Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract
Abstract
1. Introduction
2. Results
2.1. Development of the Induced Type 1 Diabetic Zebrafish Model
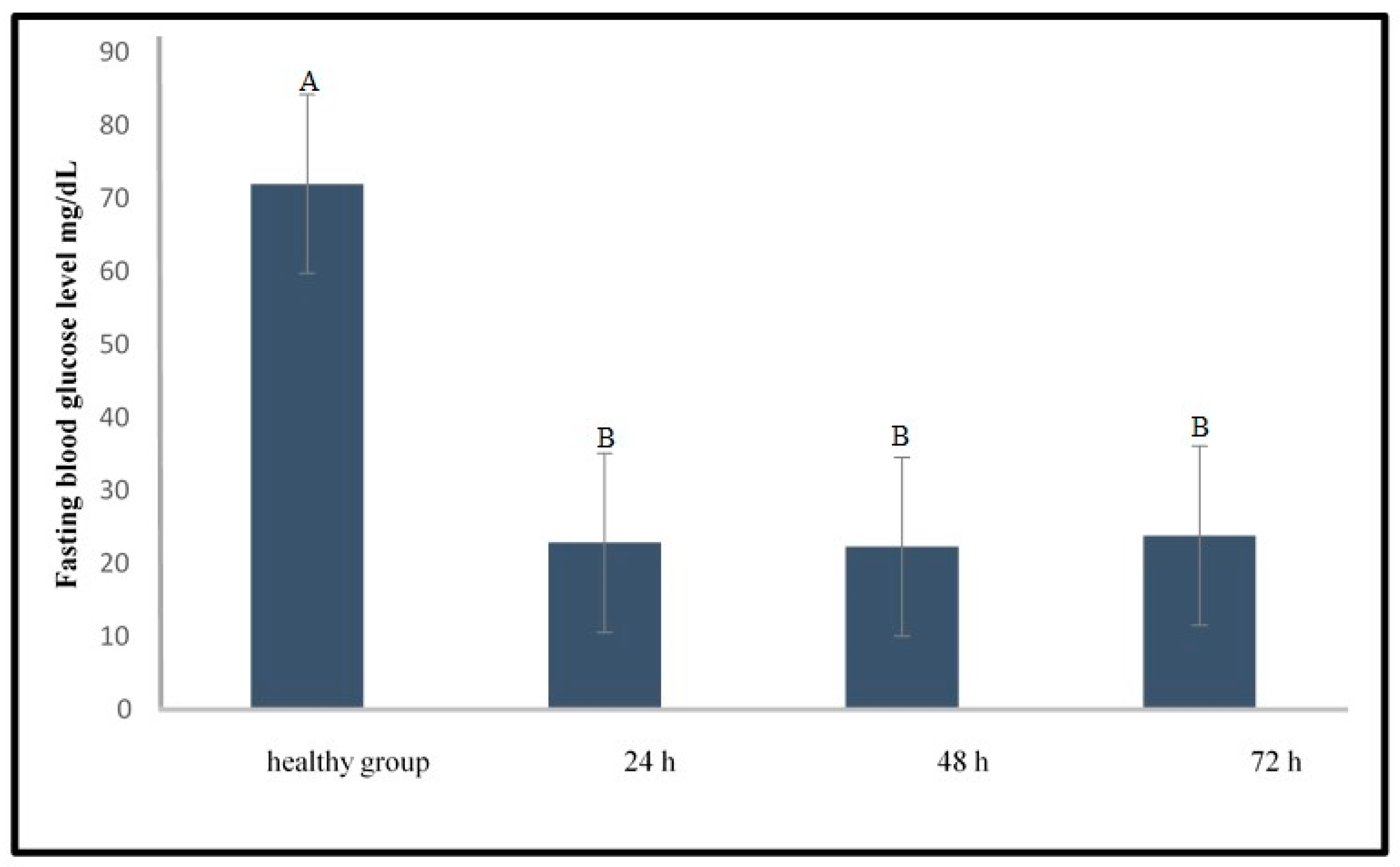
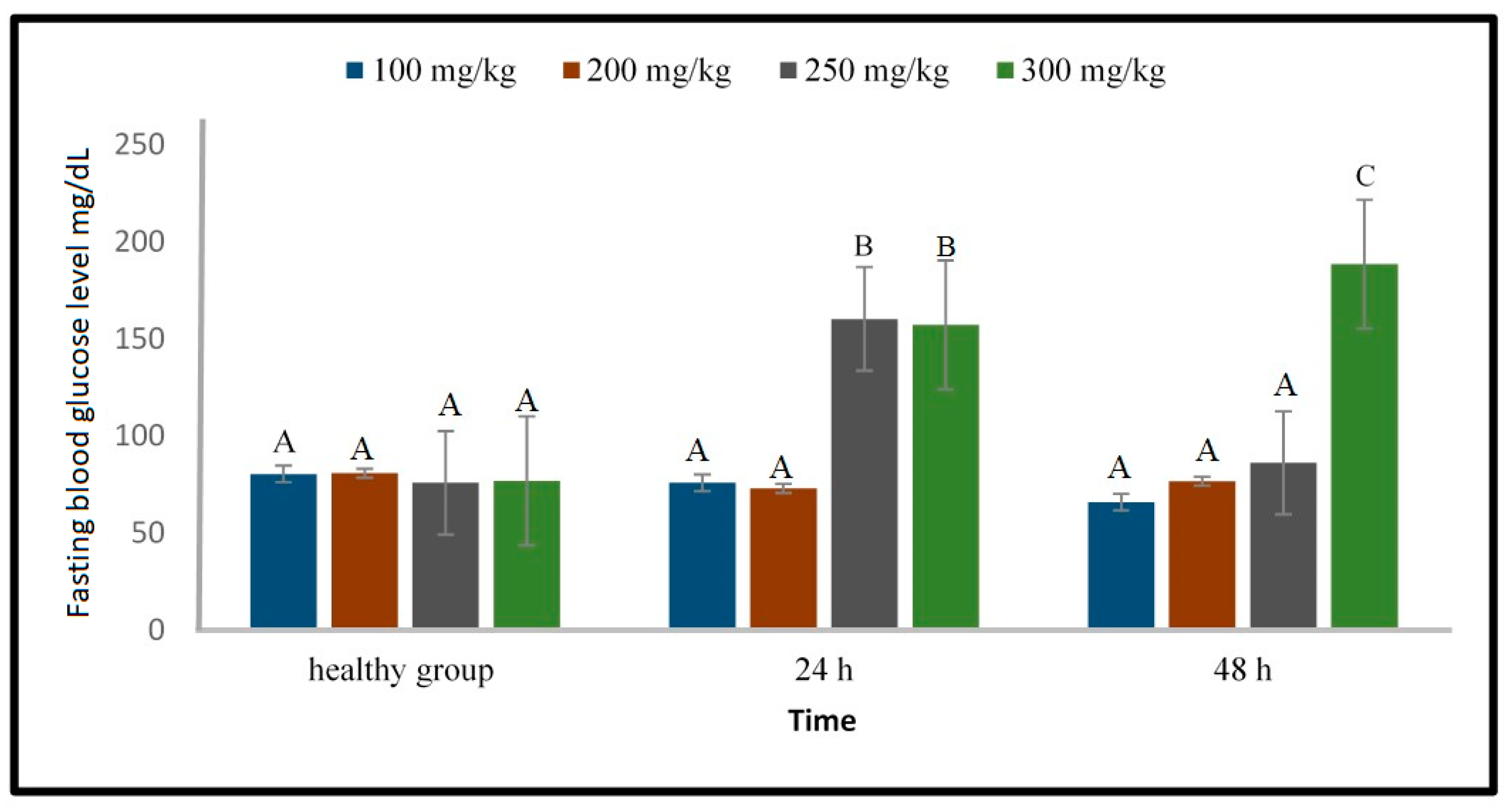
2.1.1. Induction of Type 1 Diabetes Using Streptozotocin
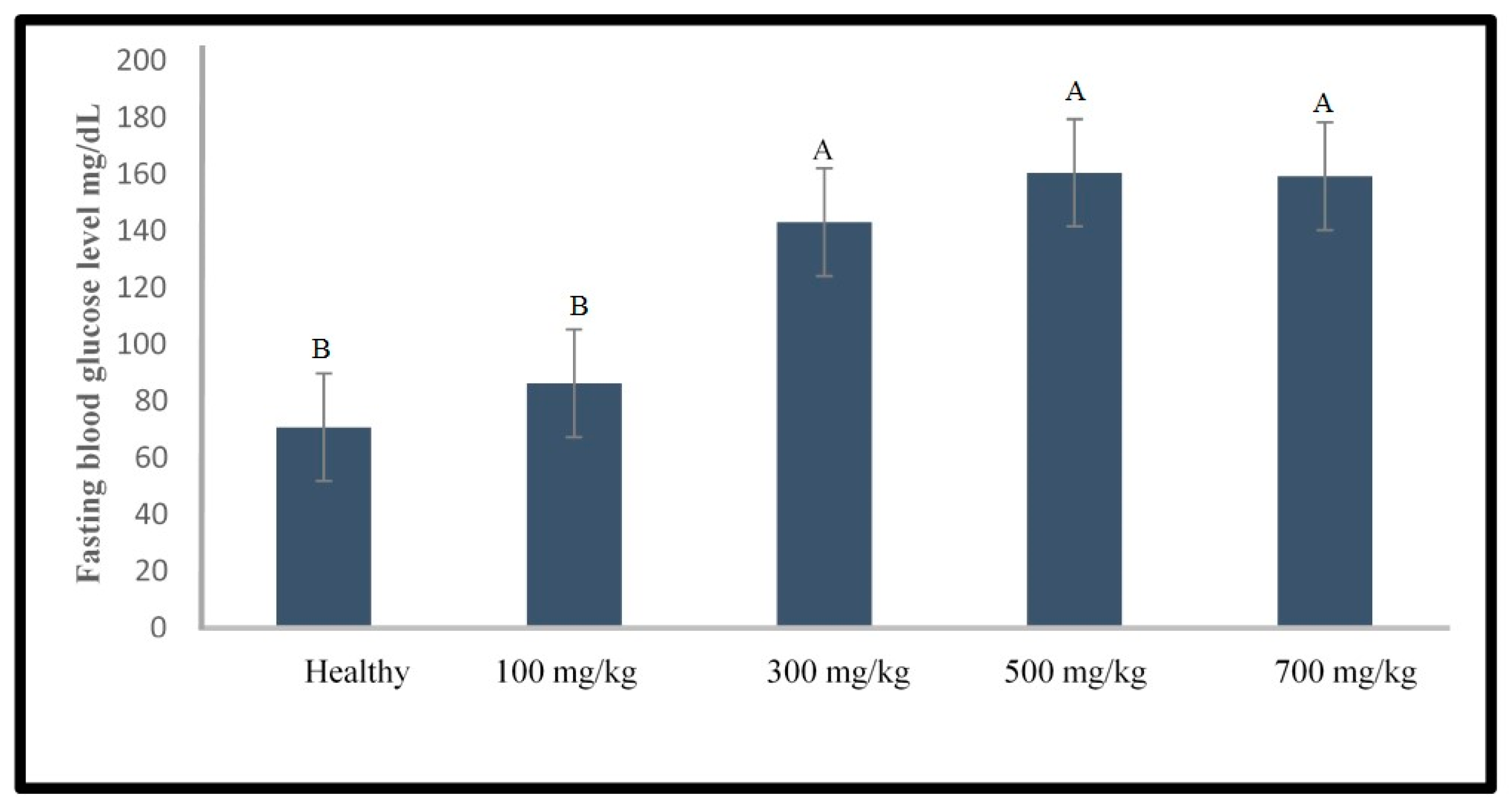
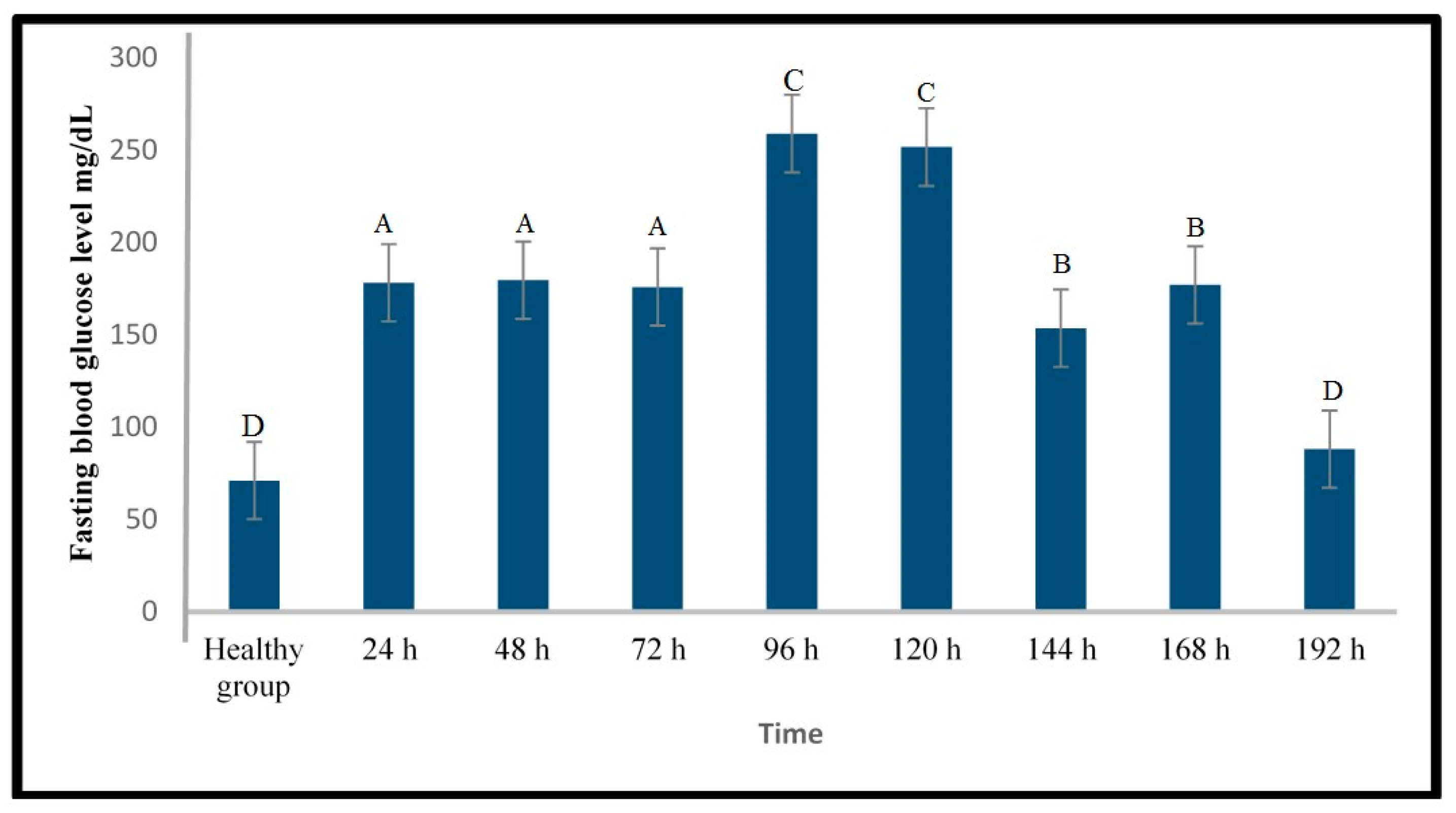
2.1.2. Induction of Type 1 Diabetes Using Alloxan
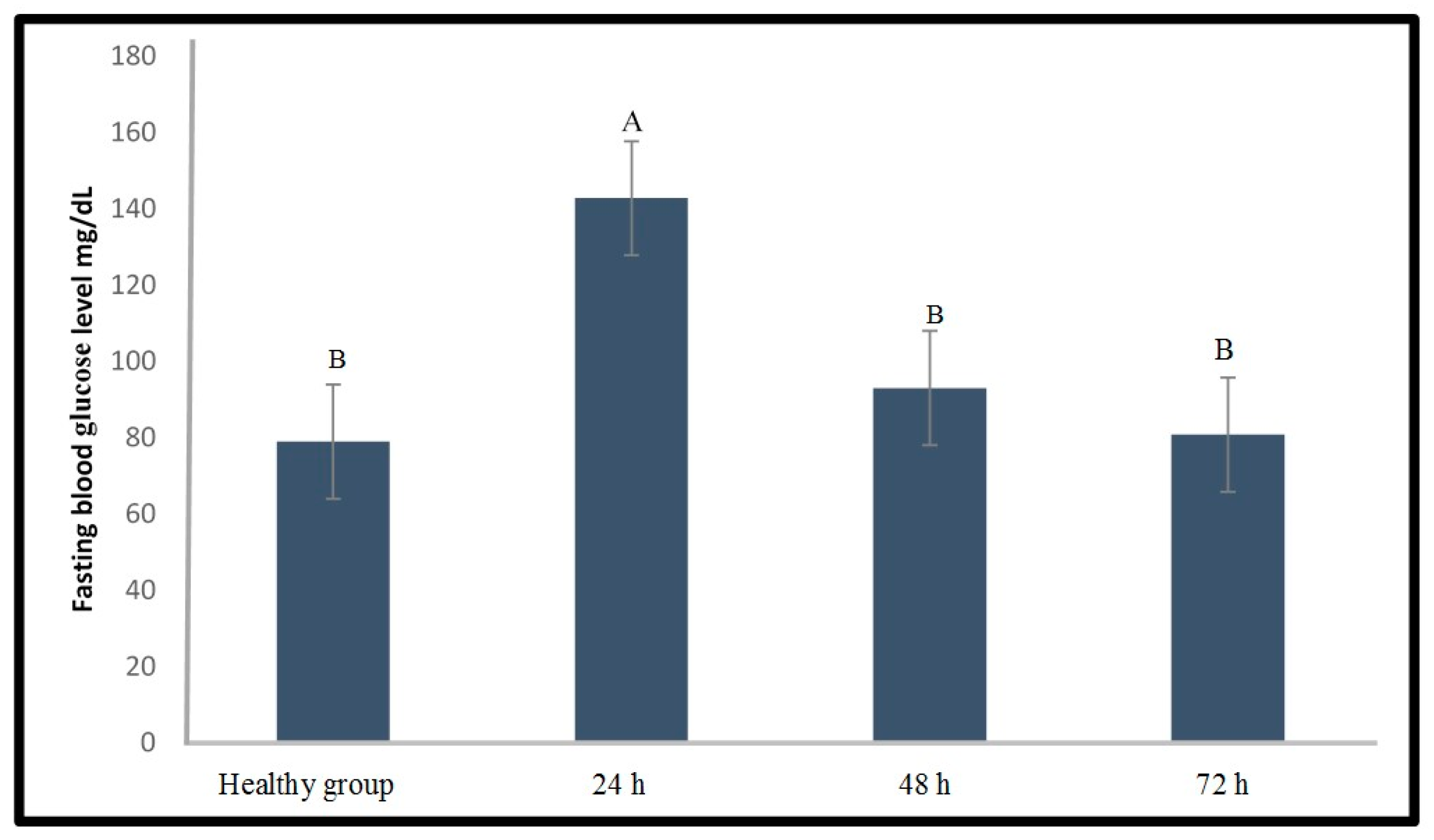
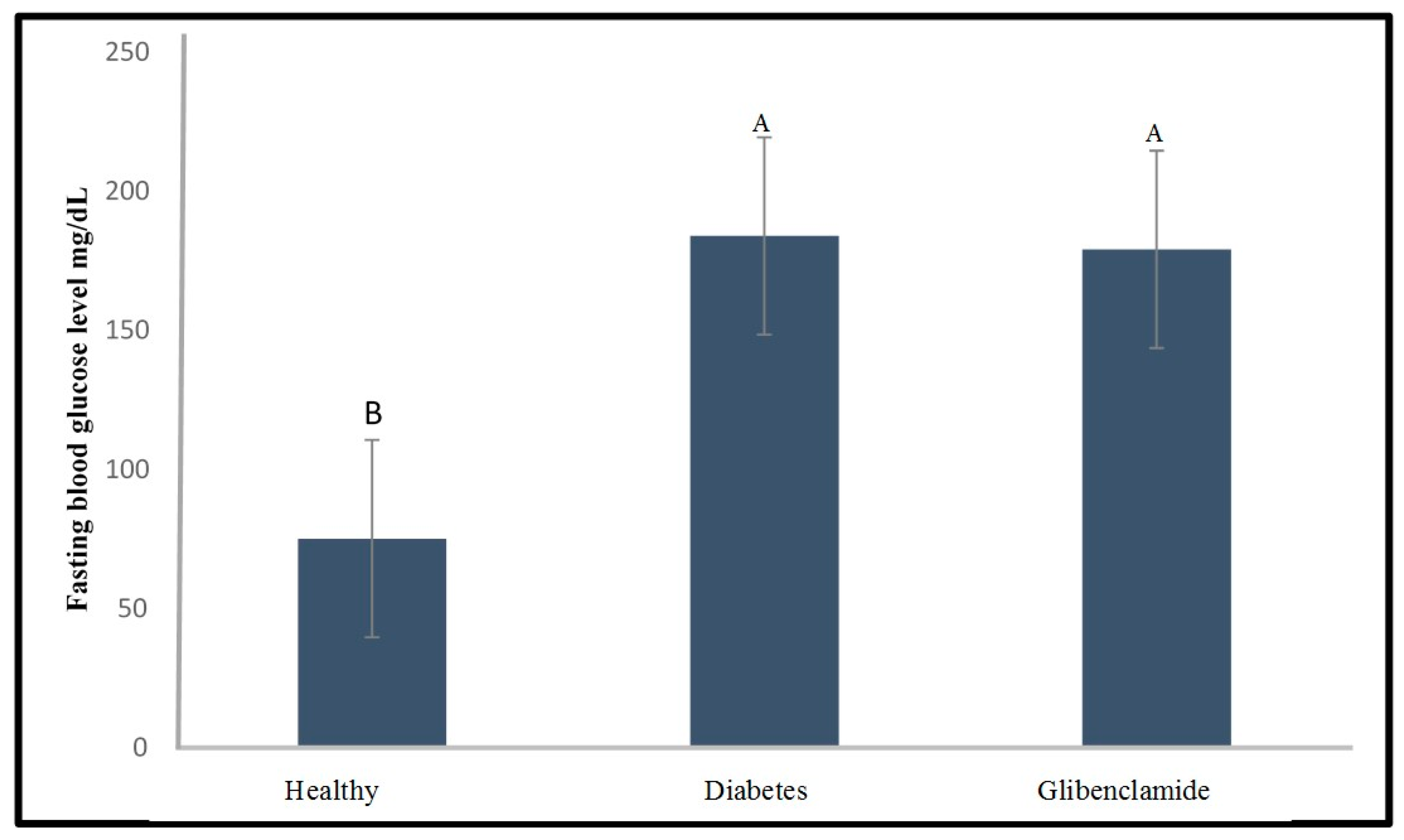
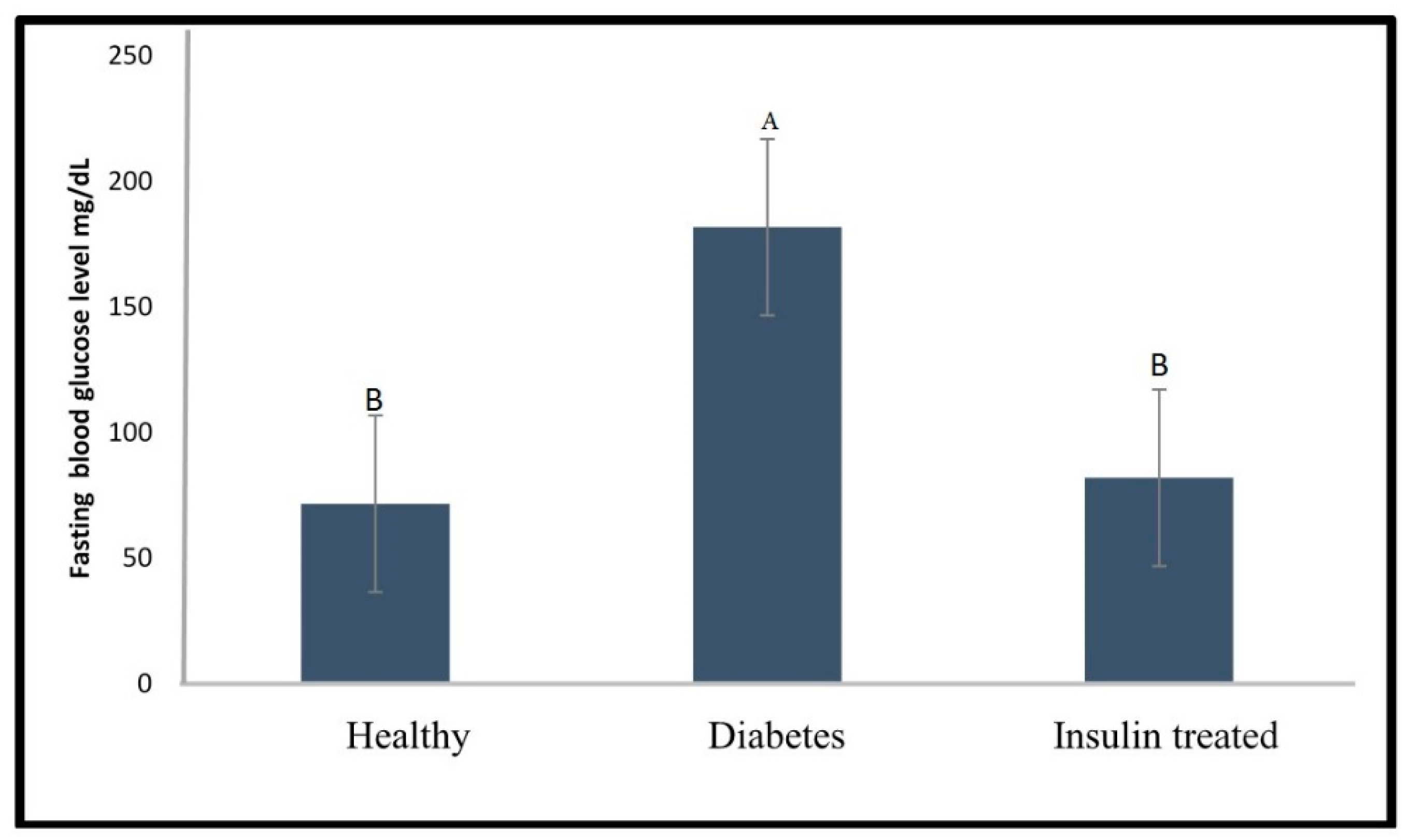
2.2. Effect of Glibenclamide and Insulin as a Positive Control on Fasting Blood Glucose Level of the Diabetic Zebrafish Model
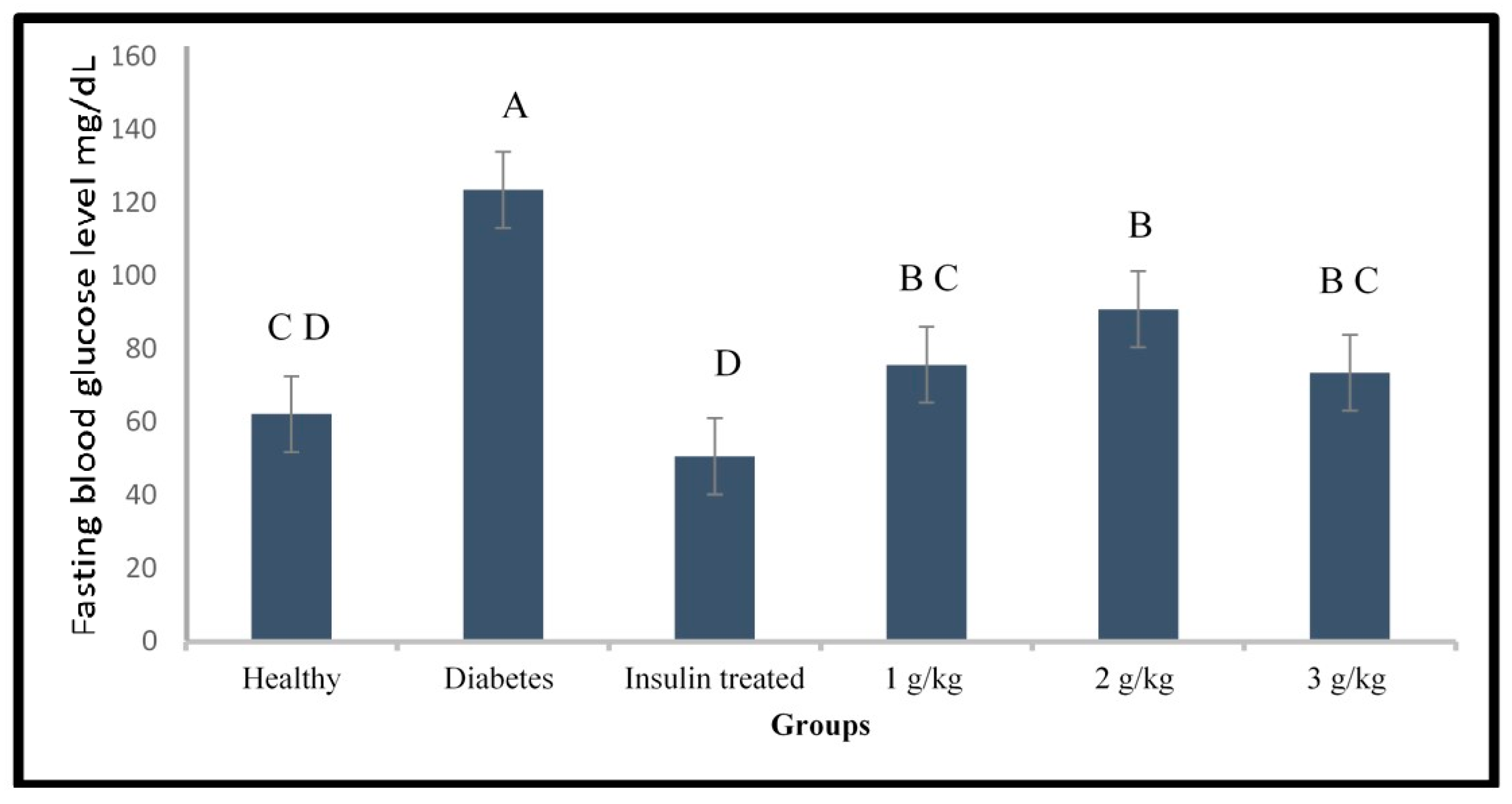
2.3. Evaluation of the Anti-Diabetic Activity of Psychotria Malayana Leaf on the Alloxan Induced Type 1 Diabetes Zebrafish
2.4. GC-MS Analysis of the P. malayana Leaf Extracts
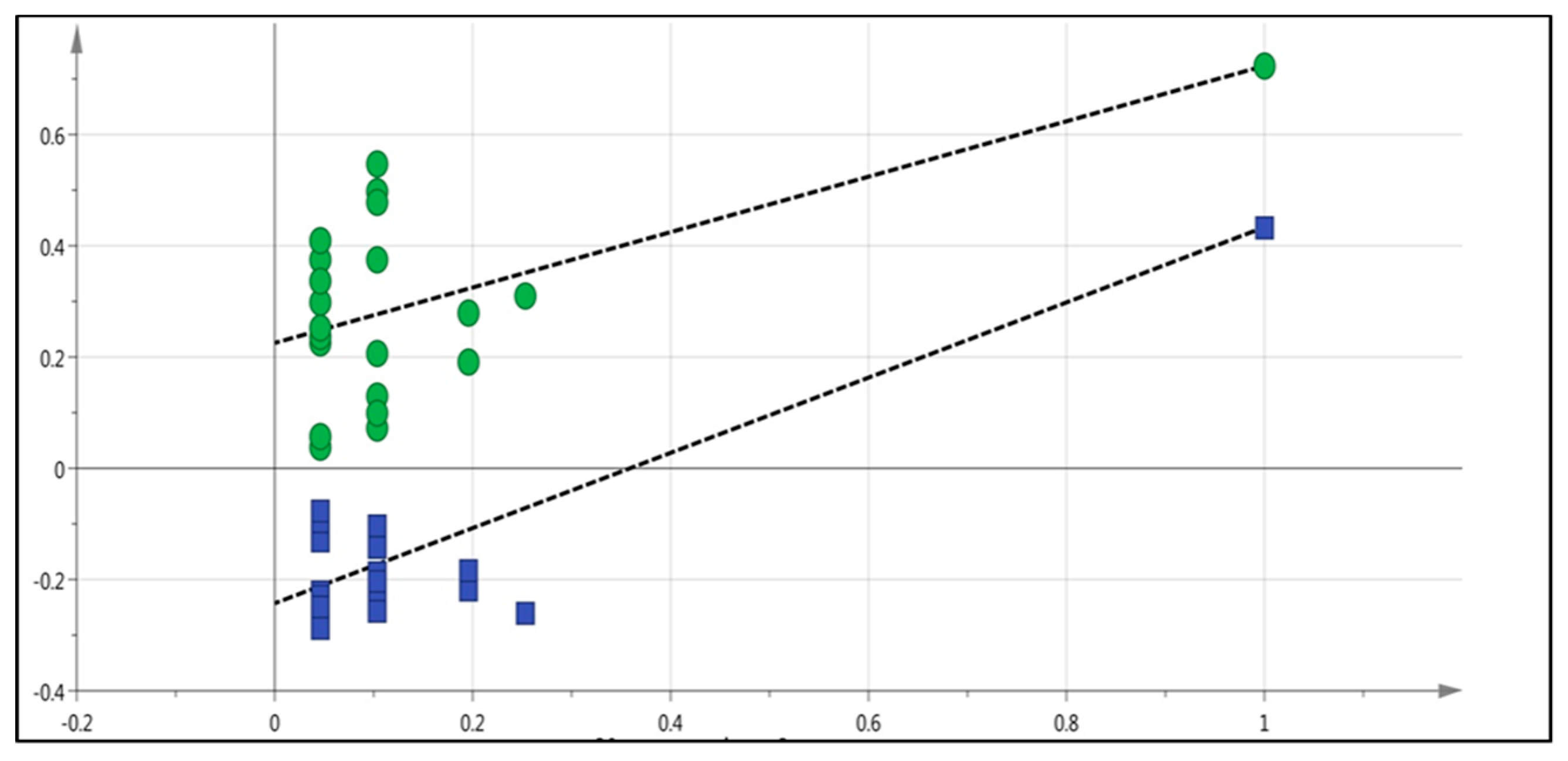
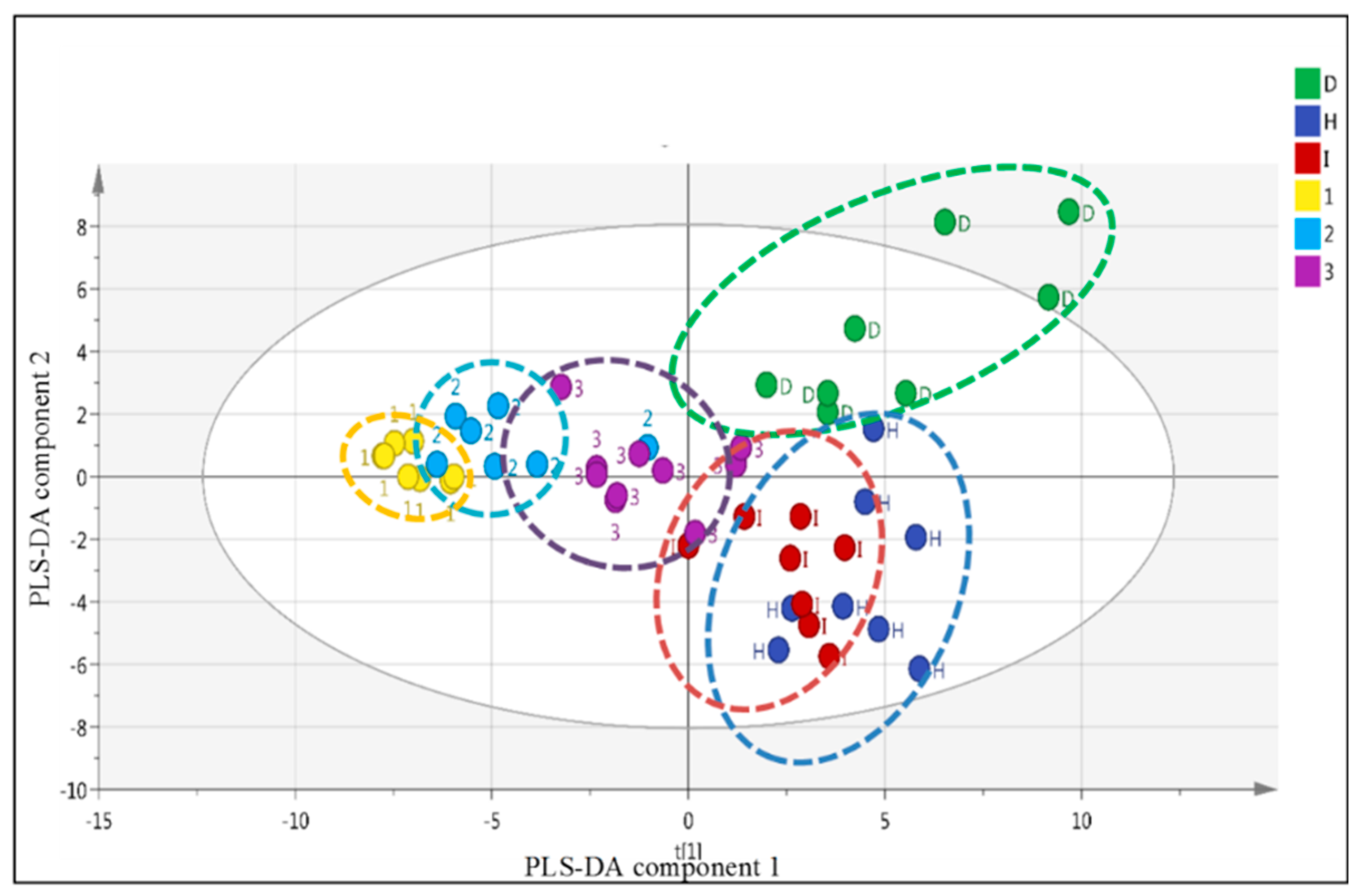
2.5. LC-MS Based Fingerprinting of Zebrafish Serum
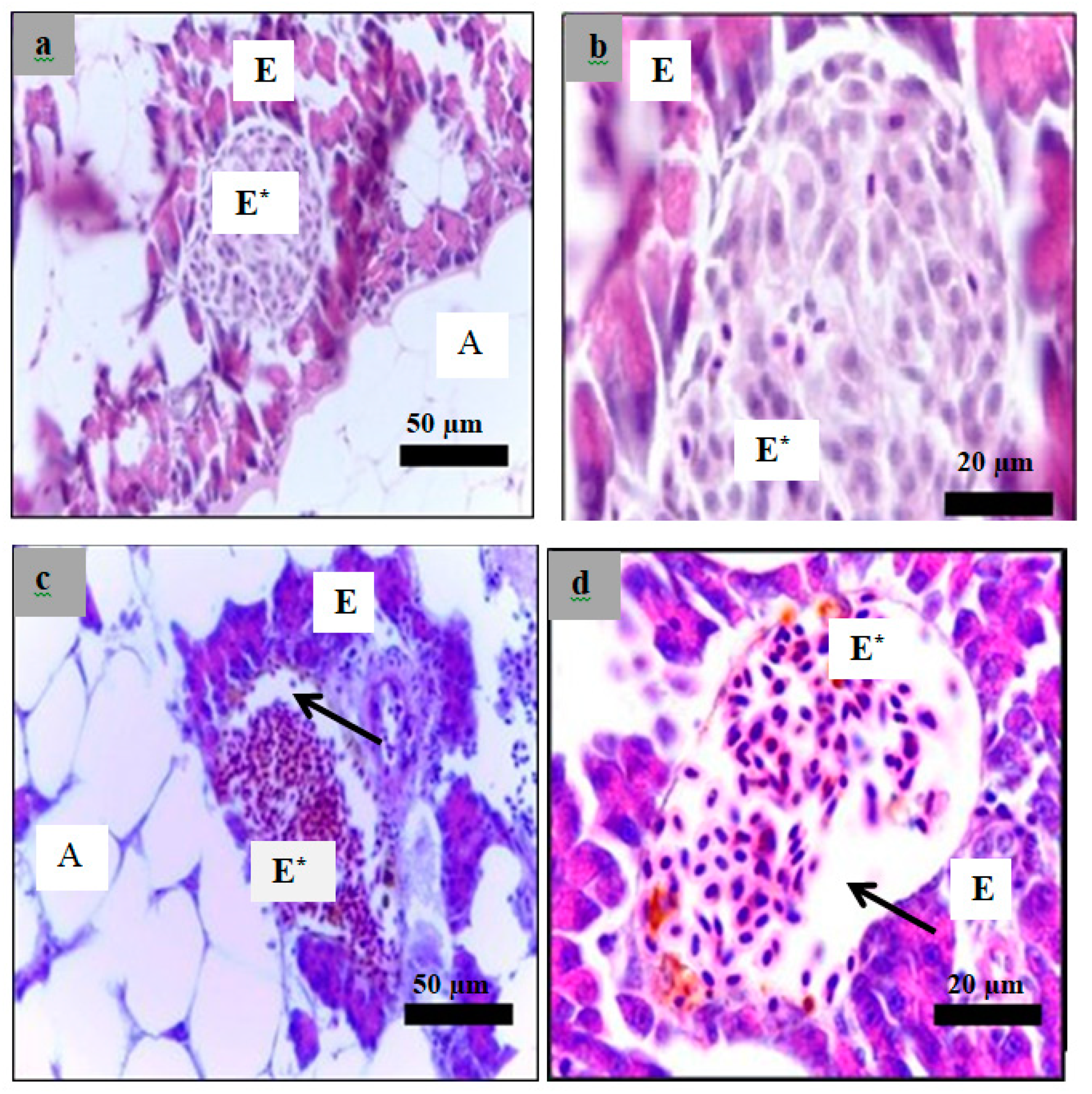
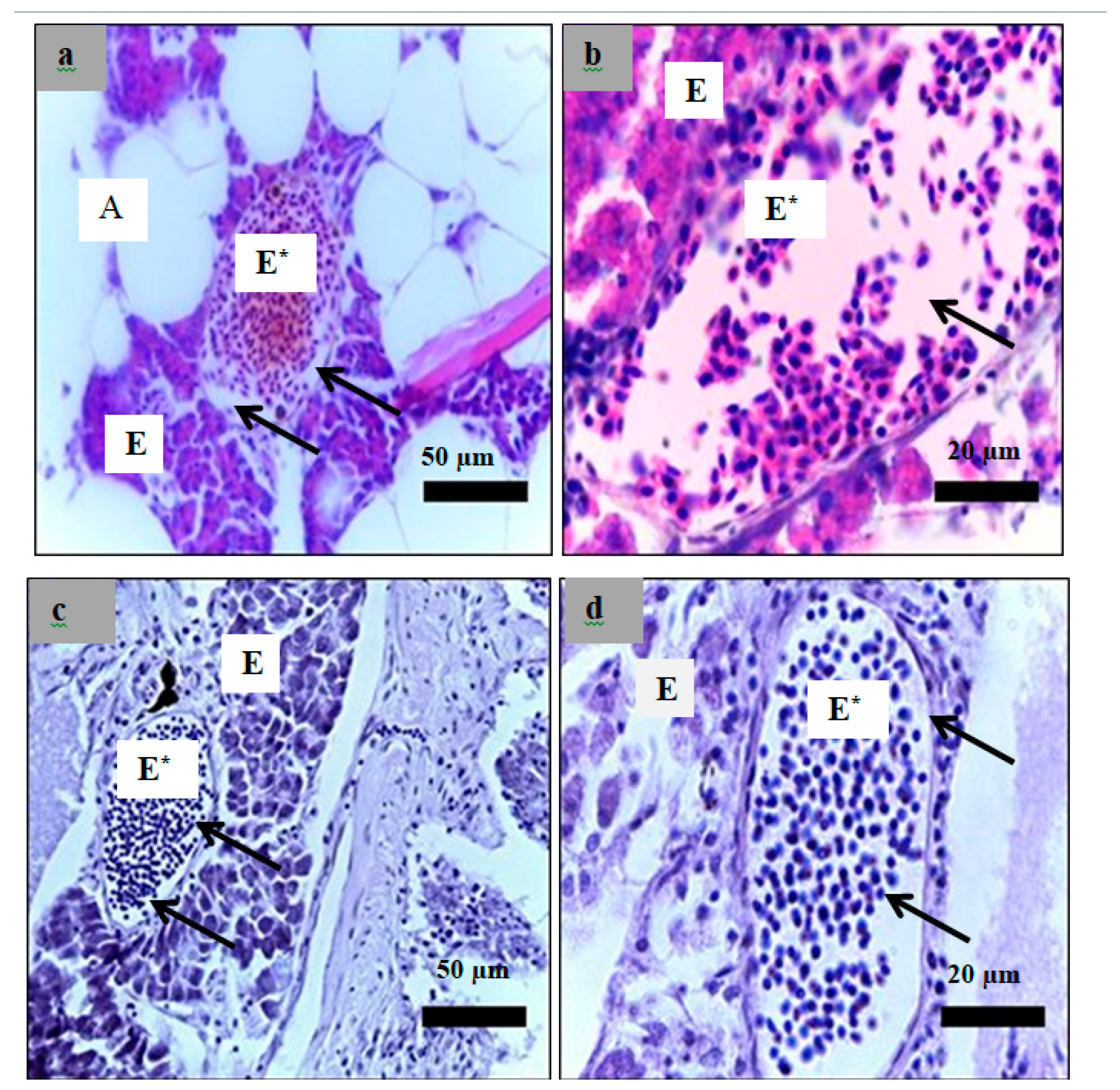
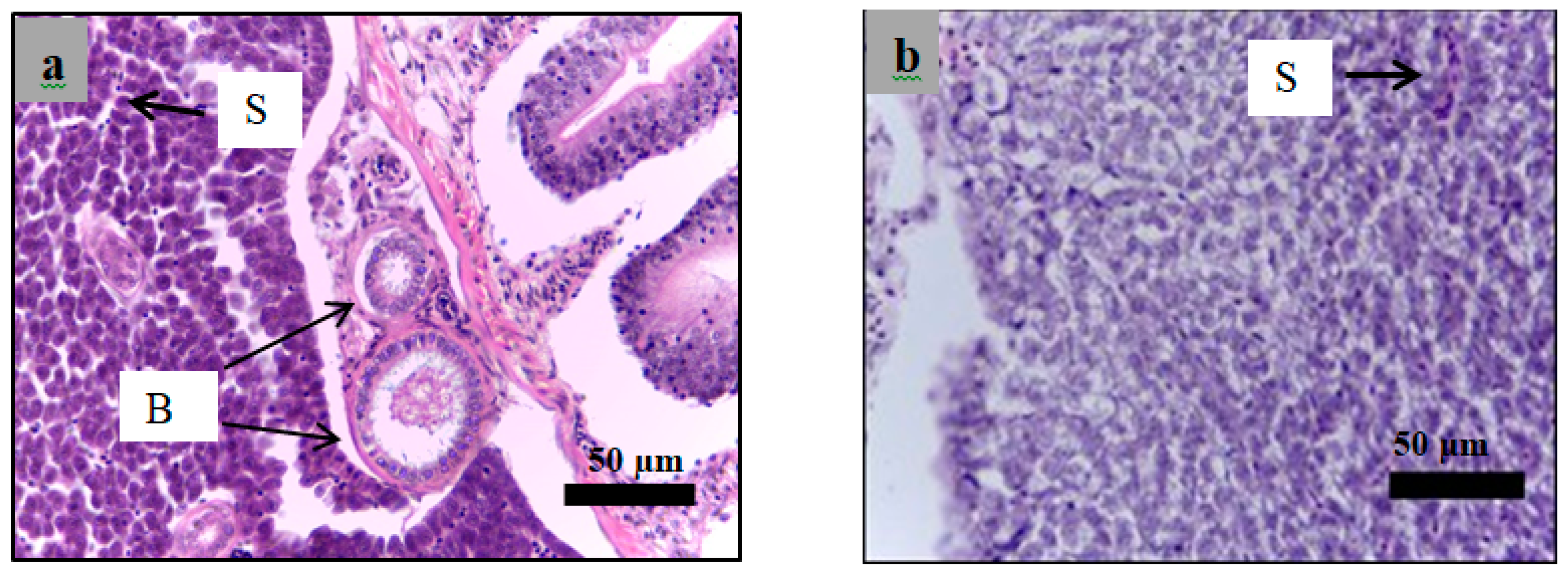
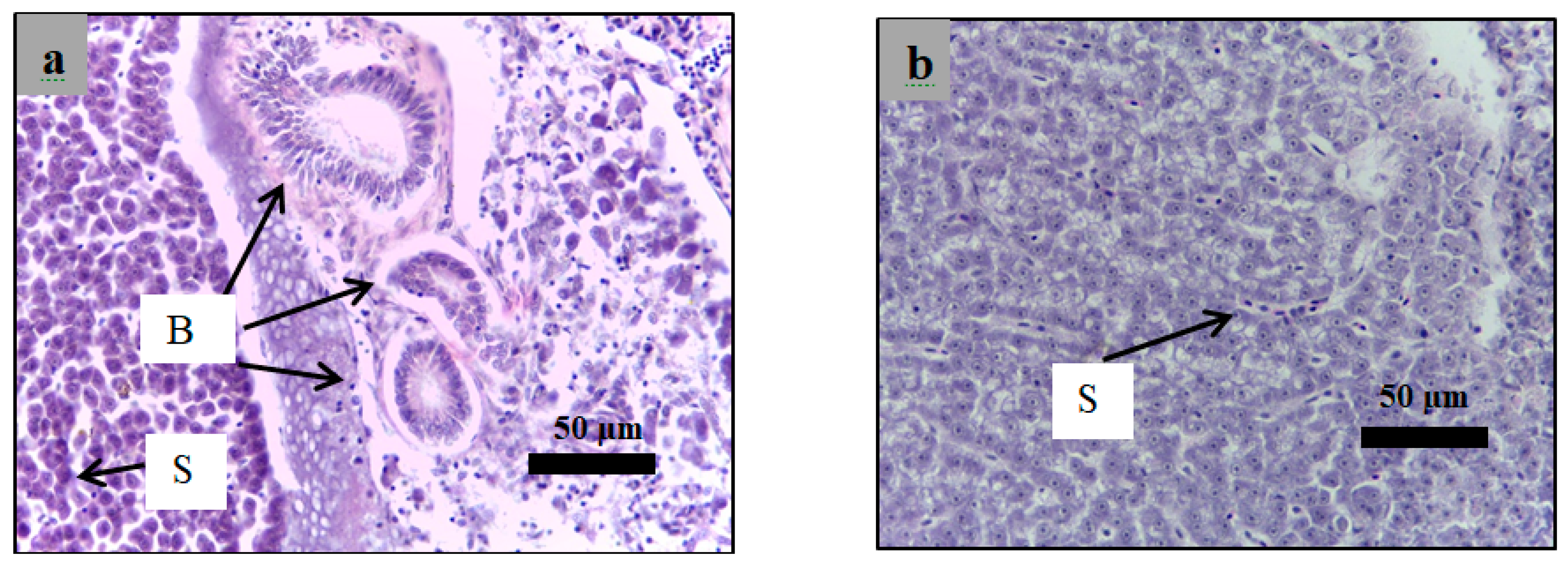
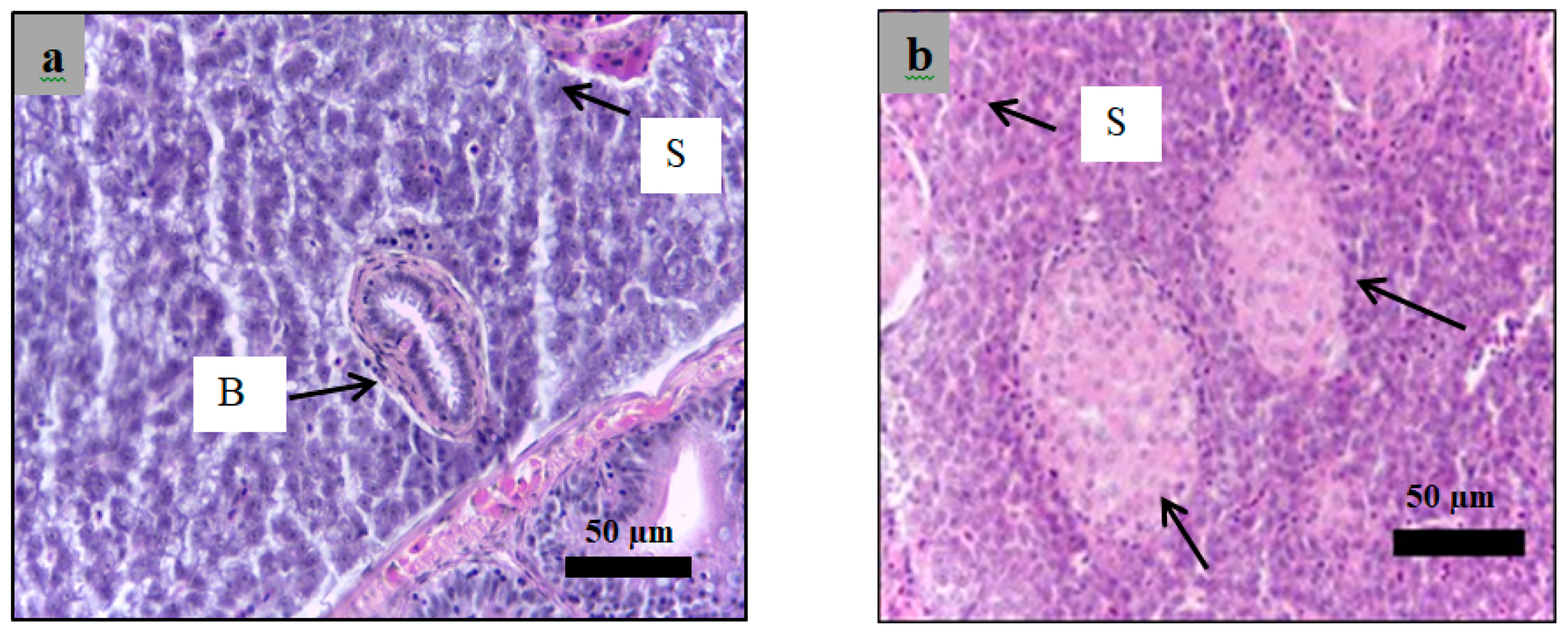
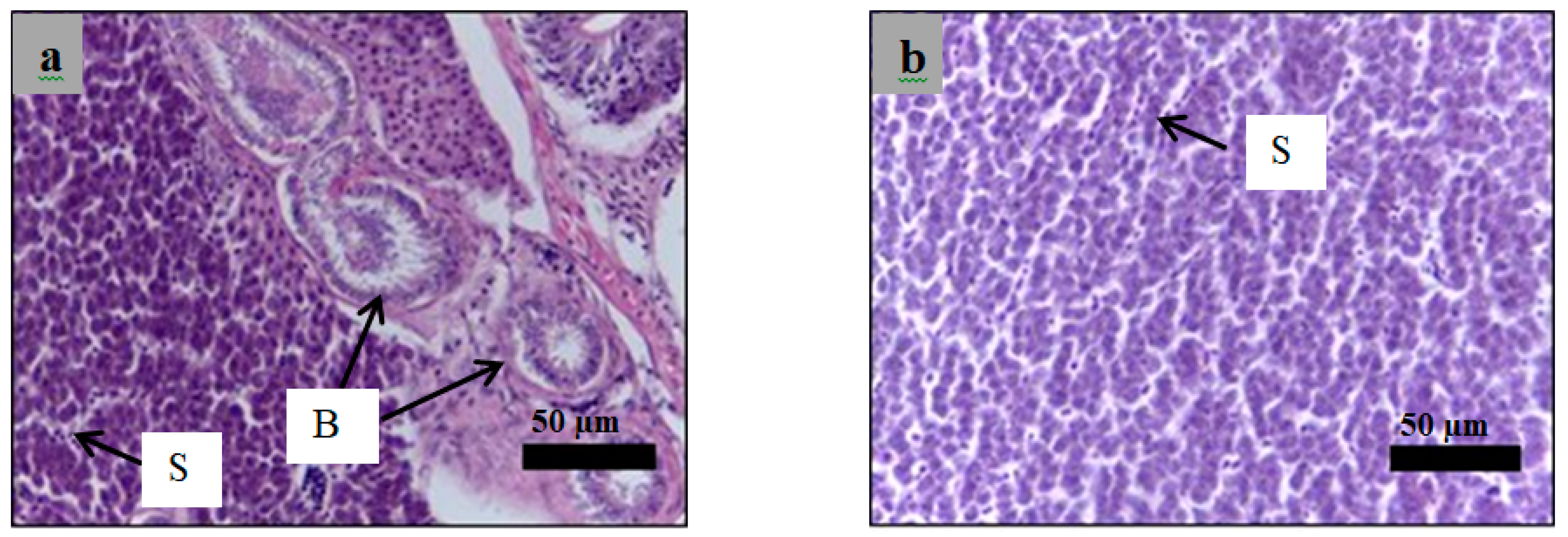
2.6. Histological Examination
3. Discussion
3.1. Development of the Induced Type 1 Diabetes Zebrafish Model
3.2. Effect of Glibenclamide and Insulin on Fasting Blood Glucose Level in Diabetic’s Zebrafish Model
3.3. Anti-Diabetic Activity of P. malayana Leaf in Alloxan-Induced Type 1 Diabetic Zebrafish
4. Materials and Methods
4.1. Chemicals
4.2. Maintenance of the Fish
4.3. Induction of Type 1 Diabetes Using Streptozotocin
4.4. Induction of Type 1 Diabetes Using Alloxan
4.5. Treatment of the Fish
4.6. Preparation of the Plant Extract and Force-Feeding Procedure
4.7. Derivatization Procedure of P. malayana Extract
4.8. GC-MS Analysis of P. malayana Extract
4.9. LC-MS-Q TOF Based Fingerprinting
4.10. Histological Study
4.10.1. Organ Harvesting and Fixation
4.10.2. Tissue Processing
4.10.3. Tissue Sectioning and Hematoxyline and Eosin (H and E) Staining
5. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.; Torroja, C.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Biemar, F.; Argenton, F.; Schmidtke, R.; Epperlein, S.; Peers, B.; Driever, W. Pancreas development in zebrafish: Early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev. Biol. 2001, 230, 189–203. [Google Scholar] [CrossRef]
- Wendik, B.; Maier, E.; Meyer, D. Zebrafish mnx genes in endocrine and exocrine pancreas formation. Dev. Biol. 2004, 268, 372–383. [Google Scholar] [CrossRef]
- Tabassum, N.; Tai, H.; Jung, D.W.; Williams, D.R. Fishing for Nature’s Hits: Establishment of the Zebrafish as a Model for Screening Antidiabetic Natural Products. Evidence-based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Junior, A.R.; Vieira, I.J.C.; de Carvalho, M.G.; Braz-Filho, R.; S Lima, M.A.; Ferreira, R.O.; José Maria, E.; de Oliveira, D.B. 13C-NMR Spectral Data of Alkaloids Isolated from Psychotria Species (Rubiaceae). Molecules 2017, 22, 103. [Google Scholar]
- Currais, A.; Chiruta, C.; Goujon-Svrzic, M.; Costa, G.; Santos, T.; Batista, M.T.; Paiva, J.; do Céu Madureira, M.; Maher, P. Screening and identification of neuroprotective compounds relevant to Alzheimer׳ s disease from medicinal plants of S. Tomé e Príncipe. J. Ethnopharmacol. 2014, 155, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.S.N.; Volobuff, C.R.F.; Santiago, M.; Cardoso, C.A.L.; Vieira, M.D.C.; Valdevina Pereira, Z. Evaluation of antioxidant activity, total flavonoids, tannins and phenolic compounds in psychotria leaf extracts. Antioxidants 2014, 3, 745–757. [Google Scholar] [CrossRef]
- Snyder, R.W.; Berns, J.S. Reviews: Use of insulin and oral hypoglycemic medications in patients with diabetes mellitus and advanced kidney disease. Semin. Dial. 2004, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Shah, A.; Ferro, A. Pharmacological and non-pharmacological treatment of endothelial dysfunction: Relevance to diabetes. Br. J. Diabetes Vasc. Dis. 2007, 7, 5–10. [Google Scholar] [CrossRef]
- Intine, R.V.; Olsen, A.S.; Sarras, M.P. A Zebrafish Model of Diabetes Mellitus and Metabolic Memory. J. Vis. Exp. 2013, 72, 50232. [Google Scholar] [CrossRef]
- Mostafavinia, A.; Amini, A.; Ghorishi, S.K.; Pouriran, R.; Bayat, M. The effects of dosage and the routes of administrations of streptozotocin and alloxan on induction rate of type1 diabetes mellitus and mortality rate in rats. Lab. Anim. Res. 2016, 32, 160–165. [Google Scholar] [CrossRef]
- Sharif, K.M.; Rahman, M.M.; Azmir, J.; Khatib, A.; Hadijah, S.; Mohamed, A.; Sahena, F.; Zaidul, I.S.M. Orthogonal partial least squares model for rapid prediction of antioxidant activity of Pereskia bleo by Fourier transform infrared spectroscopy. Anal. Lett. 2014, 47, 2061–2071. [Google Scholar] [CrossRef]
- Elsner, M.; Guldbakke, B.; Tiedge, M.; Munday, R.; Lenzen, S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia 2000, 43, 1528–1533. [Google Scholar] [CrossRef]
- Parkman, H.P.; Fass, R.; Foxx-Orenstein, A.E. Treatment of patients with diabetic gastroparesis. Gastroenterol. Hepatol. 2010, 6, 1. [Google Scholar]
- Jörgens, K.; Hillebrands, J.L.; Hammes, H.P.; Kroll, J. Zebrafish: A model for understanding diabetic complications. Exp. Clin. Endocrinol. Diabetes 2012, 120, 186–187. [Google Scholar] [CrossRef]
- Shin, E.; Hong, B.N.; Kang, T.H. An optimal establishment of an acute hyperglycemia zebrafish model. Afr. J. Pharm. Pharmacol. 2012, 6, 2922–2928. [Google Scholar] [CrossRef]
- Moss, J.B.; Koustubhan, P.; Greenman, M.; Parsons, M.J.; Walter, I.; Moss, L.G. Regeneration of the pancreas in adult zebrafish. Diabetes 2009, 58, 1844–1851. [Google Scholar] [CrossRef]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar]
- Gorus, F.K.; Malaisse, W.J.; Pipeleers, D.G. Selective uptake of alloxan by pancreatic B-cells. Biochem. J. 1982, 208, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S.; Mirzaie-petri, M. Inhibition of glucokinase and hexokinase from pancreatic b-cells and liver by alloxan, alloxantin, dialuric acid, and i-butylhydroperoxide. Biomed. Res. 1991, 12, 297–307. [Google Scholar] [CrossRef]
- Danial, N.N.; Gramm, C.F.; Scorrano, L.; Zhang, C.-Y.; Krauss, S.; Ranger, A.M.; Robert Datta, S.; Greenberg, M.E.; Licklider, L.J.; Lowell, B.B.; et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 2003, 424, 952–956. [Google Scholar] [CrossRef]
- Bedoya, F.J.; Solano, F.; Lucas, M. N-monomethyl-arginine and nicotinamide prevent streptozotocin-induced double strand DNA break formation in pancreatic rat islets. Experientia 1996, 52, 344–347. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Roselló-Catafau, J.; Jawerbaum, A.; Sinner, D.; Pustovrh, C.; Vela, J.; White, V.; Xaus, C.; Peralta, C.; Gimeno, M. Pancreatic nitric oxide and oxygen free radicals in the early stages of streptozotocin-induced diabetes mellitus in the rat. Brazilian J. Med. Biol. Res. 2000, 33, 1335–1342. [Google Scholar] [CrossRef][Green Version]
- Lu, J.; Liu, K.; Schulz, N.; Karampelias, C.; Charbord, J.; Hilding, A.; Rautio, L.; Bertolino, P.; Östenson, C.; Brismar, K. IGFBP1 increases β-cell regeneration by promoting α-to β-cell transdifferentiation. EMBO J. 2016, 35, 2026–2044. [Google Scholar] [CrossRef]
- Beer, R.L.; Parsons, M.J.; Rovira, M. Centroacinar cells: At the center of pancreas regeneration. Dev. Biol. 2016, 413, 8–15. [Google Scholar] [CrossRef]
- Luzi, L.; Pozza, G. Glibenclamide: An old drug with a novel mechanism of action? Acta Diabetol. 1997, 34, 239–244. [Google Scholar] [CrossRef]
- Müller, G. The mode of action of the antidiabetic drug glimepiride-beyond insulin secretion. Curr. Med. Chem. Immunol. Endocr. Metab. Agents 2005, 5, 499–518. [Google Scholar] [CrossRef]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. AMS 2015, 11, 840. [Google Scholar] [CrossRef]
- Joya-Galeana, J.; Fernandez, M.; Cervera, A.; Reyna, S.; Ghosh, S.; Triplitt, C.; Musi, N.; DeFronzo, R.A.; Cersosimo, E. Effects of insulin and oral anti-diabetic agents on glucose metabolism, vascular dysfunction and skeletal muscle inflammation in type 2 diabetic subjects. Diabetes. Metab. Res. Rev. 2011, 27, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Capiotti, K.M.; Antonioli, R.; Kist, L.W.; Bogo, M.R.; Bonan, C.D.; Da Silva, R.S. Persistent impaired glucose metabolism in a zebrafish hyperglycemia model. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2014, 171, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Zheng, H.C.; Bukuru, J.; De Kimpe, N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J. Ethnopharmacol. 2004, 92, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mangelinckx, S.; Adams, A.; Wang, Z.T.; Li, W.L.; De, N.K. Natural flavonoids as potential herbal medication for the treatment of diabetes mellitus and its complications. Nat. Prod. Commun. 2015, 10, 187–200. [Google Scholar] [CrossRef]
- Hui, H.; Tang, G.; Go, V.L.W. Hypoglycemic herbs and their action mechanisms. Chin. Med. 2009, 4, 11. [Google Scholar] [CrossRef]
- Jemain, M.R.M.; Musa’adah, M.N.; Rohaya, A.; Rashid, L.A.; Hadiani, I.N. In vitro antihyperglycaemic effects of some Malaysian plants. J. Trop. For. Sci. 2011, 23, 467–472. [Google Scholar]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Yanardağ, R.; Bolkent, Ş.; Tabakoğlu-Oğuz, A.; Özsoy-Saçan, Ö. Effects of Petroselinum crispum extract on pancreatic B cells and blood glucose of streptozotocin-induced diabetic rats. Biol. Pharm. Bull. 2003, 26, 1206–1210. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yagi, N.; Taguchi, K. Inhibitory compounds of α-glucosidase activity from Arctium lappa L. J. Oleo Sci. 2005, 54, 589–594. [Google Scholar] [CrossRef]
- Liu, L.; Deseo, M.A.; Morris, C.; Winter, K.M.; Leach, D.N. Investigation of α-glucosidase inhibitory activity of wheat bran and germ. Food Chem. 2011, 126, 553–561. [Google Scholar] [CrossRef]
- Ortiz-Andrade, R.R.; Garcia-Jimenez, S.; Castillo-Espana, P.; Ramirez-Avila, G.; Villalobos-Molina, R.; Estrada-Soto, S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: An anti-hyperglycemic agent. J. Ethnopharmacol. 2007, 109, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, O.; Ying, S.; Yoshikai, K.; Matsuura, Y.; Yamada, E.; Minematsu, T.; Tanabe, G.; Matsuda, H.; Yoshikawa, M. Synthesis of a nitrogen analogue of salacinol and its α-glucosidase inhibitory activity. Chem. Pharm. Bull. 2001, 49, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. α-Glucosidase and α-amylase inhibitors from seed oil: A review of liposoluble substance to treat diabetes. Crit. Rev. Food Sci. Nutr. 2017, 57, 3438–3448. [Google Scholar] [CrossRef]
- Kandra, L.; Gyémánt, G.; Zajácz, Á.; Batta, G. Inhibitory effects of tannin on human salivary α-amylase. Biochem. Biophys. Res. Commun. 2004, 319, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.-U.; Nik Yusoff, N.-I.; Uzir, B.-F.; Perumal, V.; Abas, F.; Saari, K.; El-Seedi, H.; Khatib, A. Characterization of α-Glucosidase Inhibitors from Clinacanthus nutans Lindau Leaves by Gas Chromatography-Mass Spectrometry-Based Metabolomics and Molecular Docking Simulation. Molecules 2018, 23, 2402. [Google Scholar] [CrossRef]
- Bursell, S.-E.; King, G.L. Can protein kinase C inhibition and vitamin E prevent the development of diabetic vascular complications? Diabetes Res. Clin. Pract. 1999, 45, 169–182. [Google Scholar] [CrossRef]
- Joshi, S.R.; Standl, E.; Tong, N.; Shah, P.; Kalra, S.; Rathod, R. Therapeutic potential of α-glucosidase inhibitors in type 2 diabetes mellitus: An evidence-based review. Expert Opin. Pharmacother. 2015, 16, 1959–1981. [Google Scholar] [CrossRef]
- Chougale, A.D. Optimization of Alloxan Dose is Essential to Induce Stable Diabetes for Prolonged Period Ashok D. Chougale, Shrimant N. Panaskar, Pradeep M. Gurao and Akalpita U. Arvindekar Department of Biochemistry, Shivaji University, Kolhapur, 416 004, India. Asian J. Biochem. 2007, 2, 402–408. [Google Scholar]
- Capiotti, K.M.; De Moraes, D.A.; Menezes, F.P.; Kist, L.W.; Bogo, M.R.; Da Silva, R.S. Hyperglycemia induces memory impairment linked to increased acetylcholinesterase activity in zebrafish (Danio rerio). Behav. Brain Res. 2014, 274, 319–325. [Google Scholar] [CrossRef]
- Elo, B.; Villano, C.M.; Govorko, D.; White, L.A. Larval zebrafish as a model for glucose metabolism: Expression of phosphoenolpyruvate carboxykinase as a marker for exposure to anti-diabetic compounds. J. Mol. Endocrinol. 2007, 38, 433–440. [Google Scholar] [CrossRef]
- Javadi, N.; Abas, F.; Hamid, A.A.; Simoh, S.; Shaari, K.; Ismail, I.S.; Mediani, A.; Khatib, A. GC-MS-Based Metabolite Profiling of Cosmos caudatus Leaves Possessing Alpha-Glucosidase Inhibitory Activity. J. Food Sci. 2014, 79, C1130–C1136. [Google Scholar] [CrossRef] [PubMed]
- Khatib, A.; Yuliana, N.D.; Jinap, S.; Sarker, M.Z.I.; Jaswir, I.; Wilson, E.G.; Chung, S.-K.; Verpoorte, R. Identification of possible compounds possessing adenosine A1 receptor binding activity in the leaves of Orthosiphon stamineus using TLC and multivariate data analysis. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 2906–2916. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 6th ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2008; pp. 53–121. [Google Scholar]
- Tsao-Wu, G.S.; Weber, C.H.; Budgeon, L.R.; Cheng, K.C. Agarose-embedded tissue arrays for histologic and genetic analysis. Biotechniques 1998, 25, 614–618. [Google Scholar] [CrossRef]
- Copper, J.E.; Budgeon, L.R.; Foutz, C.A.; van Rossum, D.B.; Vanselow, D.J.; Hubley, M.J.; Clark, D.P.; Mandrell, D.T.; Cheng, K.C. Comparative analysis of fixation and embedding techniques for optimized histological preparation of zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 208, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Matthews, M.; Varga, Z.M. Anesthesia and euthanasia in zebrafish. ILAR J. 2012, 53, 192–204. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Sample of the P. malayana leaf is not available from the authors. |
| Tentative Compounds | Retention Time (min) | Similarity Index | Peak Area (%) |
|---|---|---|---|
| Erythrono-1,4-lactone | 21.1 | 95 | 0.03 |
| Erythritol | 29.4 | 90 | 0.09 |
| Xylonic acid | 35.9 | 86 | 0.02 |
| 1,3,5-Benzetriol | 37.2 | 99 | 0.03 |
| Xylitol | 41.6 | 93 | 0.09 |
| Shikimic acid | 46.7 | 98 | 0.49 |
| Quininic acid | 48.8 | 91 | 1.31 |
| Cyanuric acid | 49.0 | 80 | 0.02 |
| d-Fructose | 49.6 | 91 | 4.91 |
| d-Galactose | 50.5 | 91 | 0.44 |
| d-Allose | 50.7 | 91 | 0.68 |
| d-Mannose | 50.9 | 91 | 9.81 |
| Galactose oxime | 51.7 | 93 | 1.80 |
| l-Sorbose | 52.9 | 93 | 0.72 |
| 3,4-Dihydroxyphenylglycol | 56.9 | 91 | 0.08 |
| Palmitic acid | 57.9 | 99 | 0.01 |
| Myo-inositol | 60.1 | 90 | 5.94 |
| Stearic acid | 67.3 | 98 | 0.05 |
| Cholesta-7,9(11)-dien-3-ol | 78.3 | 95 | 1.09 |
| 1-Monopalmitin | 82.0 | 93 | 0.14 |
| Sucrose | 84.1 | 93 | 33.53 |
| d-Trehalose | 87.8 | 93 | 0.43 |
| Glycerol monostearate | 89.3 | 90 | 0.10 |
| Supraene | 90.3 | 93 | 0.10 |
| 4-(1H-Pyrrol-1-yl)-piperidine | 98.3 | 93 | 0.08 |
| Alpha-tocopherol | 101.2 | 98 | 0.22 |
| Beta-sitosterol | 107.2 | 99 | 0.27 |
| 5-Beta-cholest-24-en-12-one | 108.5 | 90 | 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benchoula, K.; Khatib, A.; Quzwain, F.M.C.; Che Mohamad, C.A.; Wan Sulaiman, W.M.A.; Abdul Wahab, R.; Ahmed, Q.U.; Abdul Ghaffar, M.; Saiman, M.Z.; Alajmi, M.F.; et al. Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract. Molecules 2019, 24, 1506. https://doi.org/10.3390/molecules24081506
Benchoula K, Khatib A, Quzwain FMC, Che Mohamad CA, Wan Sulaiman WMA, Abdul Wahab R, Ahmed QU, Abdul Ghaffar M, Saiman MZ, Alajmi MF, et al. Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract. Molecules. 2019; 24(8):1506. https://doi.org/10.3390/molecules24081506
Chicago/Turabian StyleBenchoula, Khaled, Alfi Khatib, Fairuz M. C. Quzwain, Che Anuar Che Mohamad, Wan Mohd Azizi Wan Sulaiman, Ridhwan Abdul Wahab, Qamar Uddin Ahmed, Majid Abdul Ghaffar, Mohd Zuwairi Saiman, Mohamed F. Alajmi, and et al. 2019. "Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract" Molecules 24, no. 8: 1506. https://doi.org/10.3390/molecules24081506
APA StyleBenchoula, K., Khatib, A., Quzwain, F. M. C., Che Mohamad, C. A., Wan Sulaiman, W. M. A., Abdul Wahab, R., Ahmed, Q. U., Abdul Ghaffar, M., Saiman, M. Z., Alajmi, M. F., & El-Seedi, H. (2019). Optimization of Hyperglycemic Induction in Zebrafish and Evaluation of Its Blood Glucose Level and Metabolite Fingerprint Treated with Psychotria malayana Jack Leaf Extract. Molecules, 24(8), 1506. https://doi.org/10.3390/molecules24081506