Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides
Abstract
1. Introduction
2. Anti-Diabetic Potentials of Polysaccharides
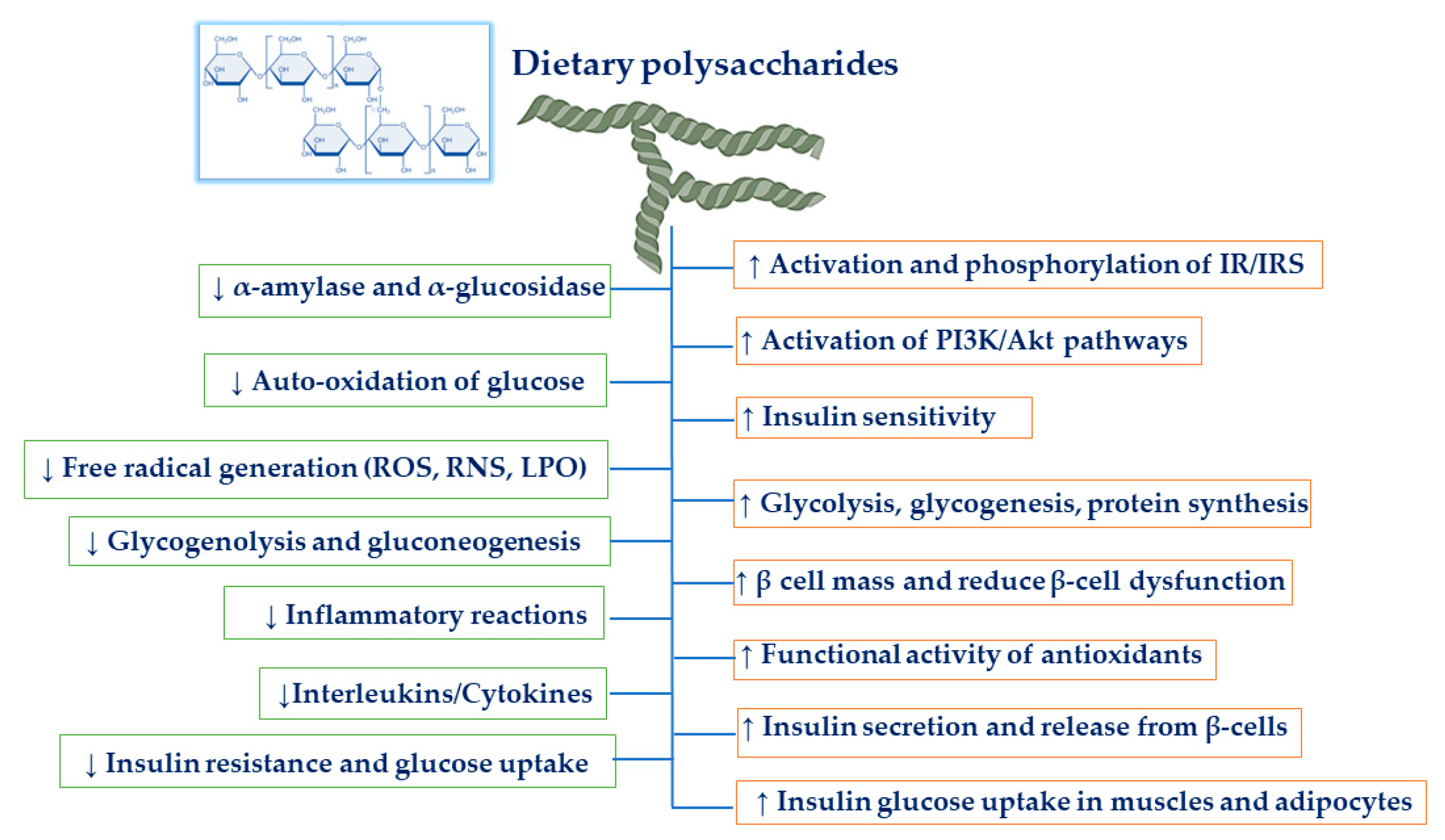
3. Mechanism of Dietary Polysaccharides on Anti-Diabetic Activities
3.1. Hypoglycemic and Hypolipidemic Effects
3.2. Increasing β-Cell Mass and Reducing β-Cell Dysfunction
3.3. Antioxidant Effects
3.4. Anti-Cholesterolemic and Anti-Triglyceridemic Effects
3.5. Anti-Inflammatory Effects
3.6. Inhibition of α-Amylase and α-Glucosidase
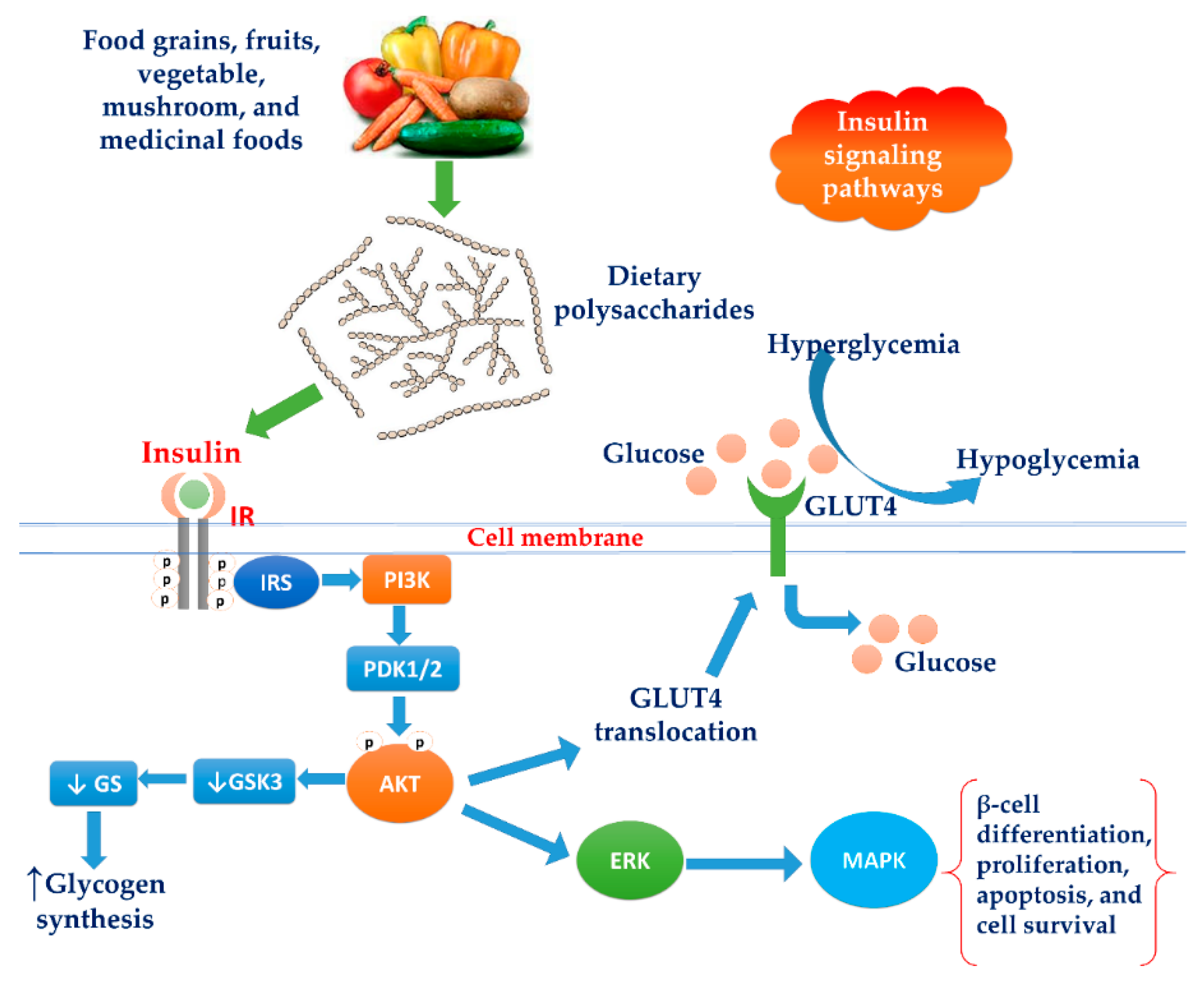
3.7. Increasing Insulin Signaling Pathways
3.7.1. Activation of the PI3K/Akt Pathway
3.7.2. Modulation of the MAPK Pathway
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACC | acetyl-CoA carboxylase |
| ACE | angiotensin converting enzyme |
| AGEs | advanced glycation end products |
| Akt | serine/threonine-specific protein kinase |
| ALP | alkaline phosphatase |
| ALT | alanine transaminase |
| AMPK | serine/threonine protein kinase |
| AST | aspartate transaminase |
| Bax | BCL2 associated X protein |
| Bcl-2 | B-cell lymphoma 2 |
| BUN | blood urea nitrogen |
| CAT | catalase |
| CPT1 | carnitine palmitoyltransferase-1 |
| CRE | creatinine |
| CVD | cardiovascular diseases |
| CRP | c-reactive protein |
| eNOS | endothelial nitric oxide synthase |
| ERK | extracellular-signal-regulated kinase |
| FBG | fasting blood glucose |
| FFA | free fatty acids |
| GLP-1 | glucagon-like peptide-1 |
| GLUT4 | glucose transporter 4 |
| GSH-Px | glutathione peroxidase |
| GSH-R | glutathione reductase |
| GSK- 3 | glycogen synthase kinase-3 |
| HbA1c | glycated hemoglobin |
| HDL-C | high-density lipoprotein –C |
| HepG2 | human liver cancer cell line |
| HFD | high-fat diet |
| IF-γ | interferon γ |
| IL | interleukin |
| IL-6 | interleukin-6 |
| InsR | insulin receptor |
| IPITT | Intraperitoneal Insulin Tolerance Test |
| IRS1 | Insulin receptor substrate 1 |
| IRS-1,2 | insulin receptor-1,2 |
| JNK | c-Jun N-terminal kinases |
| K | potassium |
| KDa | kilodaltons |
| LDL-C | low-density lipoprotein -C |
| LPO | lipid peroxidation |
| LPS | lipopolysaccharides |
| MAPK | mitogen-activated protein kinases |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | malondialdehyde |
| mRNA | messenger RNA |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium |
| Na | sodium |
| NAG | n-acetyl-β-d-glucosaminidase |
| NO | nitric oxide |
| OGTT | oral glucose tolerance test |
| OGTT | oral glucose tolerance test |
| PBG | postprandial glucose |
| PDX-1 | insulin promoter factor 1 |
| PI3K | Phosphoinositide 3-kinases |
| PK | pyruvate kinase |
| PKC | protein kinase C |
| PPAR-α | peroxisome proliferator-activated receptor-alpha |
| PYY | peptide YY hormone |
| SD | Sprague-Dawley |
| SOD | superoxide dismutase |
| SREBP-1c | sterol regulatory element binding protein -1c |
| SREBP-2 | sterol regulatory element binding protein -2 |
| STZ | streptozotocin |
| T2DM | T2DM |
| TC | total cholesterol |
| TG | triglycerides |
| TGF-β1 | transforming growth factor-β1 |
| TNF-α | tumor necrosis factor-α |
| UA | uric acid |
| γGT | gamma-glutamyltransferase |
References
- Ganesan, K.; Chung, S.K.; Vanamala, J.; Xu, B. Causal relationship between diet-induced gut microbiota changes and diabetes: A novel strategy to transplant Faecalibacterium prausnitzii in preventing diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Sharmila Banu, G.; Murugesan, A.G. Attenuation of Helicteres isora L. bark extracts on streptozotocin-induced alterations in glycogen and carbohydrate metabolism in albino rats. Hum. Exp. Toxicol. 2009, 28, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Zhang, T.; Ganesan, K.; Xu, B.; Chung, S.S.M. Isoquercetin ameliorates hyperglycemia and regulates key enzymes of glucose metabolism via insulin signaling pathway in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2018, 829, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Wu, Z.; Ganesan, K.; Khalid, S.; Chung, S.M.; Xu, B. Isoquercetin upregulates antioxidant genes, suppresses inflammatory cytokines and regulates AMPK pathway in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2019, 303, 62–69. [Google Scholar] [CrossRef]
- Sukalingam, K.; Ganesan, K.; Ponnusamy, K. Evaluation of antidiabetic activity of polyherbal formulations on type 2 diabetic patients: A single blinded randomized study. Int. J. Integ. Med. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Ganesan, K.; Jayachandran, M.; Xu, B. A critical review on hepatoprotective effects of bioactive food components. Crit. Rev. Food Sci. Nutr. 2017, 58, 1165–1229. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-rich lentils and their health promoting effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Wang, D.; Li, C.; Fan, W.; Yi, T.; Wei, A.; Ma, Y. Hypoglycemic and hypolipidemic effects of a polysaccharide from Fructus Corni in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2019, 133, 420–427. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, C.; Li, C.; Huang, Q.; Fu, X. Physicochemical characterization, antioxidant and hypoglycemic activities of selenized polysaccharides from Sargassum pallidum. Int. J. Biol. Macromol. 2019, 132, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Yu, M.; Fang, Z.; Xiao, B.; Guo, L.; Wang, W.; Li, J.; Wang, S.; Zhang, Y. Preparation of the controlled acid hydrolysates from pumpkin polysaccharides and their antioxidant and antidiabetic evaluation. Int. J. Biol. Macromol. 2019, 121, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Mao, G.-H.; Zhang, M.; Li, F.; Zou, Y.; Zhou, Y.; Zheng, W.; Zheng, D.-H.; Yang, L.-Q.; Wu, X.-Y. Anti-diabetic effects of polysaccharides from ethanol-insoluble residue of Schisandra chinensis (Turcz.) Baill on alloxan-induced diabetic mice. Chem. Res. Chin. Univ. 2012, 29, 99–102. [Google Scholar] [CrossRef]
- Liu, C.; Song, J.; Teng, M.; Zheng, X.; Li, X.; Tian, Y.; Pan, M.; Li, Y.; Lee, R.J.; Wang, D. Antidiabetic and antinephritic activities of aqueous extract of Cordyceps militaris fruit body in diet-streptozotocin-induced diabetic Sprague Dawley rats. Oxid. Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Sarker, M.M.R.; Yan, X.; Yang, C.; Zhao, L.; Lv, X.; Liu, B.; Zhao, C. Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydr. Polym. 2018, 198, 452–461. [Google Scholar] [CrossRef]
- Tang, H.-L.; Chen, C.; Wang, S.-K.; Sun, G.-J. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int. J. Biol. Macromol. 2015, 77, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Molecular weight and sulfate content modulate the inhibition of α-amylase by fucoidan relevant for type 2 diabetes management. Pharma. Nutr. 2015, 3, 108–114. [Google Scholar] [CrossRef]
- Ye, M.; Qiu, T.; Peng, W.; Chen, W.-X.; Ye, Y.-W.; Lin, Y.-R. Purification, characterization and hypoglycemic activity of extracellular polysaccharides from Lachnum calyculiforme. Carbohydr. Polym. 2011, 86, 285–290. [Google Scholar] [CrossRef]
- Wang, J.; Jin, W.; Zhang, W.; Hou, Y.; Zhang, H.; Zhang, Q. Hypoglycemic property of acidic polysaccharide extracted from Saccharina japonica and its potential mechanism. Carbohydr. Polym. 2013, 95, 143–147. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, D.; Geng, L.; Wang, Z.; Xu, C. Production, fractionation, characterization of extracellular polysaccharide from a newly isolated Trametes gibbosa and its hypoglycemic activity. Carbohydr. Polym. 2013, 96, 460–465. [Google Scholar] [CrossRef]
- Xu, W.; Zhou, Q.; Yin, J.-J.; Yao, Y.; Zhang, J.-L. Anti-diabetic effects of polysaccharides from Talinum triangulare in streptozotocin (STZ)-induced type 2 diabetic male mice. Int. J. Biol. Macromol. 2015, 72, 575–579. [Google Scholar] [CrossRef]
- Hong, T.; Zhao, J.; Dong, M.; Meng, Y.; Mu, J.; Yang, Z. Composition and bioactivity of polysaccharides from Inula britannica flower. Int. J. Biol. Macromol. 2012, 51, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lai, Q.; Zhang, J.; Huang, C.; Jia, L. Antioxidant and hypoglycemic effects of acidic-extractable polysaccharides from Cordyceps militaris on type 2 diabetes mice. Oxid. Med. Cell. Longev. 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, Y.; Bian, Y.; Wong, J.H.; Ng, T.B.; Wang, H. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats. Appl. Microbiol. Biotechnol. 2006, 72, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Jing, T.; Meng, Q.; Liu, C.; Hu, S.; Ma, Y.; Liu, Y.; Lu, J.; Cheng, Y.; Wang, D.; et al. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley rats. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, D.; You, Y.; Zeng, S.; Hu, Y.; Duan, X.; Liu, A.; Chen, H.; Hu, X.; Chen, S.; et al. Structural characterization and antidiabetic activity of a glucopyranose-rich heteropolysaccharide from Catathelasma ventricosum. Carbohydr. Polym. 2016, 149, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Zhang, G.H.; Zeng, Q.; Huang, Z.G.; Wang, Y.T.; Dong, T.T.X.; Tsim, K.W.K. Hypoglycemic activity of polysaccharide, with antioxidation, isolated from cultured Cordyceps mycelia. Phytomedicine 2006, 13, 428–433. [Google Scholar] [CrossRef]
- Yu, S.-H.; Dubey, N.K.; Li, W.-S.; Liu, M.-C.; Chiang, H.-S.; Leu, S.-J.; Shieh, Y.-H.; Tsai, F.-C.; Deng, W.-P. Cordyceps militaris treatment preserves renal function in type 2 diabetic nephropathy mice. PLoS ONE 2016, 11, e0166342. [Google Scholar] [CrossRef]
- Wang, J.; Teng, L.; Liu, Y.; Hu, W.; Chen, W.; Hu, X.; Wang, Y.; Wang, D. Studies on the antidiabetic and antinephritic activities of Paecilomyces hepiali water extract in diet-streptozotocin-induced diabetic Sprague Dawley rats. J. Diabet. Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Xue, J.; Tong, S.; Wang, Z.; Liu, P. Chemical characterization and hypoglycaemic activities in vitro of two polysaccharides from Inonotus obliquus by submerged culture. Molecules 2018, 23, 3261. [Google Scholar] [CrossRef]
- Huang, H.; Wang, S.-L.; Nguyen, V.; Kuo, Y.-H. Isolation and identification of potent antidiabetic compounds from Antrodia cinnamomea—An edible Taiwanese mushroom. Molecules 2018, 23, 2864. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, L.; Wang, B.; Zhang, Z.; Liu, H.; Zhang, Y.; Liu, J. Synergistic hypoglycemic effects of pumpkin polysaccharides and puerarin on type II diabetes mellitus mice. Molecules 2019, 24, 955. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, M.; Kwon, D.; Kim, D.; Zhang, T.; Ha, C.; Park, S. Combination of aronia, red ginseng, shiitake mushroom and nattokinase potentiated insulin secretion and reduced insulin resistance with improving gut microbiome dysbiosis in insulin deficient type 2 diabetic rats. Nutrients 2018, 10, 948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, X.; Zhao, L.Q.; Zhao, J.; Qi, Z.; Wang, L.-A. A primary study of the antioxidant, hypoglycemic, hypolipidemic, and antitumor activities of ethanol extract of brown slimecap mushroom, Chroogomphus rutilus (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Nyam, K.L.; Chow, C.F.; Tan, C.S.; Ng, S.T. Antidiabetic properties of the Tiger’s Milk medicinal mushroom, Lignosus rhinocerotis (Agaricomycetes), in streptozotocin-induced diabetic rats. Int. J. Med. Mushrooms 2017, 19, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Vitak, T.Y.; Wasser, S.P.; Nevo, E.; Sybirna, N.O. Enzymatic system of antioxidant protection of erythrocytes in diabetic rats treated with medicinal mushrooms Agaricus brasiliensis and Ganoderma lucidum (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Huang, Y.; Liu, Y.; Huang, M.; Song, G.; Ming, Q.; Ma, X.; Yang, J.; Deng, S.; Wen, Y.; et al. Antidiabetic activity of ergosterol from Pleurotus ostreatus in KK-Ay mice with spontaneous type 2 diabetes mellitus. Mol. Nutr. Food Res. 2018, 62, 1700444. [Google Scholar] [CrossRef]
- Ikewuchi, C.C.; Ikewuchi, J.C.; Ifeanacho, M.O. Restoration of plasma markers of liver and kidney functions/integrity in alloxan-induced diabetic rabbits by aqueous extract of Pleurotus tuberregium sclerotia. Biomed. Pharmacother. 2017, 95, 1809–1814. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Li, S.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed. Pharmacother. 2017, 95, 1669–1677. [Google Scholar] [CrossRef]
- Chen, P.-H.; Weng, Y.-M.; Lin, S.-M.; Yu, Z.-R.; Wang, B.-J. Molecular weight affected antioxidant, hypoglycemic and hypotensive activities of cold water extract from Pleurotus citrinopileatus. J. Food Sci. 2017, 82, 2456–2461. [Google Scholar] [CrossRef]
- Liu, Y.; You, Y.; Li, Y.; Zhang, L.; Yin, L.; Shen, Y.; Li, C.; Chen, H.; Chen, S.; Hu, B.; et al. The characterization, selenylation and antidiabetic activity of mycelial polysaccharides from Catathelasma ventricosum. Carbohydr. Polym. 2017, 174, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Bedi, G.K.; Shri, R. In vitro and in vivo antidiabetic evaluation of selected culinary-medicinal mushrooms (Agaricomycetes). Int. J. Med. Mushrooms 2017, 19, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.; Sha, O.; Xu, W.; Wang, S. Hypolipidaemic and hypoglycaemic activities of polysaccharide from Pleurotus eryngii in Kunming mice. Int. J. Biol. Macromol. 2016, 93, 1206–1209. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yin, R.; Hou, D.; Xue, Y.; Zhang, M.; Diao, X.; Zhang, Y.; Wu, J.; Hu, J.; Hu, X.; et al. The glucose-lowering effect of Foxtail millet in subjects with impaired glucose tolerance: A self-controlled clinical trial. Nutrients 2018, 10, 1509. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.V.; Aragaki, A.K.; Tinker, L.F.; Allison, M.; Hingle, M.D.; Johnson, K.C.; Manson, J.E.; Shadyab, A.H.; Shikany, J.M.; Snetselaar, L.G.; et al. A low-fat dietary pattern and diabetes: A secondary analysis from the Women’s Health Initiative Dietary Modification Trial. Diabetes Care 2017, 41, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Marventano, S.; Vetrani, C.; Vitale, M.; Godos, J.; Riccardi, G.; Grosso, G. Whole grain intake and glycaemic control in healthy subjects: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2017, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Mofidi, A.; Ferraro, Z.M.; Stewart, K.A.; Tulk, H.M.F.; Robinson, L.E.; Duncan, A.M.; Graham, T.E. The acute impact of ingestion of sourdough and whole-grain breads on blood glucose, insulin, and incretins in overweight and obese men. J. Nutr. Met. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Soong, Y.Y.; Quek, R.Y.C.; Henry, C.J. Glycemic potency of muffins made with wheat, rice, corn, oat and barley flours: A comparative study between in vivo and in vitro. Eur. J. Nutr. 2015, 54, 1281–1285. [Google Scholar] [CrossRef]
- Zafar, T.A.; Al-Hassawi, F.; Al-Khulaifi, F.; Al-Rayyes, G.; Waslien, C.; Huffman, F.G. Organoleptic and glycemic properties of chickpea-wheat composite breads. J. Food Sci. Technol. 2013, 52, 2256–2263. [Google Scholar] [CrossRef]
- Luhovyy, B.L.; Mollard, R.C.; Yurchenko, S.; Nunez, M.F.; Berengut, S.; Liu, T.T.; Smith, C.E.; Pelkman, C.L.; Anderson, G.H. The effects of whole grain high-amylose maize flour as a source of resistant starch on blood glucose, satiety, and food intake in young men. J. Food Sci. 2014, 79, H2550–H2556. [Google Scholar] [CrossRef]
- Poquette, N.M.; Gu, X.; Lee, S.-O. Grain sorghum muffin reduces glucose and insulin responses in men. Food Funct. 2014, 5, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Lappi, J.; Aura, A.-M.; Katina, K.; Nordlund, E.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Comparison of postprandial phenolic acid excretions and glucose responses after ingestion of breads with bioprocessed or native rye bran. Food Funct. 2013, 4, 972. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Cai, X.; Xu, M.; Li, Y. Effect of oat intake on glycaemic control and insulin sensitivity: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2014, 112, 457–466. [Google Scholar] [CrossRef] [PubMed]
- He, L.-X.; Zhao, J.; Huang, Y.-S.; Li, Y. The difference between oats and beta-glucan extract intake in the management of HbA1c, fasting glucose and insulin sensitivity: A meta-analysis of randomized controlled trials. Food Funct. 2016, 7, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, J.C.; Björck, I.M.E.; Nilsson, A.C. Effects of whole grain rye, with and without resistant starch type 2 supplementation, on glucose tolerance, gut hormones, inflammation and appetite regulation in an 11–14.5 hour perspective; a randomized controlled study in healthy subjects. Nutr. J. 2017, 16. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Chu, Y. Whole grain oats, more than just a fiber: Role of unique phytochemicals. Mol. Nutr. Food Res. 2017, 61, 1600715. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Brunius, C.; Lindelöf, M.; Shameh, S.A.; Wu, H.; Lee, I.; Landberg, R.; Moazzami, A.A. Targeted metabolomics reveals differences in the extended postprandial plasma metabolome of healthy subjects after intake of whole-grain rye porridges versus refined wheat bread. Mol. Nutr. Food Res. 2017, 61, 1600924. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Vuksan, V.; Faulkner, D.; Augustin, L.S.A.; Mitchell, S.; Ireland, C.; Srichaikul, K.; Mirrahimi, A.; Chiavaroli, L.; et al. Effect of lowering the glycemic load with Canola oil on glycemic control and cardiovascular risk factors: A randomized controlled trial. Diabetes Care 2014, 37, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Pyo, Y.-H.; Lee, K.-W. Preventive effect of monascus-fermented products enriched with ubiquinones on Type 2 diabetic rats induced by a high-fructose plus high-fat diet. J. Med. Food 2014, 17, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Kim, M.; Chae, J.; Lee, S.-H.; Lee, J. Consumption of whole grains and legumes modulates the genetic effect of the APOA5 -1131C variant on changes in triglyceride and apolipoprotein A-V concentrations in patients with impaired fasting glucose or newly diagnosed type 2 diabetes. Trials 2014, 15, 100. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Esmaillzadeh, A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: A randomized controlled clinical trial. Eur. J. Clin. Nutr. 2014, 68, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Jacobs, D.R.; Pins, J.J.; Raatz, S.K.; Gross, M.D.; Slavin, J.L.; Seaquist, E.R. Effect of whole grains on insulin sensitivity in overweight hyperinsulinemic adults. Am. J. Clin. Nutr. 2002, 75, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Hong, S.M.; Ahn, I.S.; Kim, Y.S.; Shin, D.W.; Park, S. Kochujang, a Korean fermented red pepper plus soybean paste, improves glucose homeostasis in 90% pancreatectomized diabetic rats. Nutrition 2009, 25, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.M.; McDonald, M.R.; Sullivan, J.A.; Tsao, R.; Platt, M.; Simpson, J.; Meckling, K.A. The effect of anthocyanin-rich purple vegetable diets on metabolic syndrome in obese Zucker rats. J. Med. Food 2017, 20, 1240–1249. [Google Scholar] [CrossRef]
- Wu, T.; Luo, J.; Xu, B. In vitro antidiabetic effects of selected fruits and vegetables against glycosidase and aldose reductase. Food Sci. Nutr. 2015, 3, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Hu, J.-L.; Nie, S.-P.; Xie, M.-Y. Antidiabetic mechanism of dietary polysaccharides based on their gastrointestinal functions. J. Agric. Food Chem. 2018, 66, 4781–4786. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Ganesan Murugesan, A.; Pandian, M.R. Antihyperglycaemic and antiperoxidative effect of Helicteres isora L. bark extracts in streptozotocin-induced diabetic rats. J. Appl. Biomed. 2007, 5, 97–104. [Google Scholar] [CrossRef]
- Kumar, G.; Sharmila Banu, G.; Ganesan Murugesan, A. Effect of Helicteres isora bark extracts on heart antioxidant status and lipid peroxidation in streptozotocin diabetic rats. J. Appl. Biomed. 2008, 6, 89–95. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X.; Shi, F.; Liu, Y. Comparison of antidiabetic effects of saponins and polysaccharides from Momordica charantia L. in STZ-induced type 2 diabetic mice. Biomed. Pharmacother. 2019, 109, 744–750. [Google Scholar] [CrossRef]
- Kumar Bhateja, P.; Singh, R. Antidiabetic activity of Acacia tortilis (Forsk.) Hayne ssp. raddiana polysaccharide on streptozotocin-nicotinamide induced diabetic rats. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, H.; Chang, N.; Zhang, K. Anti-hyperglycemic effect of the polysaccharide fraction isolated from Mactra veneriformis. Front Chem. Sci. Eng. 2010, 5, 238–244. [Google Scholar] [CrossRef]
- Zhao, R.; Qiu, B.; Li, Q.; Zhang, T.; Zhao, H.; Chen, Z.; Cai, Y.; Ruan, H.; Ge, W.; Zheng, X. LBP-4a improves insulin resistance via translocation and activation of GLUT4 in OLETF rats. Food Funct. 2014, 5, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhang, Y.-J.; Jiang, J.-X.; Zhu, L.-Y.; Chen, P.; Li, J.; Yao, H.-Y. Studies on the extraction of pumpkin components and their biological effects on blood glucose of diabetic mice. J. Food Drug Anal. 2013, 21, 184–189. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, X.; Jiang, X.; Kong, F.; Wang, S.; Yan, C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. J. Ethnopharmacol. 2017, 199, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Guo, S.; Han, J.; Wang, Q.; Zhang, X.; Wu, W. Hypoglycemic and hypolipidemic activities of MT-α-glucan and its effect on immune function of diabetic mice. Carbohydr. Polym. 2012, 89, 245–250. [Google Scholar] [CrossRef]
- Huang, H.-Y. Effect of Pleurotus tuber-regium polysaccharides supplementation on the progression of diabetes complications in obese-diabetic rats. Chin. J. Physiol. 2014, 57, 198–208. [Google Scholar] [CrossRef]
- Leslie, W.S.; Taylor, R.; Harris, L.; Lean, M.E.J. Weight losses with low-energy formula diets in obese patients with and without type 2 diabetes: Systematic review and meta-analysis. Int. J. Obesity. 2016, 41, 96–101. [Google Scholar] [CrossRef]
- Anoop, S.; Misra, A.; Bhatt, S.P.; Gulati, S.; Mahajan, H. High fasting C-peptide levels and insulin resistance in non-lean & non-obese (BMI > 19 to < 25 kg/m2) Asian Indians with type 2 diabetes are independently associated with high intra-abdominal fat and liver span. Diabet. Met. Synd. Clin. Res. Rev. 2019, 13, 708–715. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; McClenaghan, N.H.; McCluskey, J.T.; Flatt, P.R. Mechanisms of toxicity by proinflammatory cytokines in a novel human pancreatic beta-cell line, 1.1B4. Biochim. Biophy. Acta 2014, 1840, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: A systematic review and meta-analysis of randomized clinical trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, C.; Lu, G.; Mu, Z.; Cui, W.; Gao, H.; Wang, Y. Anti-diabetic effect of mulberry leaf polysaccharide by inhibiting pancreatic islet cell apoptosis and ameliorating insulin secretory capacity in diabetic rats. Int. Immunopharmacol. 2014, 22, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-X.; Nie, S.-P.; Li, C.; Gong, D.; Xie, M.-Y. Ganoderma atrum polysaccharide improves aortic relaxation in diabetic rats via PI3K/Akt pathway. Carbohydr. Polym. 2014, 103, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Nie, S.; Li, C.; Lin, S.; Xing, M.; Li, W.; Gong, D.; Xie, M. A newly identified polysaccharide from Ganoderma atrum attenuates hyperglycemia and hyperlipidemia. Int. J. Biol. Macromol. 2013, 57, 142–150. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Yu, Y.; Chen, Q.; Huang, T.; Li, D. Ganoderma lucidum polysaccharides exert anti-hyperglycemic effect on streptozotocin-induced diabetic rats through affecting & beta-cells. Comb. Chem. High Throughput Screen. 2012, 15, 542–550. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Zhong, Z. Antihyperglycemic Effect of Ganoderma lucidum polysaccharides on streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2011, 12, 6135–6145. [Google Scholar] [CrossRef]
- Zhu, H.-Y.; Chen, G.-T.; Meng, G.-L.; Xu, J.-L. Characterization of pumpkin polysaccharides and protective effects on streptozotocin-damaged islet cells. Chin. J. Natl. Med. 2015, 13, 199–207. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Xu, H.; Wang, Y.; Li, Z.; Xue, C. Fucosylated chondroitin sulphate from sea cucumber inhibits high-fat-sucrose diet-induced apoptosis in mouse pancreatic islets via down-regulating mitochondrial signaling pathway. J. Funct. Foods 2014, 7, 517–526. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.S.; Murugesan, A.G.; Pandian, M.R. Hypoglycaemic effect of Helicteres isora bark extract in rats. J. Ethnopharmacol. 2006, 107, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Sharmila Banu, G.; Murugesan, A.G.; Rajasekara Pandian, M. Effect of Helicteres isora. Bark extracts on brain antioxidant status and lipid peroxidation in streptozotocin diabetic rats. Pharm. Biol. 2007, 45, 753–759. [Google Scholar] [CrossRef]
- Islam, T.; Ganesan, K.; Xu, B. New Insight into mycochemical profiles and antioxidant potential of edible and medicinal mushrooms: A review. Int. J. Med. Mushrooms 2019, 21, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Maheswaran, R.; Sharmila Banu, G. Antihyperlipideamic effect of Solanum trilobatum L. leaves extract on streptozotocin induced diabetic rats. Asian J. Biomed. Pharma. Sci. 2013, 3, 51–57. [Google Scholar]
- Zhang, T.; Jayachandran, M.; Ganesan, K.; Xu, B. Black Truffle aqueous extract attenuates oxidative stress and inflammation in STZ-induced hyperglycemic rats via Nrf2 and NF-κB pathways. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, L.; Zhang, L.; Wang, T.; Zhou, Y.; Ding, C.; Yang, R.; Wang, X.; Yu, L. Optimization of extraction and antioxidant activity of polysaccharides from Salvia miltiorrhiza Bunge residue. Int. J. Biol. Macromol. 2015, 79, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Du, M.; Liu, P.; Zhang, B.; Zhang, Y.; Yang, P.; Shang, M.; Wang, X. Anti-diabetic and anti-nephritic activities of Grifola frondosa mycelium polysaccharides in diet-streptozotocin-induced diabetic rats via modulation on oxidative stress. Appl. Biochem. Biotechnol. 2018, 187, 310–322. [Google Scholar] [CrossRef]
- Chen, X.; Tang, J.; Xie, W.; Wang, J.; Jin, J.; Ren, J.; Jin, L.; Lu, J. Protective effect of the polysaccharide from Ophiopogon japonicus on streptozotocin-induced diabetic rats. Carbohydr. Polym. 2013, 94, 378–385. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, L.; Xiao, Z.; Wang, J.; Wang, Y.; Chen, J. Antidiabetic activity of polysaccharides from tuberous root of Liriope spicata var. prolifera in KKAy Mice. Evid. Based Complement. Alternat. Med. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Q.; Li, C.; Fu, X. Hypoglycemic effects of a Fructus mori polysaccharide in vitro and in vivo. Food Funct. 2017, 8, 2523–2535. [Google Scholar] [CrossRef]
- Sun, C.; Chen, Y.; Li, X.; Tai, G.; Fan, Y.; Zhou, Y. Anti-hyperglycemic and anti-oxidative activities of ginseng polysaccharides in STZ-induced diabetic mice. Food Funct. 2014, 5, 845. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Kohli, S.; Rai, G. Antidiabetic potential of polysaccharides from the white oyster culinary-medicinal mushroom Pleurotus florida (Higher Basidiomycetes). Int. J. Med. Mushrooms 2014, 16, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Ouyang, K.H.; Zhao, J.; Chen, H.; Xiong, L.; Wang, W.J. Structural characterization and hypolipidemic effect of Cyclocarya paliurus polysaccharide in rat. Int. J. Biol. Macromol. 2016, 91, 1073–1080. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, F.; Jiang, H.; Wang, Z.; Hua, C.; Zhang, Y. Chicory (Cichorium intybus L.) polysaccharides attenuate high-fat diet-induced non-alcoholic fatty liver disease via AMPK activation. Int. J. Biol. Macromol. 2018, 118, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, W.; Li, J.; Li, Y.; Song, H.; Luan, Y.; Qi, H.; Ma, L.; Lu, X.; Yang, Y. Lycium barbarum polysaccharide attenuates high-fat diet-induced hepatic steatosis by up-regulating SIRT1 expression and deacetylase activity. Sci. Rep. 2016, 6, 36209. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Huang, F.; Lin, W. Sulfated polysaccharide from Enteromorpha prolifera suppresses SREBP-1c and ACC expression to lower serum triglycerides in high-fat-diet-induced hyperlipidaemic rats. J. Funct. Foods 2018, 40, 722–728. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, F.; Wang, L.; Yang, T.; Shi, L.; Li, X.; Shen, J.; Xu, W.; Guo, T.; Lin, Q. Anti-hyperlipidemic effect of rice bran polysaccharide and its potential mechanism in high-fat diet mice. Food Funct. 2017, 8, 4028–4041. [Google Scholar] [CrossRef]
- Bruce, C.R.; Hoy, A.J.; Turner, N.; Watt, M.J.; Allen, T.L.; Carpenter, K.; Cooney, G.J.; Febbraio, M.A.; Kraegen, E.W. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet–induced insulin resistance. Diabetes 2009, 58, 550–558. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Lei, L.; Li, F.; Zhang, Y.; Chen, J.; Zhao, G.; Wu, S.; Yin, R.; Ming, J. Carboxymethylation of polysaccharide from Morchella angusticepes Peck enhances its cholesterol-lowering activity in rats. Carbohydr. Polym. 2017, 172, 85–92. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Caz, V.; Smiderle, F.R.; Martin-Hernandez, R.; Largo, C.; Tabernero, M.; Marín, F.R.; Iacomini, M.; Reglero, G.; Soler-Rivas, C. Water-soluble compounds from Lentinula edodes influencing the HMG-CoA reductase activity and the expression of genes involved in the cholesterol metabolism. J. Agric. Food Chem. 2016, 64, 1910–1920. [Google Scholar] [CrossRef]
- Park, J.; Yeom, M.; Hahm, D.H. Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation. J. Pharmacol. Sci. 2016, 131, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Kathiresan, S. Surprises from genetic analyses of lipid risk factors for atherosclerosis. Circ. Res. 2016, 118, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies Collaboration; Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [PubMed]
- Petroglou, D.; Kanellos, I.; Savopoulos, C.; Kaiafa, G.; Chrysochoou, A.; Skantzis, P.; Daios, S.; Hatzitolios, A.I.; Giannoglou, G. The LDL-receptor and its molecular properties: From theory to novel biochemical and pharmacological approaches in reducing LDL-cholesterol. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Farnier, M. PCSK9 inhibitors. Curr. Opin. Lipidol. 2013, 24, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Wang, Q.; Yang, Y. Crude extracts from Lycium barbarum suppress SREBP-1c expression and prevent diet-induced fatty liver through AMPK activation. BioMed Res. Int. 2014, 2014, 196198. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, X.; Zeng, X.; Ou, Z.; Xue, M.; Gao, D.; Liu, S.; Li, X.; Yang, S. Rheum palmatum L. Attenuates High Fat Diet-Induced Hepatosteatosis by Activating AMP-Activated Protein Kinase. Am. J. Chin. Med. 2016, 44, 551–564. [Google Scholar] [CrossRef]
- Wang, C.M.; Yuan, R.S.; Zhuang, W.Y.; Sun, J.H.; Wu, J.Y.; Li, H.; Chen, J.G. Schisandra polysaccharide inhibits hepatic lipid accumulation by down-regulating the expression of SREBPs in NAFLD mice. Lipids Health Dis. 2016, 15, 195. [Google Scholar] [CrossRef]
- Huang, X.; Tang, J.; Zhou, Q.; Lu, H.; Wu, Y.; Wu, W. Polysaccharide from fuzi (FPS) prevents hypercholesterolemia in rats. Lipids Health Dis. 2010, 9, 9. [Google Scholar] [CrossRef]
- Kim, H.; Wang, Q.; Shoemaker, C.F.; Zhong, F.; Bartley, G.E.; Yokoyama, W.H. Polysaccharide gel coating of the leaves of Brasenia schreberi lowers plasma cholesterol in hamsters. J. Tradit. Complement. Med. 2014, 5, 56–61. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed]
- Westwell-Roper, C.; Dai, D.L.; Soukhatcheva, G.; Potter, K.J.; van Rooijen, N.; Ehses, J.A.; Verchere, C.B. IL-1 Blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J. Immunol. 2011, 187, 2755–2765. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Cao, P.; Shui, W.; Yang, Q.; Tang, Z.; Zhang, Y. Angelica sinensis polysaccharide regulates glucose and lipid metabolism disorder in prediabetic and streptozotocin-induced diabetic mice through the elevation of glycogen levels and reduction of inflammatory factors. Food Funct. 2015, 6, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, G.; Yan, J.; Li, K.; Bai, Z.; Cheng, W.; Huang, K. Rehmannia glutinosa (Gaertn.) DC. polysaccharide ameliorates hyperglycemia, hyperlipemia and vascular inflammation in streptozotocin-induced diabetic mice. J. Ethnopharmacol. 2015, 164, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yan, J.; Bai, Z.; Li, K.; Huang, K. Hypoglycemic activity and potential mechanism of a polysaccharide from the loach in streptozotocin-induced diabetic mice. Carbohydr. Polym. 2015, 121, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Pang, W.; Chen, J.; Bai, S.; Zheng, Z.; Wu, X. Hypoglycemic effect of polysaccharides with different molecular weight of Pseudostellaria heterophylla. BMC Complement. Altern. Med. 2013, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabapathy, G.; Kuppusamy, U.R.; Abd Malek, S.N.; Abdulla, M.A.; Chua, K.-H.; Sabaratnam, V. Glucan-rich polysaccharides from Pleurotus sajor-caju (Fr.) Singer prevents glucose intolerance, insulin resistance and inflammation in C57BL/6J mice fed a high-fat diet. BMC Complement. Altern. Med. 2012, 12, 261. [Google Scholar] [CrossRef]
- Guo, C.; Li, R.; Zheng, N.; Xu, L.; Liang, T.; He, Q. Anti-diabetic effect of ramulus mori polysaccharides, isolated from Morus alba L. on STZ-diabetic mice through blocking inflammatory response and attenuating oxidative stress. Int. Immunopharmacol. 2013, 16, 93–99. [Google Scholar] [CrossRef]
- Yu, W.; Chen, H.; Xiang, Z.; He, N. Preparation of polysaccharides from Ramulus mori, and their antioxidant, anti-inflammatory and antibacterial activities. Molecules 2019, 24, 856. [Google Scholar] [CrossRef]
- Zhang, J.-G.; Liu, Q.; Liu, Z.-L.; Li, L.; Yi, L.-T. Antihyperglycemic activity of Anoectochilus roxburghii polysaccharose in diabetic mice induced by high-fat diet and streptozotocin. J. Ethnopharmacol. 2015, 164, 180–185. [Google Scholar] [CrossRef]
- Hou, D.; Yousaf, L.; Xue, Y.; Hu, J.; Wu, J.; Hu, X.; Feng, N.; Shen, Q. Mung bean (Vigna radiata L.): Bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients 2019, 11, 1238. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yan, X.; Zhang, M.; Chang, M.; Yun, S.; Meng, J.; Liu, J.; Feng, C.-P. Regulation of RAW 264.7 cell-mediated immunity by polysaccharides from Agaricus blazei Murill via the MAPK signal transduction pathway. Food Funct. 2017, 8, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Q.; Zang, Y.; Zhao, Y.; Liu, N.; Wang, Y.; Xu, X.; Liu, L.; Mei, Q. Apple polysaccharide inhibits microbial dysbiosis and chronic inflammation and modulates gut permeability in HFD-fed rats. Int. J. Biol. Macromol. 2017, 99, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhong, J.; Zhang, Q.; Qing, D.; Yan, C. Structural elucidation and bioactivities of a novel arabinogalactan from Coreopsis tinctoria. Carbohydr. Polym. 2019, 219, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Niu, X.; Liu, N.; Gao, Y.; Wang, L.; Xu, G.; Li, X.; Yang, Y. Characterization, antioxidant and hypoglycemic activities of degraded polysaccharides from blackcurrant (Ribes nigrum L.) fruits. Food Chem. 2018, 243, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Chen, F.; Xiao, T.; Zhang, L. Inhibitory effects of polysaccharide from Diaphragma juglandis fructus on α-amylase and α-d-glucosidase activity, streptozotocin-induced hyperglycemia model, advanced glycation end-products formation, and H2O2-induced oxidative damage. Int. J. Biol. Macromol. 2019, 124, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Senthil, S.; Chandrasekaran, R.; Arjun, H.A.; Anantharaman, P. In vitro and in silico inhibition properties of fucoidan against α-amylase and α-d-glucosidase with relevance to type 2 diabetes mellitus. Carbohydr. Polym. 2019, 209, 350–355. [Google Scholar] [CrossRef]
- Cui, J.; Gu, X.; Wang, F.; Ouyang, J.; Wang, J. Purification and structural characterization of an α-glucosidase inhibitory polysaccharide from apricot (Armeniaca sibirica L. Lam.) pulp. Carbohydr. Polym. 2015, 121, 309–314. [Google Scholar] [CrossRef]
- Hu, J.-L.; Nie, S.-P.; Li, C.; Xie, M.-Y. In vitro effects of a novel polysaccharide from the seeds of Plantago asiatica L. on intestinal function. Int. J. Biol. Macromol. 2013, 54, 264–269. [Google Scholar] [CrossRef]
- Lakshmanasenthil, S.; Vinothkumar, T.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Sindhu, N.S. Fucoidan—A novel α-amylase inhibitor from Turbinaria ornata with relevance to NIDDM therapy. Biocatal. Agric. Biotechnol. 2014, 3, 66–70. [Google Scholar] [CrossRef]
- Vinoth Kumar, T.; Lakshmanasenthil, S.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Suganya, P. Fucoidan—A α-d-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int. J. Biol. Macromol. 2015, 72, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Moullé, V.S.; Ghislain, J.; Poitout, V. Nutrient regulation of pancreatic β-cell proliferation. Biochimie 2017, 143, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, X.; Xie, C.; Luo, X.; Bao, Y.; Wu, B.; Hu, Y.; Zhong, Z.; Liu, C.; Li, M. Prevention effects and possible molecular mechanism of mulberry leaf extract and its formulation on rats with insulin-insensitivity. PLoS ONE 2016, 11, e0152728. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Wang, Y.; Xu, D.-S.; Ruan, K.-F.; Feng, Y.; Wang, S. MDG-1, a polysaccharide from Ophiopogon japonicus exerts hypoglycemic effects through the PI3K/Akt pathway in a diabetic KKAy mouse model. J. Ethnopharmacol. 2012, 143, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.-Q.; Wang, Y.-L.; Gan, S.-R.; Chen, J.-C. Polysaccharides from Liriopes radix ameliorates hyperglycemia via various potential mechanisms in diabetic rats. J. Sci. Food Agric. 2013, 94, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.-X.; Nie, S.-P.; Tan, L.-H.; Li, C.; Gong, D.-M.; Xie, M.-Y. A polysaccharide from Ganoderma atrum Improves liver function in type 2 diabetic rats via antioxidant action and short-chain fatty acids excretion. J. Agric. Food Chem. 2016, 64, 1938–1944. [Google Scholar] [CrossRef]
- Lin, W.; Wang, W.; Liao, D.; Chen, D.; Zhu, P.; Cai, G.; Kiyoshi, A. A polysaccharides from Enteromorpha prolifera improve glucose metabolism in diabetic rats. J. Diabet. Res. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, F.; Chen, Y.; Zhang, Y.; Hou, L.; Cao, X.; Wang, C. A polysaccharide from Grifola frondosa relieves insulin resistance of HepG2 cell by Akt-GSK-3 pathway. Glycoconj. J. 2014, 31, 355–363. [Google Scholar] [CrossRef]
- Zhao, C.; Liao, Z.; Wu, X.; Liu, Y.; Liu, X.; Lin, Z.; Huang, Y.; Liu, B. Isolation, purification, and structural features of a polysaccharide from Phellinus linteus and Its hypoglycemic effect in alloxan-induced diabetic mice. J. Food Sci. 2014, 79, H1002–H1010. [Google Scholar] [CrossRef]
- Hu, S.; Chang, Y.; Wang, J.; Xue, C.; Li, Z.; Wang, Y. Fucosylated chondroitin sulfate from sea cucumber in combination with rosiglitazone improved glucose metabolism in the liver of the insulin-resistant mice. Biosci. Biotechnol. Biochem. 2013, 77, 2263–2268. [Google Scholar] [CrossRef]
- Hu, S.; Xu, H.; Chen, R.; Wang, J.; Li, Z.; Xu, J. Activation of PKB and ERK, but not PI3K, is involved in fucosylated chondroitin sulphate from Acaudina molpadioides induced glucose uptake. J. Funct. Foods 2014, 10, 385–396. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, J.; Liu, B.; Yan, T.; Xu, F.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Polysaccharide from okra (Abelmoschus esculentus (L.) Moench) improves antioxidant capacity via PI3K/AKT Pathways and Nrf2 translocation in a type 2 diabetes model. Molecules 2019, 24, 1906. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Shen, Z.; Cui, J.; Zhu, Y.; Li, Y.; Chi, Y.; Wang, J.; Wang, P. Hypoglycemic activity and mechanism of the sulfated rhamnose polysaccharides chromium(III) complex in type 2 diabetic mice. Bioorg. Chem. 2019, 88, 102942. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, D.; Wang, D.; Lai, S.; Zhong, R.; Liu, Y.; Yang, C.; Liu, B.; Sarker, M.R.; Zhao, C. Hypoglycemic activity and gut microbiota regulation of a novel polysaccharide from Grifola frondosa in type 2 diabetic mice. Food Chem. Toxicol. 2019, 126, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, F.; Wang, J.; Huang, H.; Huang, Y. Anti-diabetic effect mediated by Ramulus mori polysaccharides. Carbohydr. Polym. 2015, 117, 63–69. [Google Scholar] [CrossRef] [PubMed]
| Source of Polysaccharides | Botanical Name/Composition | Model | Doses and Route of Administration | Negative Control | Investigation | Results | References |
|---|---|---|---|---|---|---|---|
| Mushroom | Cordyceps militaris | Wistar mice | 100 and 400 mg/kg, p.o. for 4 weeks | STZ (60 mg/kg, i.p) | FBG, Serum Insulin, OGTT, AST, ALT, BUN, CRE, LDL-C, TC, HDL-C, hepatic, renal, and pancreatic SOD, GSH-Px, CAT, and lipid peroxidation | Antioxidant and hypoglycemic effects | [23] |
| Mushroom | Cordyceps sinensis, Omphalia lapidescens, and Tricholomamongolicum | Wistar rats | 10 and 100 mg/kg, p.o. for 4 weeks | STZ (40 mg/kg, i.p) | FBG and PBG | Antioxidant and hypoglycemic effects | [24] |
| Mushroom | Cordyceps sinensis, Omphalia lapidescens, and Tricholomamongolicum. | SD male rats | 500 mg and 2000 mg/kg, p.o. for 3 weeks | STZ (40 mg/kg, i.p) | FBG, PK, SOD, GSH-Px, TG, TC, BUN, UA, CRE, and urine protein levels | Anti-diabetic and anti-nephropathic activities | [25] |
| Mushroom | Cordyceps militaris | Sprague-Dawley male rats | 0.5, 1.0, and 2.0 g/kg, p.o. for 4 weeks | STZ (40 mg/kg, i.p) | FBG, PK, SOD, GSH-Px, TG, TC, BUN, UA, CRE, urine protein, NAG, and MDA | Anti-diabetic and antinephritic activities | [26] |
| Mushroom | Cordyceps mycelia | Male BALB/c mice and male Sprague-Dawley rats | 200 mg, 400 mg/kg, p.o. for 1-week | STZ (60 mg/kg, i.p), Alloxan monohydrate (150 mg/kg, i.p.) | Blood glucose and insulin | Hypoglycemic activity | [27] |
| Mushroom | Cordyceps militaris | C57BL/6J mice | 360 mg/kg/p.o. for 8 weeks | HFD + STZ (60 mg/kg, i.p) + nicotinamide (180 mg/kg, i.p) | FBG, OGTT, IPITT, CRE, AGEs, TGF-β1, TC, TG, LDL-C, and HDL-C | Anti-diabetic and renoprotective activities | [28] |
| Mushroom | Paecilomyces hepiali | Sprague-Dawley male rats | 0.08, 0.4, and 2.0 g/kg/p.o. for 4 weeks | HFD + STZ (25 mg/kg, i.p) + nicotinamide (180 mg/kg, i.p) | Blood glucose, TC, LDL-C, insulin, PK, glycogen, SOD, MDA, GSH-Px IL-2, IL-6, IL-10, and TNF-α | Anti-diabetic and antinephritic Activities | [29] |
| Mushroom | Inonotus obliquus | HepG2 cells and insulin-resistant HepG2 cells | 10, 20, 40, 80, and 160 μg/mL, for 24 and 48 h. | - | Glucose, insulin | Hypoglycemic activity | [30] |
| Mushroom | Antrodia cinnamomea | - | 50 μL | - | α-glucosidase inhibitory activity | Anti-diabetic activity | [31] |
| Mushroom | Grifola frondosa | Male ICR mice, HepG2 | 75 and 150 mg/kg for 0, 14, and 28 days; 100 μg/mL | STZ (40 mg/kg, i.p) | Glucose, OGTT, insulin, IRS1, JNK1, PI3K, or GLUT4 | Anti-diabetic activity | [15,32] |
| Mushroom | Aronia melanocarpa, red ginseng, and shiitake mushroom | Male SD rats | 0.5, 1 g/kg bw | Pancreatectomy rats with 1 g dextrin/kg bw | Serum glucose, food intake, body weight, and OGTT | Anti-diabetic activity | [33] |
| Mushroom | Chroogomphus rutilus | Male SD rats | 1.0 and 2.0 g/kg bw, p.o. for 4 weeks | STZ (40 mg/kg, i.p) | α-glucosidase, blood glucose, SOD, GSH-Px, MDA, TC, TG, LDL-C, HDL-C, and MTT | Antioxidant, Hypoglycemic, Hypolipidemic, and Antitumor Activities | [34] |
| Mushroom | Lignosus rhinocerotis | Male SD rats | 0.5, 1.0, and 2.0 g/kg bw, p.o. for 8 weeks | STZ (35 mg/kg, i.v.) | Blood glucose, GSH, CAT, SOD, and LPO | Anti-diabetic activity | [35] |
| Mushroom | Agaricus brasiliensis and Ganoderma lucidum | Male SD rats | 1.0 and 2.0 g/kg bw, p.o. for 4 weeks | STZ (35 mg/kg, i.v.) | Blood glucose, GSH, CAT, SOD, LPO, TBARS, GSH-Px, and GSH-R | Anti-diabetic activity | [36] |
| Mushroom | Pleurotus Ostreatus | KK-Ay Mice | 1.0 and 2.0 g/kg bw, p.o. for 4 weeks | STZ (35 mg/kg, i.v.) | Blood glucose, AMPK, GLUT-4, Akt, and PKC | Anti-diabetic activity | [37] |
| Mushroom | Pleurotus Ostreatus | Rabbits | 100, 200, and 300 mg/kg for 4 weeks | Alloxan (120 mg/kg, p.o) | Blood glucose, ALP, γGT, ALT, AST, bilirubin, urea, BUN, CRE, Na, and K | Anti-diabetic activity | [38] |
| Mushroom | Inonotus obliquus | Male Kunming mic | 900 mg/kg for 4 weeks | STZ (60 mg/kg, i.p.) | Blood glucose, body weight, organ weight, glycogen, OGTT, TC, TG, LDL-C, HDL-C, PI3K, GLUT-4, and Akt | Anti-diabetic activity | [39] |
| Mushroom | Pleurotus citrinopileatus | In vitro | - | - | Pancreatic α-amylase, intestinal α-glucosidase, and ACE | Antioxidant, Hypoglycemic and Hypotensive Activities | [40] |
| Mushroom | Catathelasma ventricosum | Male ICR mice | 0.2 g/kg for 4 weeks | STZ (150 mg/kg, i.p.) | Blood glucose, TC, TG, LDL-C, and HDL-C | Anti-diabetic activity | [41] |
| Mushroom | Pleurotus ostreatus, Calocybe indica, and Volvariella volvacea | In vitro, in vivo (Male ICR mice) | 200 and 400 mg/kg for 6 weeks | STZ (150 mg/kg, i.p.) | α-amylase inhibition assay, glucose uptake by yeast cells, glucose adsorption capacity, and blood glucose | Anti-diabetic activity | [42] |
| Mushroom | Pleurotus eryngii | KKAy mice | 1 g/kg for 6 weeks | STZ (150 mg/kg, i.p.) | Blood glucose, insulin, FBS, OGTT, TC, TG, LDL-C, HDL-C, liver glycogen | Hypolipidemic and hypoglycemic activities | [43] |
| Grains | Foxtail Millet | Open-label, self-controlled clinical trial 64 subjects (27 male subjects and 37 female subjects) | 50–150 g of whole grain for week 6 and 12 | Diabetic patients | FBG, insulin, fructosamine, fasting C-peptide, TG, and TC HDL-C, LDL-C, apolipoprotein A1 and B, TNF-α, IL-6, leptin, GLP-1, blood pressure, body weight, waist circumference, and hip circumference | Anti-diabetic activity | [44] |
| Vegetable, fruit, and grain | Vegetable, fruit, and grain | 48,835 post-menopausal women | A 1:1:0.5–serving/day vegetable, fruit, food grains | Diabetic patients | Serum glucose, insulin, and waist circumference | Reduced the risk of diabetes | [45] |
| Whole Grain cereals | Whole grain cereals | A meta-analysis of randomized controlled trials | 50 g/day | Healthy Subjects | Serum glucose, insulin, and HbAlc | Improved the PBG and insulin homeostasis | [46] |
| Grain and Sprouted grain | Grain and sprouted grain | 12 male subjects | 50 g/day | Healthy Subjects | Serum glucose, insulin, and HbAlc | Only sprouted-grain improved PBG and insulin | [47] |
| Whole Grains muffins | Wheat, rice, corn, oat, and barley | 4 Male and 8 Female | 50 g/day | Healthy Subjects | Serum glucose, insulin, and HbAlc | Lowered the PBG | [48] |
| Whole grains bread | Chickpea-wheat composite bread | 13 female subjects | 50 g/day | Healthy Subjects | Serum glucose, insulin, and HbAlc | Reduced PBG | [49] |
| Whole grains bread | Maize | 30 male subjects | 50 g/day | Healthy Subjects | Serum glucose | Reduced PBG | [50] |
| Sorghum and Wheat muffin | Sorghum and wheat flour | 10 male subjects | 50 g/day | Healthy Subjects | Serum glucose, insulin | Improved the PBG and insulin | [51] |
| Whole rye bread | Whole rye with white wheat bread | 6 males and 9 females | 50 g/day | Healthy Subjects | Serum glucose, insulin | Improved the insulin response | [52] |
| Oat | Oat | A meta-analysis of randomized controlled trials | 50 g/day | Healthy Subjects | Serum glucose, insulin | Improved glucose and insulin response | [53] |
| Oat and beta-glucan | Oat and beta-glucan | A meta-analysis of randomized controlled trials | - | Healthy Subjects | Serum glucose, HbA1c, and insulin | Improved glucose and insulin and HbA1c response | [54] |
| Whole grain rye with starch | Whole grain rye flour and rye kernels bread | 21 subjects | 50 g/day | Healthy Subjects | Serum glucose, OGTT, insulin, PYY, FFA, and IL-6 | Improved cardiometabolic variables and glucose | [55] |
| Whole grain oats | Whole grain oats | A meta-analysis of randomized controlled trials | - | Healthy Subjects | Serum glucose, OGTT, insulin, and TC | Cholesterol-lowering and anti-diabetic effects | [56] |
| Whole-grain rye and wheat bread | Whole-grain rye porridges and refined wheat bread | 21 subjects | 40, 55 g/day | Healthy Subjects | Serum glucose, postprandial plasma amino acids and short chain fatty acids | Suppressed appetite and improved glucose metabolism. | [57] |
| Canola oil-enriched bread supplement | Canola oil-enriched bread | 141 subjects | 31 g/day | Diabetic patients | HbA1c, blood pressure, Framingham CVD risk score, and reactive hyperemia index ratio | Improved glycemic control in T2DM | [58] |
| Grains | Monascus-fermented grains | Male SD rats | 300 mg/kg bw. For 16 weeks | High-fructose (60%, w/w) plus high-fat (20%, w/w) diet | OGTT, Insulin, insulin sensitivity index, TBARS, SOD, CAT, and GPx | Anti-diabetic effect by improving insulin resistance and hepatic antioxidant enzymes. | [59] |
| Whole grains and legumes | Whole grains and legumes | 39 males, 146 females | 30–70 g for 16 weeks | Diabetic patients | BMI, waist and hip ratio, TC, TG, LDL-C, HDL-C, FBS, insulin FFA, Plasma apolipoprotein A-V, and CRP | Anti-diabetic effects | [60] |
| DASH diet | fruits, vegetables, whole grains, low-fat dairy products, low in saturated fats, cholesterol, refined grains, and sweets | 52 pregnant women | 40 g for 4 weeks | Gestational Diabetic patients | Length, weight, and head circumference of infants | Improved gestational diabetes mellitus | [61] |
| Whole grains | Cereal, bread, rice, pasta, and muffin | 11 subjects | 6–10 servings/day for 6 weeks | Diabetic/obese patients | Insulin, blood glucose, and OGTT | Reduce the risk of T2DM and heart disease. | [62] |
| Vegetables | Okra (Abelmoschus esculentus L. Moench) | Male C57BL/6 mice | 50 mg/kg, p.o for 10 days | STZ (45 mg/kg, i.p.) | blood glucose, OGTT | Hypoglycemic effect | [63] |
| Vegetables | Red pepper and soybeans | Male SD rats | 5% powder supplement | STZ (45 mg/kg, i.p.) | FBS, OGTT, body weight, visceral fat, and serum leptin | Improves glucose homeostasis by reducing insulin resistance | [64] |
| Fruits and vegetables | Fruits and vegetables | 550 children and adolescents | 257, 227 g/day for 30 days | Diabetic patients | FBS, insulin, and HbA1c | Anti-diabetic effect | [50] |
| Vegetables | Purple carrots and purple potatoes | Obese Zucker rats | Purple carrot and potatoes supplemented a high-fat diet for 8 weeks. | - | Intraperitoneal glucose and insulin tolerance test and invasive hemodynamic tests | Purple vegetables improve insulin resistance and hypertension | [65] |
| Apricot Lychee | Prunus armeniaca Lychee chinensis | In vitro | - | - | α-glycosidase, aldose reductase, and antioxidant activity | Anti-diabetic effects | [66] |
| Blueberry | Vaccinium cyanococcus | ||||||
| Plum | Prunus salicina | ||||||
| Kiwi | Kiwifruit c.v. hayward | ||||||
| Lemon pulp | Citrus limon | ||||||
| Lemon peel | Citrus limon | ||||||
| Pear | Pyrus bretschneider | ||||||
| Wolfberry | Lycium chinensis | ||||||
| Watermelon | Citrullus lanatusus | ||||||
| Lettuce | Lactuca sativa | In vitro | - | - | α-glycosidase, aldose reductase, and antioxidant activity | Anti-diabetic effects | [66] |
| Cucumber | Cucumis sativus | ||||||
| Red onion | Allium cepa | ||||||
| Bitter gourd | Momordica charantia | ||||||
| Eggplant | Solanum melongena | ||||||
| Celery | Apium graveolens | ||||||
| Kelp | Laminaria japonica | ||||||
| Wax gourd | Benincasa pruriens | ||||||
| Garlic | Allium sativum | ||||||
| Tomato | Solanum lycopeersicum | ||||||
| Vegetables | Momordica charantia | SD rats | 50 mg/kg, p.o for 10 days | STZ (45 mg/kg, i.p.) | FBS, insulin, and HbA1c | Anti-diabetic effects | [67] |
| Sources of Polysaccharides | Monosaccharide Units/Active Compounds | Effects on Metabolism | Molecular Mechanisms | Results | References |
|---|---|---|---|---|---|
| Cyclocarya paliurus | Rhamnose, arabinose, xylose, mannose, glucose, and galactose | Triglyceride metabolism | ↑ATGL, ↑PPAR-α, ↑PPARɣ coactivator-1 α, ↓FAS, ↓HMG-CoA reductase | Anti-hyperlipidemic effects | [103] |
| Cichorium intybus L. | Sorbin, glucose, fructose, and glucitol | Triglyceride metabolism | ↑p-AMPK, ↑ATGL, ↑CAPT1, ↑p-ACC, ↓FAS, | Anti-hyperlipidemic effects | [104] |
| Lycium barbarum | Rhamnose, arabinose, xylose, mannose, glucose, galactose, and galacturonic acid | Triglyceride metabolism | ↑p-AMPK, ↑p-ACC, ↑ATGL, ↑CAPT1, ↓FAS | Anti-hyperlipidemic effects | [105] |
| Enteromorpha prolifera | Rhamnose, glucuronic acid, arabinose, fucose, xylose, and glucose | Cholesterol metabolism | ↓SREBP-2, ↓HMG-CoA reductase | Cholesterol-lowering effects | [106] |
| Oryza sativa L. | Xylose, rhamnose, mannose, galactose, arabinose, and glucose | Triglyceride and cholesterol metabolism | ↑PPAR-α, ↑PPARɣ coactivator-1 α, ↓SREBP-1c | Anti-hyperlipidemic effects | [107] |
| Morchella angusticepes | Arabinose, mannose, glucose, and galactose | Cholesterol metabolism | ↓HMG-CoA reductase | Cholesterol-lowering effects | [109] |
| Lentinula edodes | α- and β-glucans and fucomannogalactans | Cholesterol metabolism | ↓HMG-CoA reductase | Cholesterol-lowering effects | [110] |
| Fucus vesiculosus | Sulfated polysaccharide with fucose | Triglycerides and cholesterol metabolism | ↓FAS, ↓ACC, ↓SREBP -1c, ↓SREBP-2, ↓HMG-CoA reductase | Triglyceride and Cholesterol-lowering effects | [111] |
| Lycium barbarum | Rhamnose, arabinose, xylose, mannose, glucose, galactose, and galacturonic acid | Triglyceride and cholesterol metabolism | ↑p-AMPK, ↑PPARɣ coactivator-1 α, ↑p-ACC, ↓FAS, ↓SREBP-1c | Anti-hyperlipidemic effects | [116] |
| Rheum palmatum L. | Rhamnose, mannose, and galactose | Triglyceride metabolism | ↑p-AMPK, ↑p-ACC | Anti-hyperlipidemic effects | [117] |
| Schisandra Chinensis | Galactose, arabinose, and glucose | Triglyceride and cholesterol metabolism | ↓SREBP-1c, ↓SREBP-2, ↓FAS ↓ACC, ↓HMG-CoA reductase | Anti-hyperlipidemic effects | [118] |
| Aconiti Lateralis Radix Praeparata | α-d-glucan | Cholesterol metabolism | ↑LDL receptor, ↓HMG-CoA reductase | Cholesterol-lowering effects | [119] |
| Brasenia schreberi | Galactose, mannose, fucose, rhamnose, arabinose, xylose, glucose, and alduronic acids | Cholesterol metabolism | ↑LDL receptor, ↑PPAR-α | Cholesterol-lowering effects | [120] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesan, K.; Xu, B. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. https://doi.org/10.3390/molecules24142556
Ganesan K, Xu B. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules. 2019; 24(14):2556. https://doi.org/10.3390/molecules24142556
Chicago/Turabian StyleGanesan, Kumar, and Baojun Xu. 2019. "Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides" Molecules 24, no. 14: 2556. https://doi.org/10.3390/molecules24142556
APA StyleGanesan, K., & Xu, B. (2019). Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules, 24(14), 2556. https://doi.org/10.3390/molecules24142556







