Lipophilicity as a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives
Abstract
:1. Introduction
2. Results and Discussion
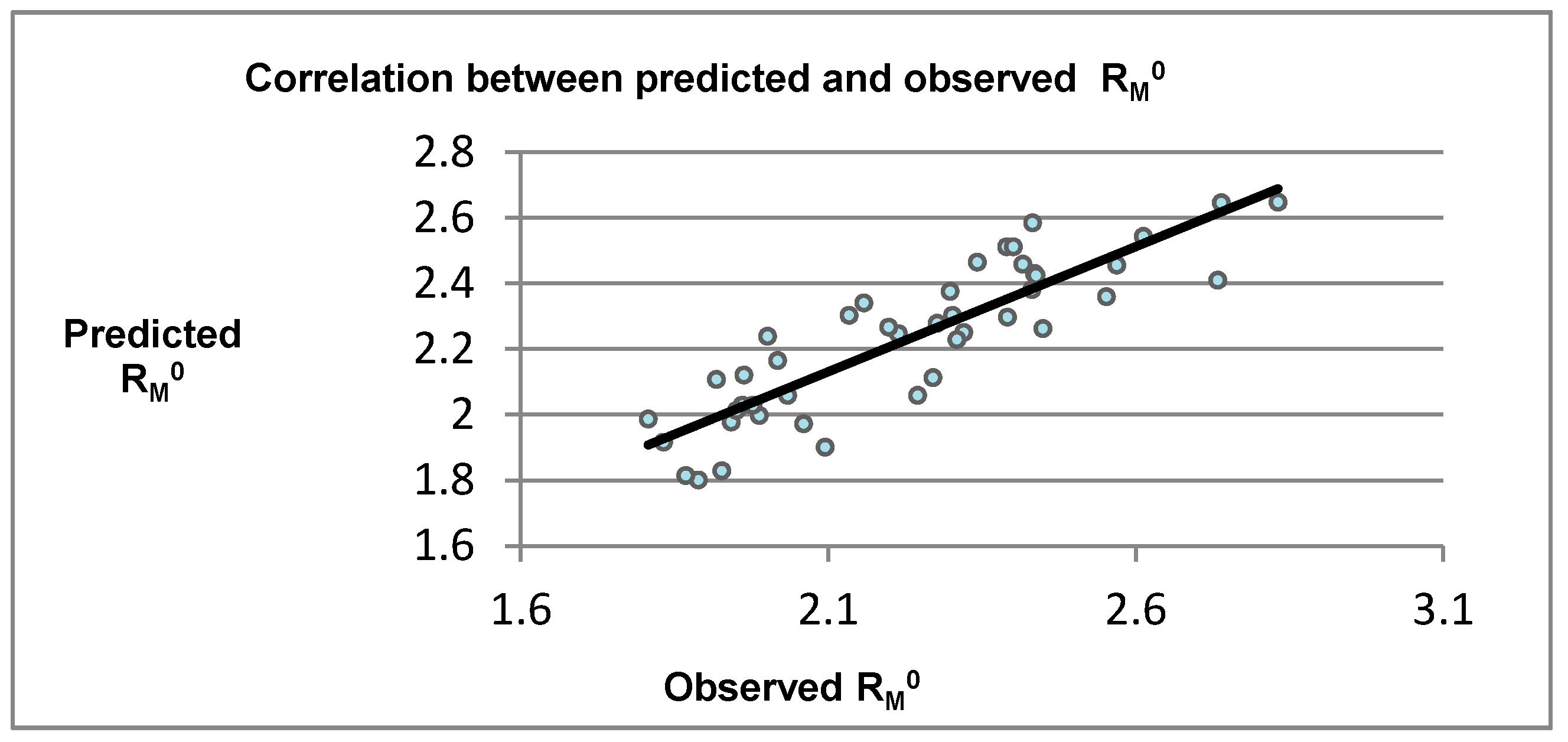
2.1. Chromatographic Evaluation
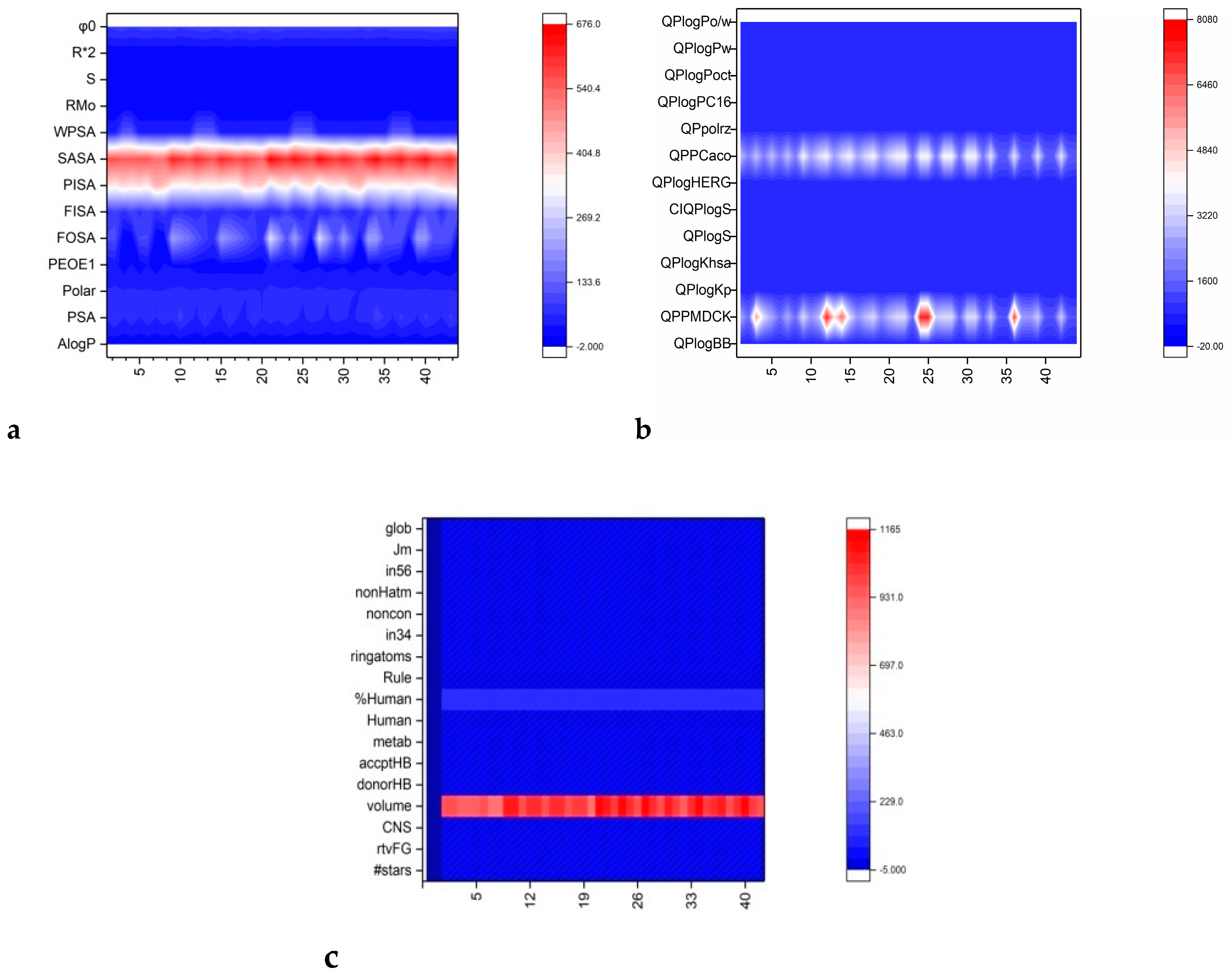
2.2. Computational Evaluation
3. Materials and Methods
3.1. Experimental Evaluation
3.2. Computational Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| #stars | number of property or descriptors values that fall outside the 95% range of similar values for known drugs. |
| %Human | percent human oral absorption |
| accptHB | estimated number of hydrogen bounds that would be donated, |
| AlogP | octanol/water partition coefficient |
| ClQPlogS | conformation independent predicted aquenos solubility |
| CNS | predicted central nervous system activity |
| donorHB | estimated number of hydrogen bounds |
| FISA | a component of SASA |
| FOSA | hydrophobic component of the SASA |
| Glob | globularity descriptor |
| Human | human oral absorbtion |
| in34 | number of atoms in 3 or 4 membered rings |
| in56 | number of atoms in 5 or 6 membered rings |
| Jm | predicted maximum transdermal transport |
| metab | number of likely metabolic reactions |
| noncom | number of ring atoms not able to form conjugated aromatic systems |
| nonHatm | number of heavy atoms |
| PEOE1 | total positive van der Waals surface area |
| PISA | number of carbon atoms and the attached hydrogens |
| Polar | polarity |
| PSA | polar surface area |
| QPlogBB | predicted brain blood partition coefficient |
| QPlogHERG | predicted IC50 value for blok HERG chanels |
| QPlogKhsa | prediction of binding to serum albumin |
| QplogKp | predicted skin permeability |
| QPlogPC16 | predicted hexadecane gas partition coefficient |
| QPlogPo/w | predicted octanol water partition coefficient |
| QPlogPoct | predicted octanolgass partition coefficient |
| QPlogPw | predicted water gass partition coefficient |
| Qpolrz | predicted polarizability in cubic angstroms |
| QPPCaco | predicted Caco-2 cell permeability in nm/s |
| QPPMDCK | predicted MDCK cell permeability in nm/s |
| R2 | correlation coefficient |
| Ringatoms | number of atoms in a ring |
| RM0 | relative lipophilycity |
| rtvFG | number of reactive functional groups |
| Rule | rule of five |
| SASA | total solvent accessible surface area |
| S | slope |
| Volume | total solvent accessible area in cubic Angstroms |
| WPSA | weakly polar component of the SASA |
| Φ0 | chromatographic hydrophobic index |
References
- Tόth, G.; Mazák, K.; Hosztafi, S.; Kökösi, J.; Noszál, B. Species-specific lipophilicity of thyroid hormones and their precursors in view of their membrane transport properties. J. Pharm. Biomed. Anal. 2013, 76, 112–118. [Google Scholar] [CrossRef]
- Jevrić, L.R.; Karadžić, M.Ž.; Mandić, A.I.; Podunavac Kuzmanović, S.O.; Kovačević, S.Z.; Nikolić, A.R.; Oklješa, A.M.; Sakač, M.N.; Penov Gaši, K.M.; Stojanović, S.Z. Lipophilicity estimation and characterization of selected steroid derivatives of biomedical importance applying RP HPLC. J. Pharm. Biomed. Anal. 2017, 134, 27–35. [Google Scholar] [CrossRef]
- Mazák, K.; Kökösi, J.; Naszál, B. Lipophlicity of zwitterions and related species: a new insight. Eur. J. Pharm. Sci. 2011, 44, 68–73. [Google Scholar] [CrossRef]
- Santos, Á.; Soares, Y.X.; Cravo, S.; Tiritan, M.; Reis, S.; Afonso, C.; Fernandes, C.; Pinto, M. Lipophilicity assessment in drug discovery: experimental and theoretical methods applied to xanthone derivatives. J. Chromatogr. B 2018, 1072, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Klose, M.; Theiner, S.; Varbanov, H.; Hoefer, D.; Pichler, V.; Galanski, M.; Meier-Menches, S.; Keppler, B. Development and validation of liquid chromatography-based methods to assess the lipophilicity of citotoxic Platinum (IV) complexes. Inorganics 2018, 6, 130. [Google Scholar] [CrossRef]
- Łączkowski, K.; Biernasiuk, A.; Baranowska-Łączkowska, A.; Zavyalova, O.; Redka, M.; Malm, A. Synthesis, lipophilicity determination, DFT calculation, antifungal and DPPH radical scaverging activities of tetrahydrothiophen-3-one based thiazole. J. Mol. Struct. 2018, 1171, 717–725. [Google Scholar] [CrossRef]
- Malinowska, I.; Studziński, M.; Malinoski, H. Change of 1,2,4-triazole retention and lipophilicity descriptor values in RP-TLC and MLC-TLC systems in the presence of an external magnetic field. J. Planar Chromatogr. 2017, 30, 106–112. [Google Scholar] [CrossRef]
- Pastuszko, A.; Majchrzak, K.; Czyz, M.; Kupcewicz, B.; Budzisz, E. The synthesis, lipophilicity and cytotoxic effects of new ruthenium(II) arene complexes with chromone derivatives. J. Inorg. Biochem. 2016, 159, 133–141. [Google Scholar] [CrossRef]
- Moreira, J.; Ribeiro, D.; Silva, P.; Nazareth, N.; Monteiro, M.; Palmeira, A.; Saraiva, L.; Pinto, M.; Bousbaa, H.; Cidade, H. New alcoxy flavone derivatives targeting caspases: Synthesis and antitumor activity evaluation. Molecules 2019, 24, 129. [Google Scholar] [CrossRef]
- Bakht, M.A.; Alajmi, M.F.; Alam, P.; Alam, A.; Alam, P.; Aljarba, T.M. Theoretical and experimental study on lipophilicity and wound healing activity on ginger compounds. Asian Pac. J. Trop. Biomed. 2014, 4, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, N.; Chavada, V.; Sanyal, M.; Shrivastav, P. Influence of organic modifier and separation modes for lipophilicity assessment of drugs using thin layer chromatography indices. J. Chromatogr. A 2018, 1571, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Numviyimana, C.; Chmiel, T.; Kot-Wasik, A.; Namieśnik, J. Study of pH temperature effect on lipophilicity of catechol-containing antioxidants by reversed phase liquid chromatography. Microchem. J. 2019, 145, 380–387. [Google Scholar] [CrossRef]
- Vastag, G.; Apostolov, S.; Matjević, B. Prediction of lipophilicity and pharmacokinetics of chloroacetamides by chemometric approach. Iran. J. Pharm. Res. 2018, 17, 100–114. [Google Scholar] [PubMed]
- Xin, X.; Zhang, M.; Li, X.; Lai, F.; Zhao, G. Biocatalytic synthesis of acylated derivatives of troxerutin: Their bioavailability and antioxidant properties in vitro. Microb. Cell Fact. 2018, 17, 130. [Google Scholar] [CrossRef]
- Linton, M.A.; Burke, B.; Johnson, T.; Ninkovic, S.; Gajiwala, K.; Richardson, P.; Le, P. Effect of water solvation on the lipophilicity of isomeric pyrimidine-carboxamides. Bioorg. Med. Chem. 2015, 23, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Martel, S.; Begnaud, F.; Schuler, W.; Gillerat, F.; Oberhauser, N.; Nurisso, A.; Carrupt, P.A. Limits of rapid log P determination methods for highly lipophilic and flexible compounds. Anal. Chim. Acta 2016, 915, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Lian, H.-Z. Recent advances in lipophilicity measurement by reversed-phase high-performance liquid chromatography. Trends Anal. Chem. 2015, 68, 28–36. [Google Scholar] [CrossRef]
- Mc Bride, E.; Kretsch, A.; Garibay, L.; Brigance, K.; Frey, B.; Buss, B.; Verbeck, G. Rapid experimental and computational determination of phenethylamine drug analogue lipophilicity. Forensic Chem. 2016, 1, 58–65. [Google Scholar] [CrossRef]
- Rageh, A.H.; Atia, N.N.; Abdel-Rahman, A.M. Lipophilicity estimation of statins as a decisive physicochemical parameter for their hepato-selectivity using reversed-phase thin layer chromatography. J. Pharm. Biomed. Anal. 2017, 142, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Karadžić, M.; Lončar, D.; Benedeković, G.; Kovačević, I.; Popsavin, V.; Kocačević, S.; Jevrić, L.; Podunavac-Kuzmanavić, S. A comparative study of chromatographic behavior and lipophilicity of selected natural styryl lactones, their derivatives and analogues. Eur. J. Pharm. Sci. 2017, 105, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.B.; Su, C.R.; Chiou, W.F.; Liu, Y.N.; Chen, R.Y.H.; Bastaw, K.F.; Lee, K.H.; Wu, T.S. Design, synthesis and biological evaluation of Mannich bases of heterocyclic chalcone analogs as cytotoxic agents. Bioorg. Med. Chem. 2018, 16, 7358–7370. [Google Scholar] [CrossRef]
- Evangelista, F.C.G.; Bandeira, M.O.; Silva, G.D.; Silva, M.G.; Andrade, S.N.; Marques, D.R.; Silva, L.M.; Castro, W.V.; Santos, F.V.; Viana, G.H.R.; et al. Synthesis and in vitro evaluation of novel triazole/azide chalcones. Med. Chem. Res. 2017, 26, 27–43. [Google Scholar] [CrossRef]
- Venkataramana Reddy, P.O.; Hridhay, M.; Nikhil, K.; Khan, S.; Jha, P.N.; Shah, K.; Kumar, D. Synthesis and invastigations into the anticancer and antibacterial activity studies of β-carboline chalcones and their bromide salts. Bioorg. Med. Chem. Lett. 2018, 28, 1278–1282. [Google Scholar] [CrossRef]
- Mellado, M.; Madrid, A.; Reyna, M.; Weinstein-Oppenheimer, C.; Mella, J.; Sales, C.; Sanchez, E.; Cuellar, M. Synthesis of chalcones with antiproliferative activity on the SH-5Y5Y neuroblastoma cell line: Quantitative Structure-Activity Relationship Models. Med. Chem. Res. 2018, 27, 2414–2425. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N. Chalcones lack of specificity: Tubuline, a multitargeted molecule. RJLBPCD 2018, 4, 260–268. [Google Scholar]
- Chen, P.Y.; Wang, T.P.; Chiang, M.Y.; Huang, K.S.; Tzeng, C.C.; Chen, Y.L.; Wang, E.C. Environmentally benign syntheses of flavanones. Tetrahedron 2011, 67, 4155–4160. [Google Scholar] [CrossRef]
- Lahyani, A.; Trabelsi, M. Ultrasonic-assisted synthesis of flavones by oxidative cyclization of 2′-hydroxychalcones using iodine monochloride. Ultrason. Sonochem. 2016, 31, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Pawar, C.D.; Sarkate, A.P.; Karnik, K.S.; Bahekar, S.S.; Pansare, D.N.; Shelke, R.N.; Jawale, C.S.; Shinde, D.B. Synthesis and antimicrobial evaluation of novel ethyl 2-(2-(4-substituted)acetamido)-4-subtituted-thiazole-5-carboxylate derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 3525–3528. [Google Scholar] [CrossRef]
- Lungu, C.N.; Diudea, M.V.; Putz, M.V.; Grudzinski, I.P. Linear and branched PEIs (polyethylenimines) and their property space. Int. J. Mol. Sci. 2016, 17, 555. [Google Scholar] [CrossRef] [PubMed]
- Lungu, C.N. C-C Chemokine receptor type 3 inhibitors: bioactivity prediction using local vertex invariants based on thermal conductivity layer matrix. Studia UBB Chemia 2018, 1, 177–188. [Google Scholar] [CrossRef]
- Majumdar, S.; Basak, S.C.; Lungu, C.N.; Diudea, M.V.; Grunwald, G.D. Mathematical structural descriptors and mutagenicity assessment: A study with congeneric and diverse datasets. SAR QSAR Environ. Res. 2018, 29, 579–590. [Google Scholar] [CrossRef]
- Raghav, N.; Kaur, R. Chalcones, semicarbazones and pyrazolines as inhibitors of cathepsins B, H and L. Int. J. Biol. Macromol. 2015, 80, 710–724. [Google Scholar] [CrossRef] [PubMed]
- Bano, S.; Javed, K.; Ahmad, S.; Rathish, I.G.; Singh, S.; Chaitanya, M.; Arunasree, K.M.; Alam, M.S. Synthesis of some novel chalcones, flavanones and flavones and evaluation of their anti-inflammatory activity. Eur. J. Med. Chem. 2013, 65, 51–59. [Google Scholar] [CrossRef]
- Safavi, M.; Esmati, N.; Ardestani, S.K.; Emami, S.; Ajdari, S.; Davoodi, J.; Shafiee, A.; Foroumadi, A. Halogenated flavanones as potential apoptosis-inducing agents: synthesis and biological activity evaluation. Eur. J. Med. Chem. 2012, 58, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Simoens, M.; Falchi, G.; Lavaggi, M.L.; Piro, O.E.; Castellano, E.E.; Vidal, A.; Azqueta, A.; Monge, A.; de Ceráin, A.L.; et al. Synthetic chalcones, flavanones, and flavones as antitumoral agents: Biological evaluation and structure-activity relationships. Bioorg. Med. Chem. 2007, 15, 3356–3367. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, F.; Fioravanti, R.; Bolasco, A.; Chimenti, P.; Secci, D.; Rossi, F.; Yáñez, M.; Orallo, F.; Ortuso, F.; Alcaro, S.; et al. A new series of flavones, thioflavones, and flavanones as selective monoamine oxidase-B inhibitors. Bioorg. Med. Chem. 2010, 18, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, S.; Goren, A.C.; Ozturk, T. Facile syntheses of 3-hydroxyflavones. Org. Lett. 2012, 14, 1576–1579. [Google Scholar] [CrossRef]
- Ghuarpure, M.; Choudhary, R.; Ingle, V.; Juneja, H. Synthesis of new series of 3-hydroxy/acetoxy-2-phenyl-4H-chromen-4-ones and their biological importance. J. Chem. Sci. 2013, 125, 575–582. [Google Scholar] [CrossRef]
- Lungu, C.N.; Diudea, M.V.; Putz, M.V. Ligand shaping in induced fit docking of Mra Y inhibitors. Polynomial discriminant and laplacian operator as biologicaly activity descriptors. Int. J. Mol. Sci. 2017, 18, 1377. [Google Scholar] [CrossRef] [PubMed]
- Diudea, M.V.; Lungu, C.N.; Nagy, C.L. Cube-Rhombellane related structures: A drug perspective. Molecules 2018, 23, 2533. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinescu, T.; Lungu, C.N.; Lung, I. Lipophilicity as a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives. Molecules 2019, 24, 1505. https://doi.org/10.3390/molecules24081505
Constantinescu T, Lungu CN, Lung I. Lipophilicity as a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives. Molecules. 2019; 24(8):1505. https://doi.org/10.3390/molecules24081505
Chicago/Turabian StyleConstantinescu, Teodora, Claudiu Nicolae Lungu, and Ildiko Lung. 2019. "Lipophilicity as a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives" Molecules 24, no. 8: 1505. https://doi.org/10.3390/molecules24081505
APA StyleConstantinescu, T., Lungu, C. N., & Lung, I. (2019). Lipophilicity as a Central Component of Drug-Like Properties of Chalchones and Flavonoid Derivatives. Molecules, 24(8), 1505. https://doi.org/10.3390/molecules24081505





