctional dihydropyrrole via an organocatalytic tandem michael/cyclization sequence. Org. Lett. 2011, 13, 3806–3809. [Google Scholar] [CrossRef]
- Reddy, B.V.S.; Swain, M.; Reddy, S.M.; Yadav, J.S.; Sridhar, B. Asymmetric Michael/hemiketalization of 5-hydroxy-2-methyl-4H-pyran-4-one to β,γ-unsaturated α-ketoesters catalyzed by a bifunctional rosin–indane amine thiourea catalyst. RSC Adv. 2014, 4, 42299–42307. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Wu, L.; Zhang, G.; Liu, X.; Zhang, H.; Fu, D.; Wang, R. Doubly stereocontrolled asymmetric aza-Henry reaction with in situ generation of N-Boc-imines catalyzed by novel rosin-derived amine thiourea catalysts. Adv. Synth. Catal. 2009, 351, 2096–2100. [Google Scholar] [CrossRef]
- Zhang, H.-R.; Xue, J.-J.; Chen, R.; Tang, Y.; Li, Y. A bifunctional rosin-derived thiourea catalyzed asymmetric tandem reaction and its new mechanism. Chin. Chem. Lett. 2014, 25, 710–714. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Zhang, G.; Fu, D.; Zhang, F.; Kai, M.; Wang, R. Enantioselective synthesis of cyclic thioureas via mannich reaction and concise synthesis of highly optically active methylthioimidazolines: discovery of a more potent antipyretic agent. Adv. Synth. Catal. 2011, 353, 1787–1796. [Google Scholar] [CrossRef]
- Jiang, X.; Fu, D.; Zhang, G.; Cao, Y.; Liu, L.; Song, J.; Wang, R. Highly diastereo- and enantioselective Mannich reaction of lactones with N-Boc-aldimines catalyzed by bifunctional rosin-derived amine thiourea catalysts. Chem. Commun. 2010, 46, 4294–4296. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, L.; Xing, Y.; Wang, L.; Wang, S.; Chen, Z.; Wang, R. Highly enantioselective Friedel–Crafts alkylation reaction catalyzed by rosin-derived tertiary amine–thiourea: synthesis of modified chromanes with anticancer potency. Chem. Commun. 2011, 48, 446–448. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Liu, X.; Zhang, G.; Lai, L.; Wu, L.; Zhang, J.; Wang, R. Enantio- and diastereoselective asymmetric addition of 1,3-dicarbonyl compounds to nitroalkenes in a doubly stereocontrolled manner catalyzed by bifunctional rosin-derived amine thiourea catalysts. J. Org. Chem. 2009, 74, 5562–5567. [Google Scholar] [CrossRef]
- Zhao, X.; Kang, T.; Shen, J.; Sha, F.; Wu, X. Enantioselective allylic amination of morita-baylis-hillman acetates catalyzed by chiral thiourea-phosphine. Chin. J. Chem. 2015, 33, 1333–1337. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, X.; Li, X.; Hou, C.; Jiang, Y.; Hou, K.; Wang, R.; Li, Y. Highly enantioselective synthesis of N-protected β-amino malonates catalyzed by magnetically separable heterogeneous rosin-derived amino thiourea catalysts: a stereocontrolled approach to β-amino acids. ChemCatChem 2013, 5, 2187–2190. [Google Scholar] [CrossRef]
- Lin, N.; Long, X.W.; Chen, Q.; Zhu, W.R.; Wang, B.C.; Chen, K.B.; Jiang, C.W.; Weng, J.; Lu, G. Highly efficient construction of chiral dispirocyclic oxindole/thiobutyrolactam/chromanone complexes through Michael/cyclization cascade reactions with a rosin-based squaramide catalyst. Tetrahedron 2018, 74, 3734–3741. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, X.; Liu, L.; Shen, F.; Zhang, F.; Wang, R. Enantioselective Michael/cyclization reaction sequence: Scaffold-inspired synthesis of spirooxindoles with multiple stereocenters. Angew. Chem. Int. Ed. 2011, 50, 9124–9127. [Google Scholar] [CrossRef]
- Pan, D.; Wu, A.; Li, P.; Xu, H.; Lei, F.; Shen, L. Palladium-loaded renewable polymer as a green heterogeneous catalyst for cross-coupling reactions under microwave irradiation. J. Chem. Res. 2014, 38, 715–718. [Google Scholar] [CrossRef]
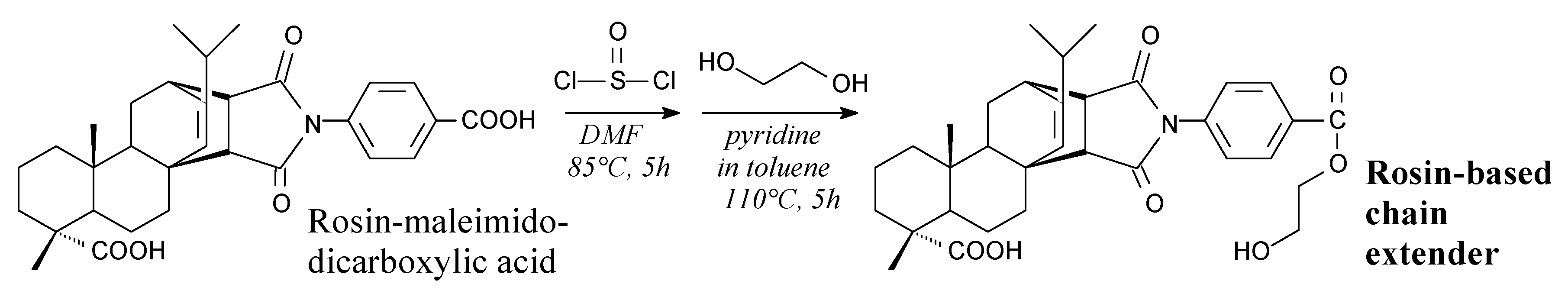
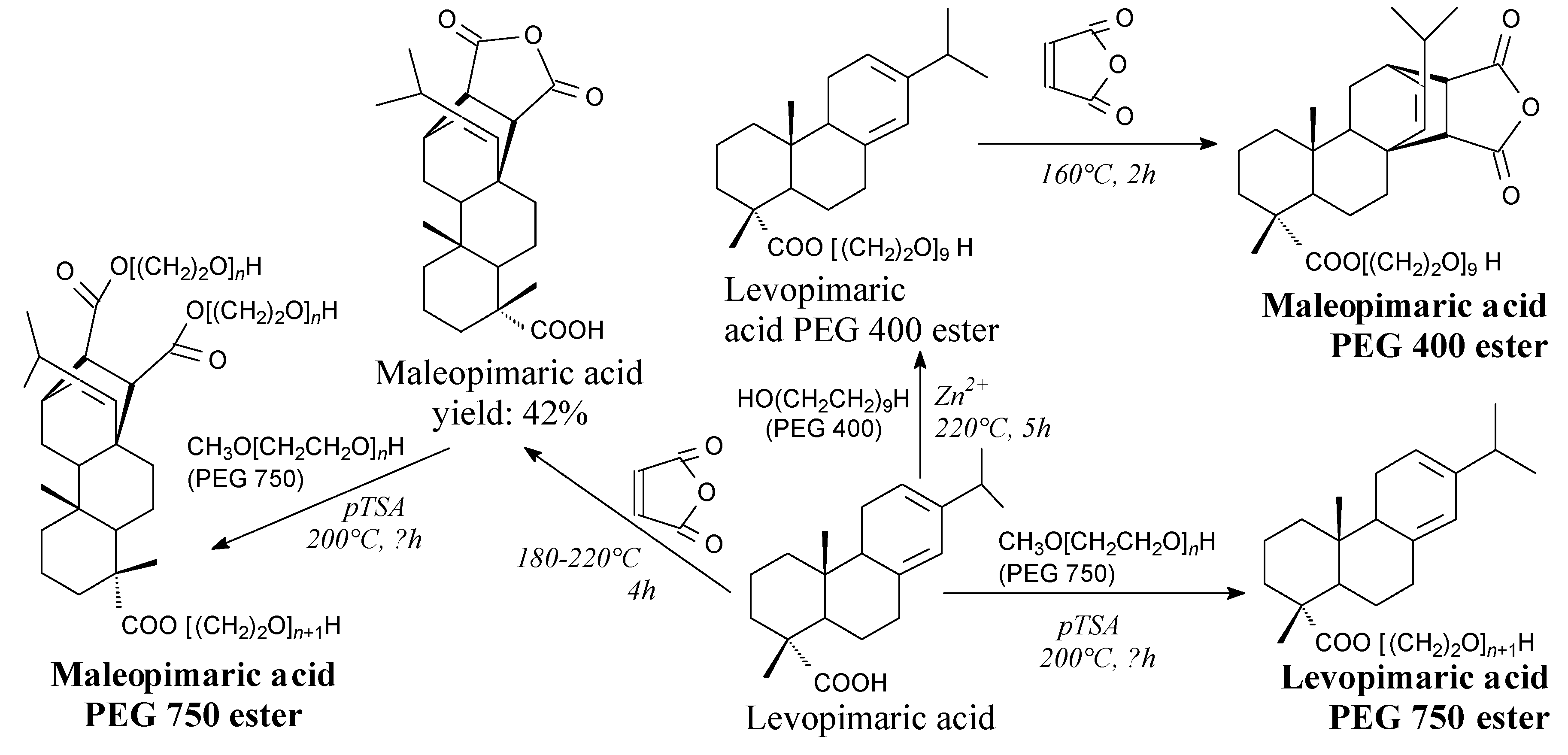
- Morkhade, D.M.; Nande, V.S.; Barabde, U.V.; Patil, A.T.; Joshi, S.B. PEGylated rosin derivatives: Novel microencapsulating materials for sustained drug delivery. AAPS PharmSciTech 2007, 8, E134. [Google Scholar] [CrossRef]
- Morkhade, D.M.; Nande, V.S.; Barabde, U.V.; Joshi, S.B. Study of biodegradation and biocompatibility of PEGylated rosin derivatives. J. Bioact. Compat. Polym. 2017, 32, 628–640. [Google Scholar] [CrossRef]
- Morkhade, D.M.; Nande, V.S.; Barabde, U.V.; Patil, A.T.; Joshi, S.B. Design and evaluation of dental films of PEGylated rosin derivatives containing sparfloxacin for periodontitis. Drug. Dev. Ind. Pharm. 2018, 44, 914–922. [Google Scholar] [CrossRef]
- El-Mahdy, G.A.; Atta, A.M.; Al-Lohedan, H.A. Water soluble nonionic rosin surfactants as corrosion inhibitor of carbon steel in 1 M HCl. Int. J. Electrochem. Sci. 2013, 8, 5052–5066. [Google Scholar]
- Chen, Y.; Wilbon, P.A.; Chen, Y.P.; Zhou, J.; Nagarkatti, M.; Wang, C.; Chu, F.; Decho, A.W.; Tang, C. Amphipathic antibacterial agents using cationic methacrylic polymers with natural rosin as pendant group. RSC Adv. 2012, 2, 10275–10282. [Google Scholar] [CrossRef]
- Kaith, B.S.; Jindal, R.; Sharma, R. Synthesis of a gum rosin alcohol-poly(acrylamide) based adsorbent and its application in removal of malachite green dye from waste water. RSC Adv. 2015, 5, 43092–43104. [Google Scholar] [CrossRef]
- Jindal, R.; Kaith, B.S.; Sharma, R. Central composite design model to study swelling of GrA-cl-poly(AAm) hydrogel and kinetic investigation of colloidal suspension. J. Polym. Environ. 2018, 26, 999–1011. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Ding, W.; Rahman, M.A.; Yuan, L.; Wang, Z.; Hamidi, N.; Robertson, M.L.; Tang, C. Biobased plastics and elastomers from renewable rosin via “living” ring-opening metathesis polymerization. Macromolecules 2016, 49, 7155–7164. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lokupitiya, H.N.; Ganewatta, M.S.; Yuan, L.; Stefik, M.; Tang, C. Designing block copolymer architectures toward tough bioplastics from natural rosin. Macromolecules 2017, 50, 2069–2077. [Google Scholar] [CrossRef]
- Choi, S.J.; Yim, T.; Cho, W.; Mun, J.; Jo, Y.N.; Kim, K.J.; Jeong, G.; Kim, T.-H.; Kim, Y.-J. Rosin-embedded poly(acrylic acid) binder for silicon/graphite negative electrode. ACS Sustain. Chem. Eng. 2016, 4, 6362–6370. [Google Scholar] [CrossRef]
- Carbonell-Blasco, P.; Antoniac, I.V.; Martín-Martínez, J.M. New polyurethane sealants containing rosin for non-invasive disc regeneration surgery. Key Eng. Mater. 2014, 583, 67–79. [Google Scholar] [CrossRef]
- Carbonell-Blasco, P.; Martín-Martínez, J.M.; Antoniac, I.V. Synthesis and characterization of polyurethane sealants containing rosin intended for sealing defect in annulus for disc regeneration. Int. J. Adhes. Adhes. 2013, 42, 11–20. [Google Scholar] [CrossRef]
- Atta, A.M.; Elsaeed, A.M. Use of rosin-based nonionic surfactants as petroleum crude oil sludge dispersants. J. Appl. Polym. Sci. 2011, 122, 183–192. [Google Scholar] [CrossRef]
- Atta, A.M.; Ramadan, A.M.; Shaffei, K.A.; Nassar, A.M.; Ahmed, N.S.; Fekry, M. Synthesis and properties of nonionic surfactants from rosin-imides maleic anhydride adduct. J. Disper. Sci. Technol. 2009, 30, 1100–1110. [Google Scholar] [CrossRef]
- Kaith, B.S.; Jindal, R.; Sharma, R. Study of ionic charge dependent salt resistant swelling behavior and removal of colloidal particles using reduced gum rosin-poly(acrylamide)-based green flocculant. Iran. Polym. J. 2016, 25, 349–362. [Google Scholar] [CrossRef]
- Jindal, R.; Sharma, R.; Maiti, M.; Kaur, A.; Sharma, P.; Mishra, V.; Jana, A.K. Synthesis and characterization of novel reduced gum rosin-acrylamide copolymer-based nanogel and their investigation for antibacterial activity. Polym. Bull. 2017, 74, 2995–3014. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Properties Enhancement of Room Temperature Vulcanized Silicone Rubber by Rosin Modified Aminopropyltriethoxysilane as a Cross-linking Agent. ACS Sustain. Chem. Eng. 2017, 5, 10002–10010. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Preparation and properties of room temperature vulcanized silicone rubber based on rosin-grafted polydimethylsiloxane. RSC Adv. 2018, 8, 14684–14693. [Google Scholar] [CrossRef]
- Xu, T.; Liu, H.; Song, J.; Shang, S.-B.; Song, Z.-Q.; Chen, X.-J.; Yang, C. Synthesis and characterization of imide modified poly(dimethylsiloxane) with maleopimaric acid as raw material. Chin. Chem. Lett. 2015, 26, 572–574. [Google Scholar] [CrossRef]
- Lin, R.; Li, H.; Long, H.; Su, J.; Huang, W. Structure and characteristics of lipase-catalyzed rosin acid starch. Food Hydrocoll. 2015, 43, 352–359. [Google Scholar] [CrossRef]
- Lin, R.; Li, H.; Long, H.; Su, J.; Huang, W. Synthesis of rosin acid starch catalyzed by lipase. BioMed Res. Int. 2014, 2014, 647068. [Google Scholar] [CrossRef]
- De Castro, D.O.; Bras, J.; Gandini, A.; Belgacem, N. Surface grafting of cellulose nanocrystals with natural antimicrobial rosin mixture using a green process. Carbohydr. Polym. 2016, 137, 1–8. [Google Scholar] [CrossRef]
- Niu, X.; Liu, Y.; Song, Y.; Han, J.; Pan, H. Rosin modified cellulose nanofiber as a reinforcing and co-antimicrobial agents in polylactic acid /chitosan composite film for food packaging. Carbohydr. Polym. 2018, 183, 102–109. [Google Scholar] [CrossRef]
- George, M.; Mussone, P.G.; Bressler, D.C. Utilization of tall oil to enhance natural fibers for composite applications and production of a bioplastic. J. Appl. Polym. Sci. 2016, 133, 44327. [Google Scholar] [CrossRef]
- Sacripante, G.G.; Zhou, K.; Farooque, M. Sustainable polyester resins derived from rosins. Macromolecules 2015, 48, 6876–6881. [Google Scholar] [CrossRef]
- Yuan, L.; Hamidi, N.; Smith, S.; Clemons, F.; Hamidi, A.; Tang, C. Molecular characterization of biodegradable natural resin acid-substituted polycaprolactone. Eur. Polym. J. 2015, 62, 43–50. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.P.; Yao, K.; Wilbon, P.A.; Zhang, W.; Ren, L.; Zhou, J.; Nagarkatti, M.; Wang, C.; Chu, F.; et al. Robust antimicrobial compounds and polymers derived from natural resin acids. Chem. Commun. 2011, 48, 916–918. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, T.; Wu, J.; Hou, C.; Ling, L.; Wang, B. Preparation and properties of glycerin ester of tung oil modified rosin. J. Appl. Polym. Sci. 2013, 130, 1700–1706. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, T.; Ma, G.; Li, P.; Ling, L.; Wang, B. Synthesis of a tung oil–rosin adduct via the diels–alder reaction: Its reaction mechanism and properties in an ultraviolet-curable adhesive. J. Appl. Polym. Sci. 2013, 130, 4201–4208. [Google Scholar] [CrossRef]
- Yu, C.; Chen, C.; Gong, Q.; Zhang, F.-A. Preparation of polymer microspheres with a rosin moiety from rosin ester, styrene and divinylbenzene. Polym. Int. 2012, 61, 1619–1626. [Google Scholar] [CrossRef]
- Huang, J.F.; Shi, Q.S.; Feng, J.; Chen, M.J.; Li, W.R.; Li, L.Q. Facile pyrolysis preparation of rosin-derived biochar for supporting silver nanoparticles with antibacterial activity. Compos. Sci. Technol. 2017, 145, 89–95. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Wang, Y.; Zhang, H.; Zhou, H.; Wei, Y.; Tao, S.; Ma, T. Composite catalyst of rosin carbon/Fe3O4: Highly efficient counter electrode for dye-sensitized solar cells. Chem. Commun. 2014, 50, 1701–1703. [Google Scholar] [CrossRef]
- Ruan, Z.; Wu, J.; Huang, J.F.; Lin, Z.T.; Li, Y.F.; Liu, Y.-L.; Cao, P.-Y.; Fang, Y.-P.; Xie, J.; Jiang, G.-B. Facile preparation of rosin-based biochar coated bentonite for supporting α-Fe2O3 nanoparticles and its application for Cr(VI) adsorption. J. Mater. Chem. A 2015, 3, 4595–4603. [Google Scholar] [CrossRef]
- Zeng, C.; Lin, Q.; Fang, C.; Xu, D.; Ma, Z. Preparation and characterization of high surface area activated carbons from co-pyrolysis product of coal-tar pitch and rosin. J. Anal. Appl. Pyrol. 2013, 104, 372–377. [Google Scholar] [CrossRef]
- Liu, H.; Du, S.; Chen, Y. Preparing mesoporous carbon and silica with rosin-silica composite gel. J. Nanosci. Nanotechnol. 2009, 9, 799–802. [Google Scholar] [CrossRef]



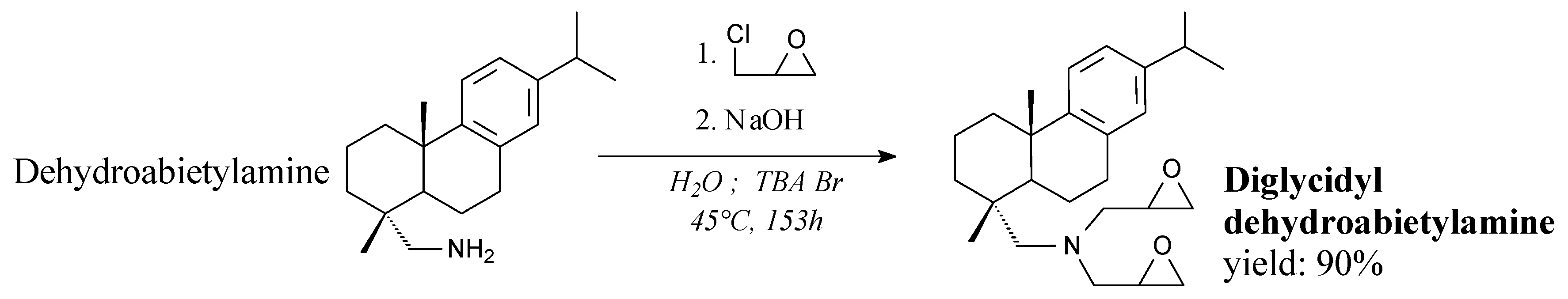
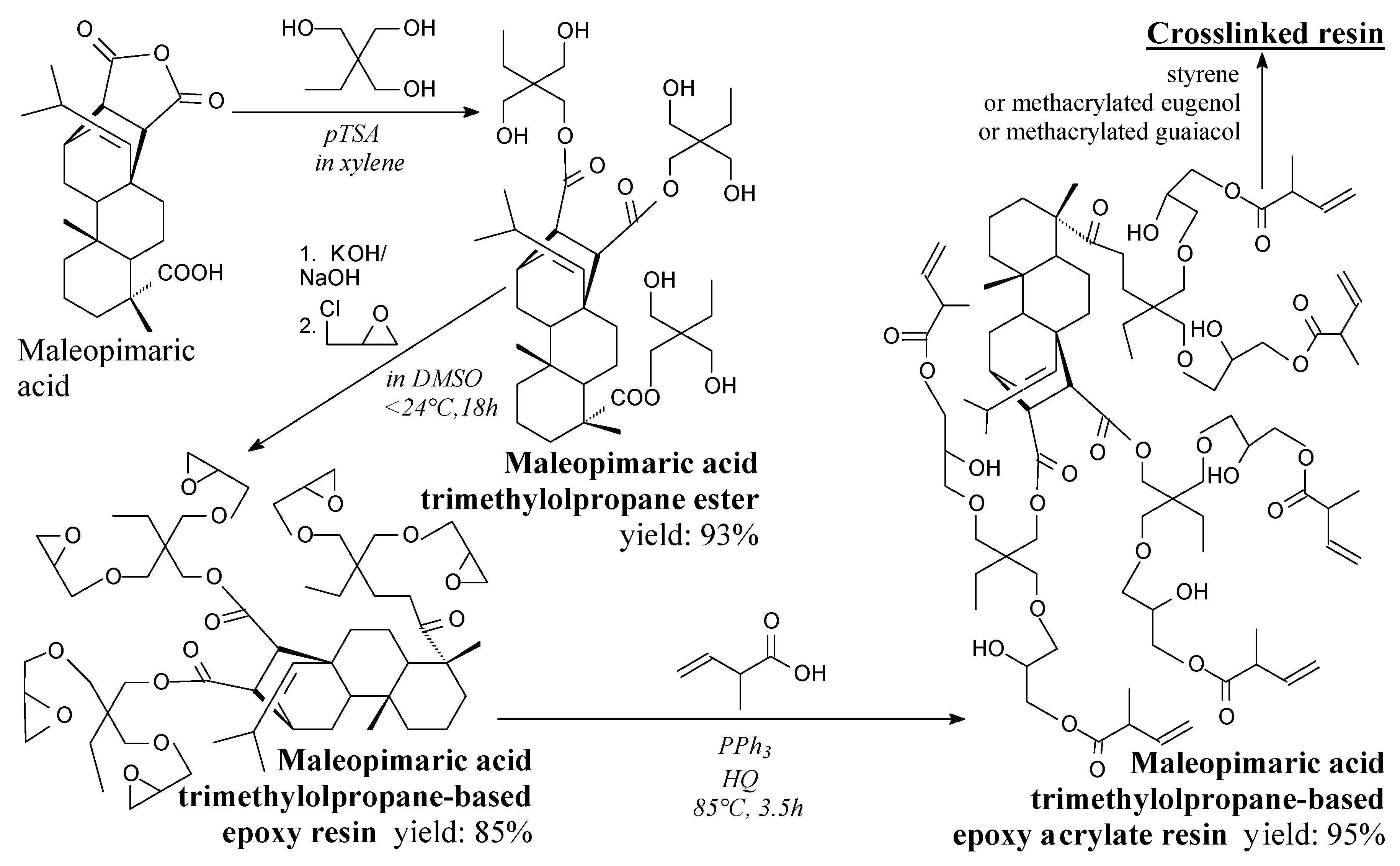


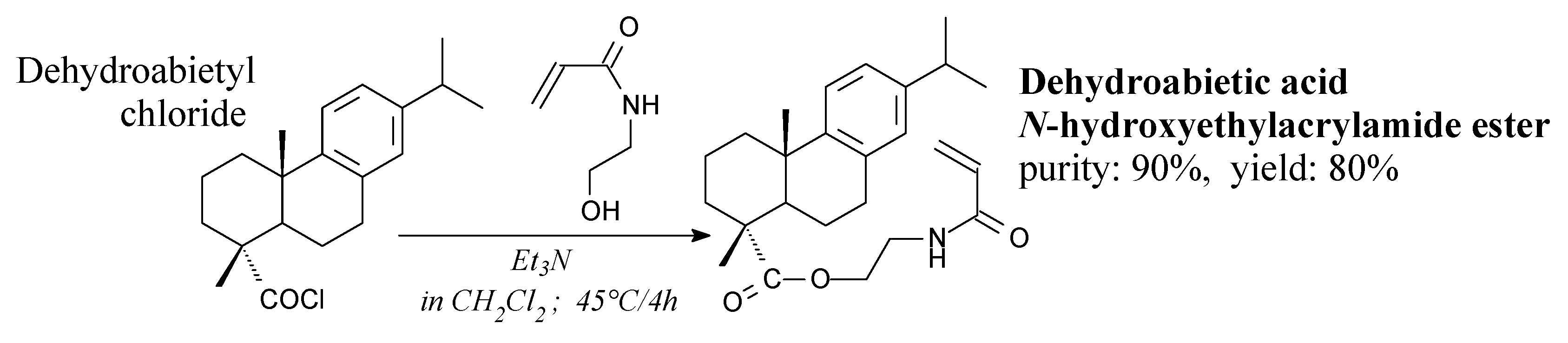

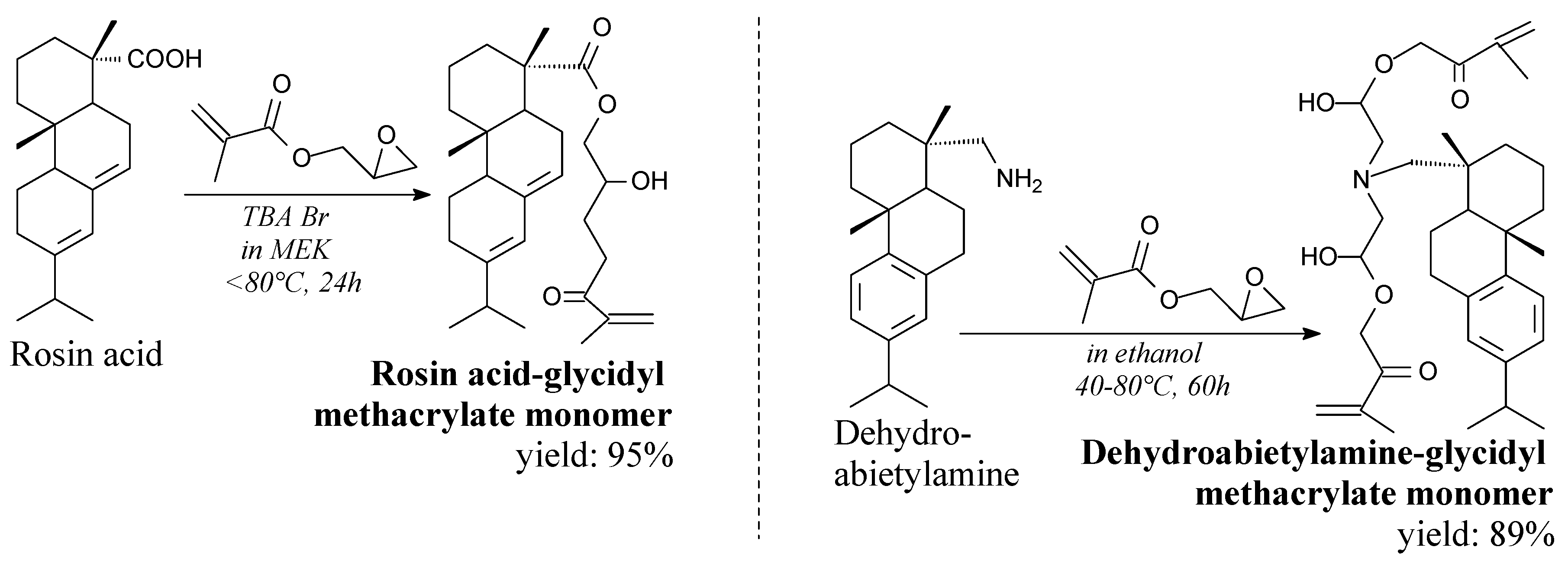
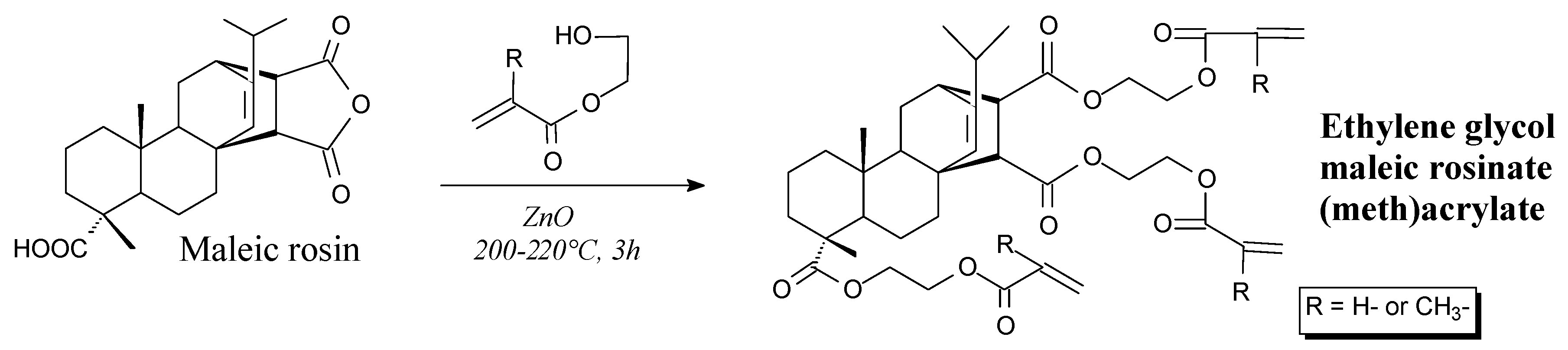
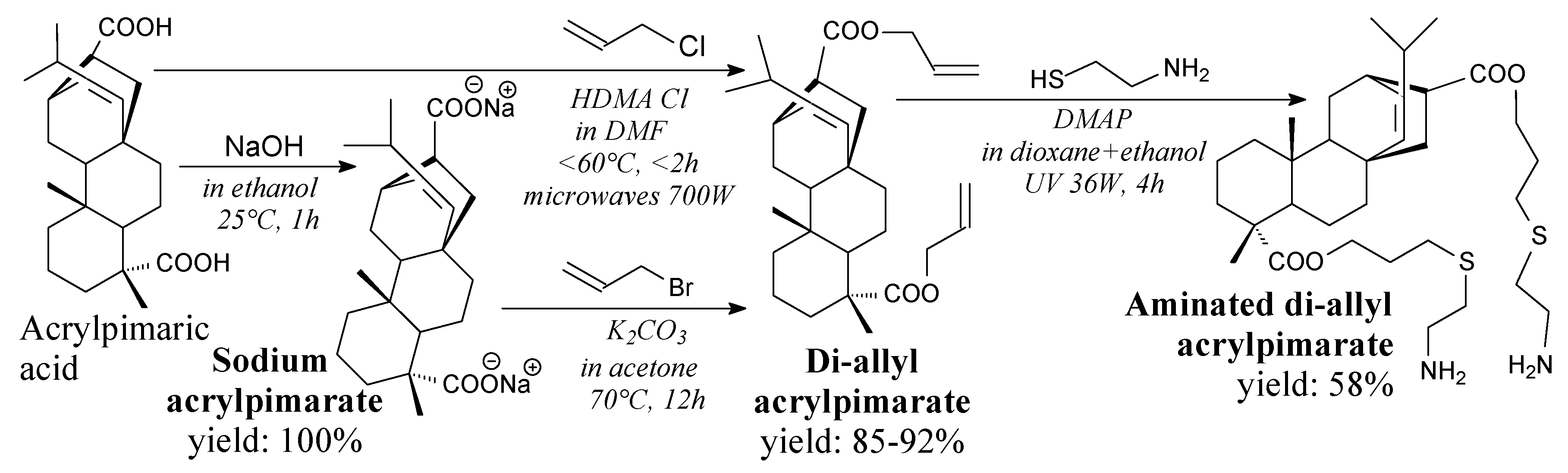
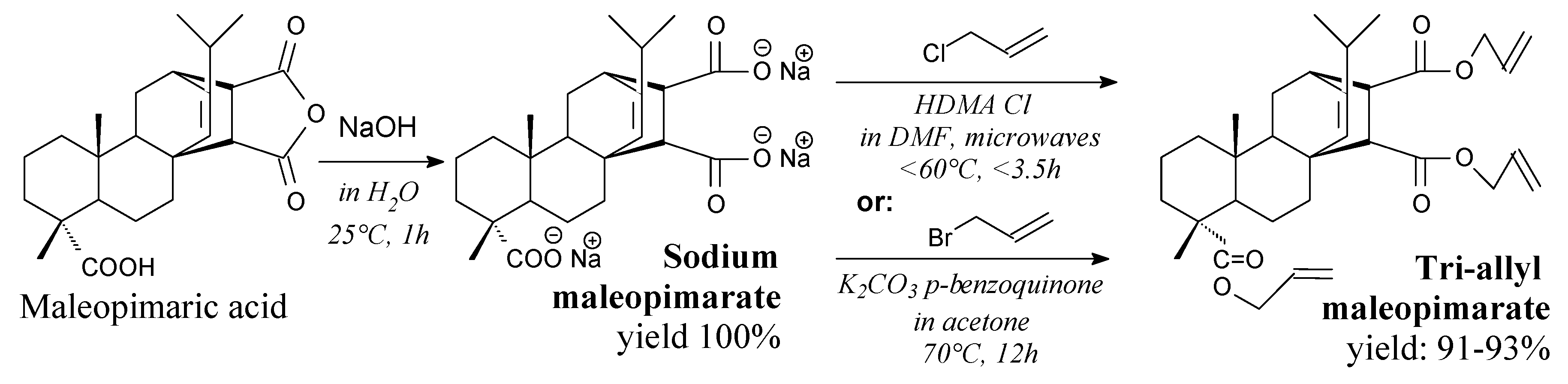
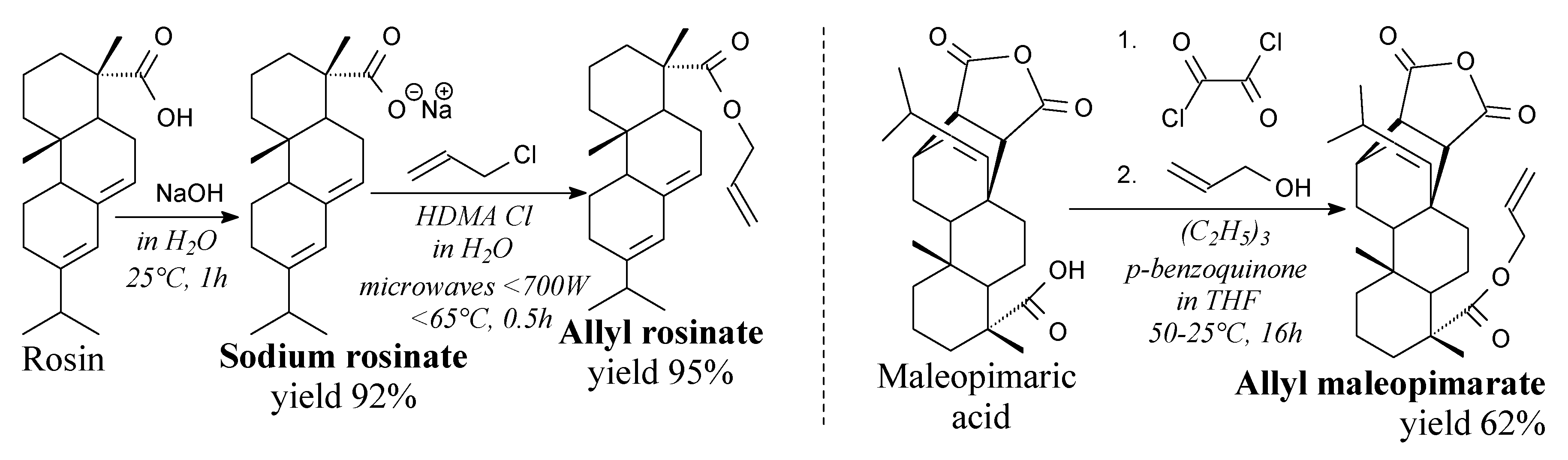
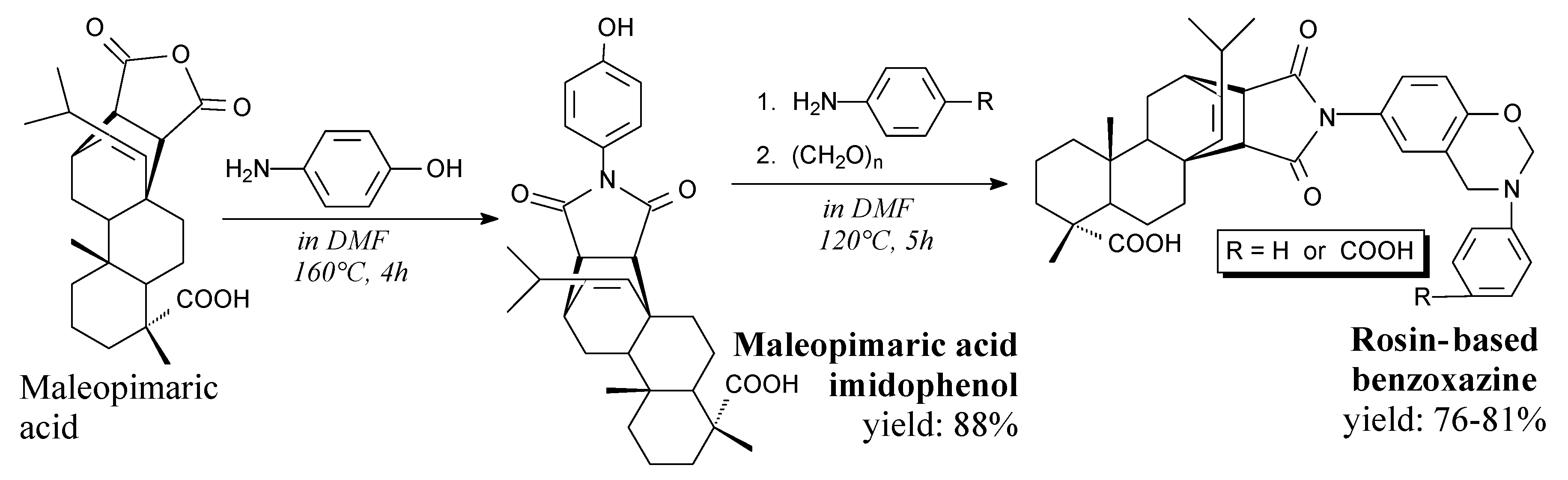








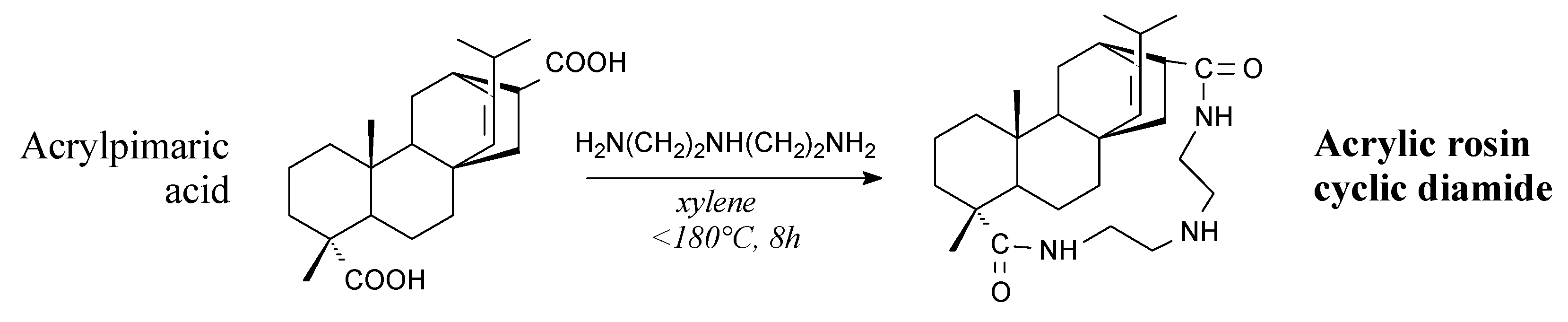



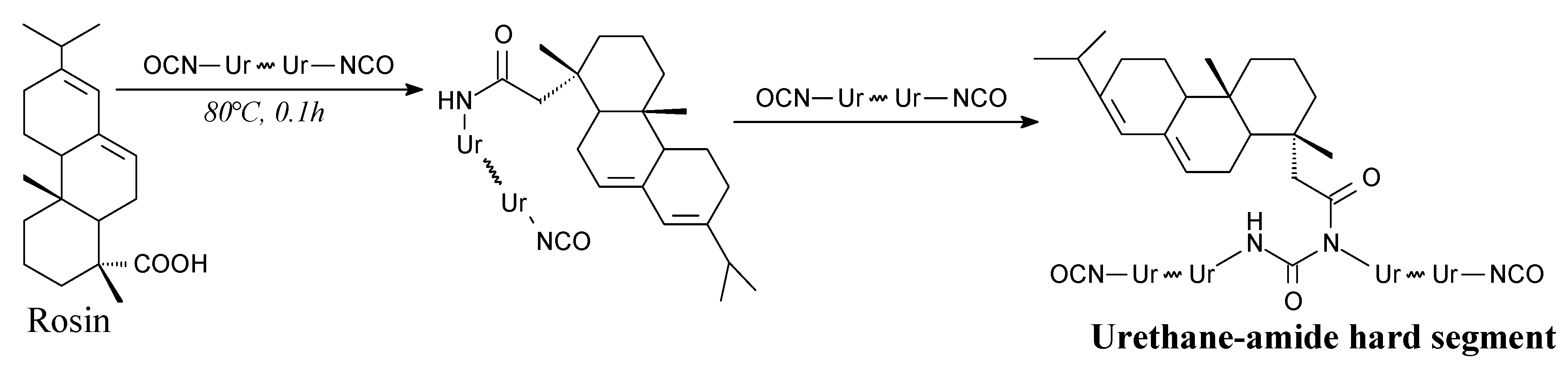

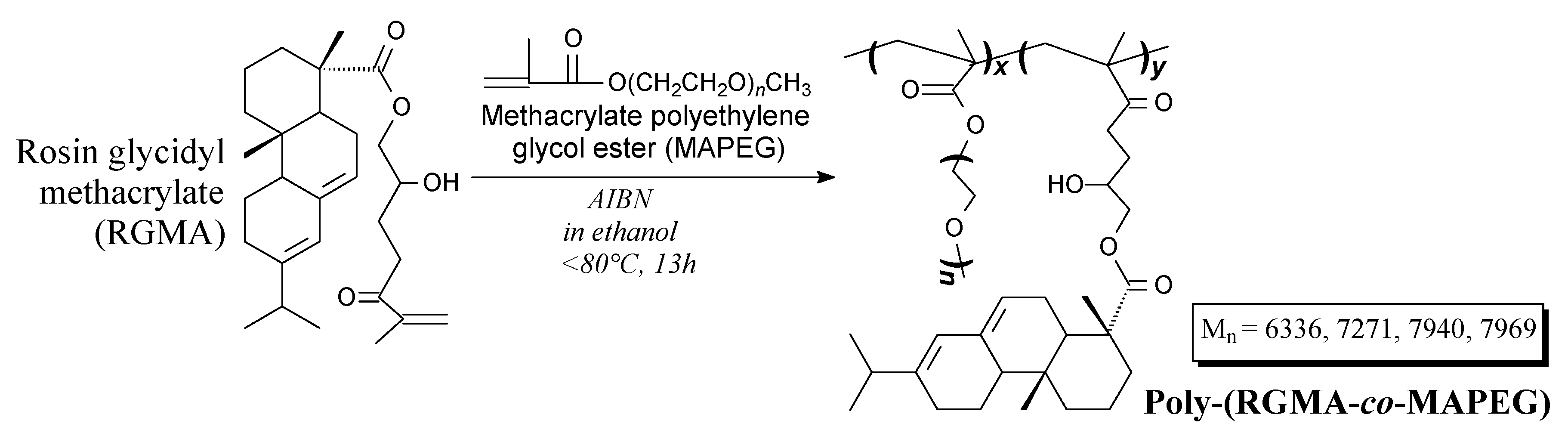
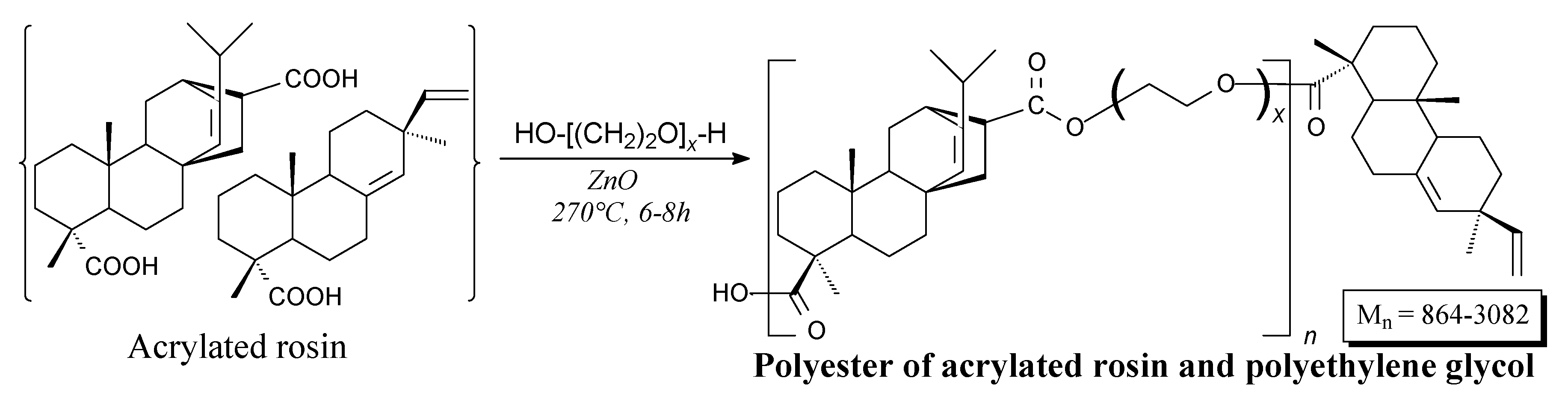
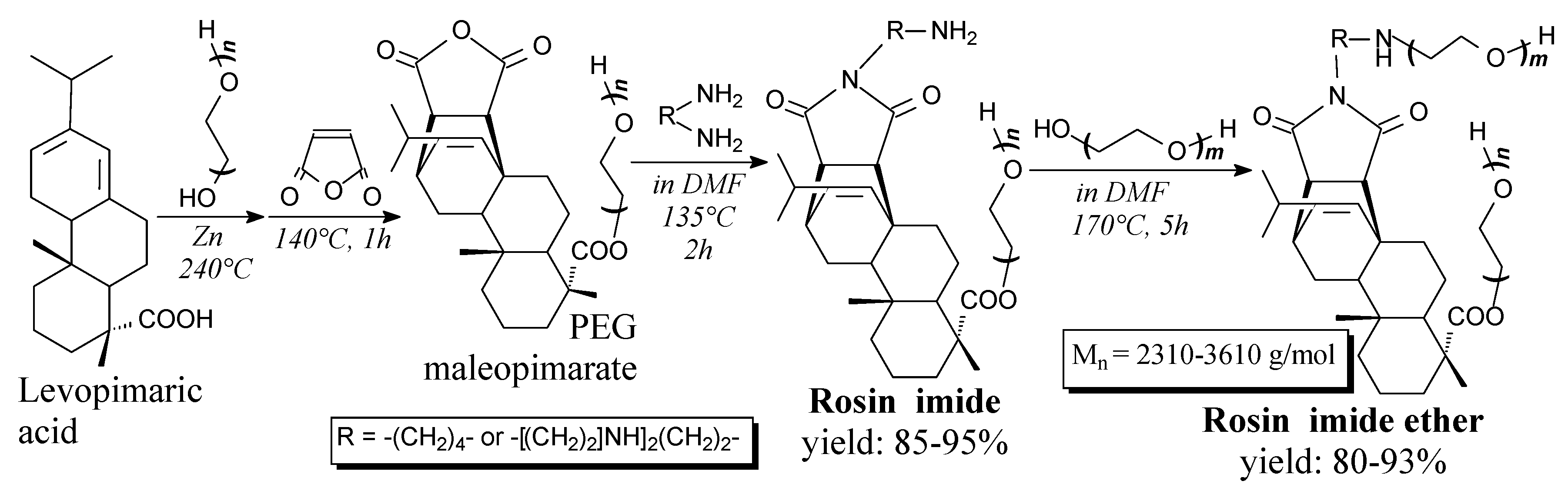











| Species | Origin | Abietane-type Acids Content (wt.%) | Other Acids (wt.%) | |||
|---|---|---|---|---|---|---|
| Abietic | Palustric/Levopimaric | Neoabietic | Dehydroabietic | |||
| Pinus massoniana | China | 39 | 25 | 16 | 7 | 13 |
| Pinus elliotti | Brazil | 37 | 15 | 16 | 5 | 27 |
| Pinus merkusii | Indonesia | 28 | 27 | 5 | 4 | 36 |
| Pinus sylvestris | Russia | 35 | 23 | 15 | 10 | 17 |
| Pinus halepensis | Greece | 45 | 23 | 13 | 5 | 14 |
| Pinus pinaster | France | 35 | 20 | 15 | 9 | 21 |
| Portugal | 34 | 21 | 19 | 9 | 17 | |
| Spain | 26 | 22 | 27 | 6 | 19 | |
| USA | 14 | 39 | 18 | 4 | 25 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in Rosin-Based Chemicals: The Latest Recipes, Applications and Future Trends. Molecules 2019, 24, 1651. https://doi.org/10.3390/molecules24091651
Kugler S, Ossowicz P, Malarczyk-Matusiak K, Wierzbicka E. Advances in Rosin-Based Chemicals: The Latest Recipes, Applications and Future Trends. Molecules. 2019; 24(9):1651. https://doi.org/10.3390/molecules24091651
Chicago/Turabian StyleKugler, Szymon, Paula Ossowicz, Kornelia Malarczyk-Matusiak, and Ewa Wierzbicka. 2019. "Advances in Rosin-Based Chemicals: The Latest Recipes, Applications and Future Trends" Molecules 24, no. 9: 1651. https://doi.org/10.3390/molecules24091651
APA StyleKugler, S., Ossowicz, P., Malarczyk-Matusiak, K., & Wierzbicka, E. (2019). Advances in Rosin-Based Chemicals: The Latest Recipes, Applications and Future Trends. Molecules, 24(9), 1651. https://doi.org/10.3390/molecules24091651







