Synthesis and Characterization of Healable Waterborne Polyurethanes with Cystamine Chain Extenders
Abstract
1. Introduction
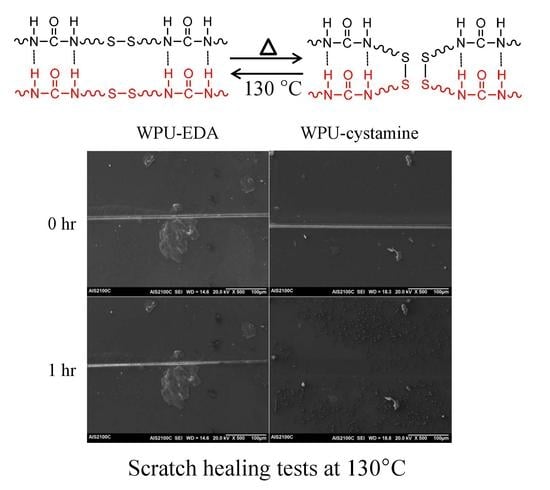
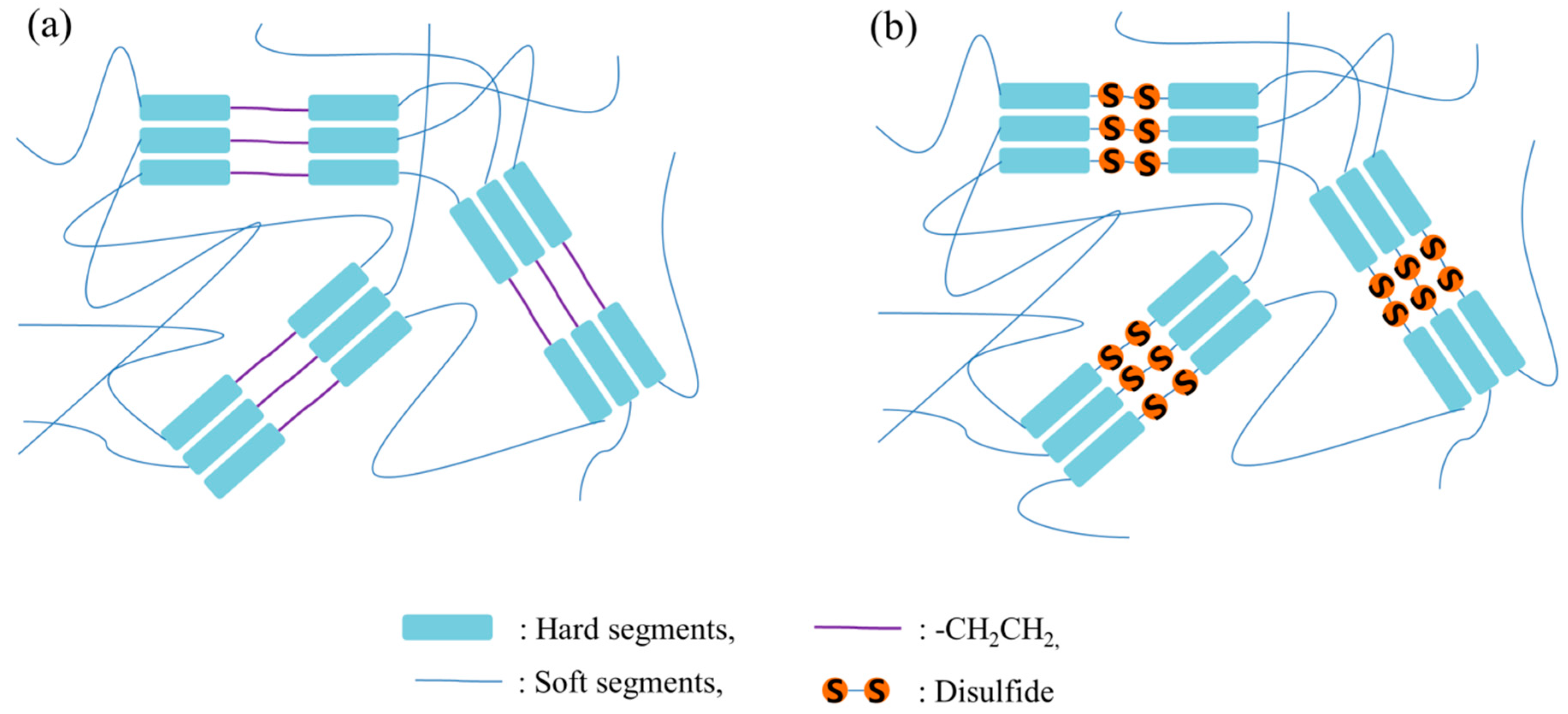
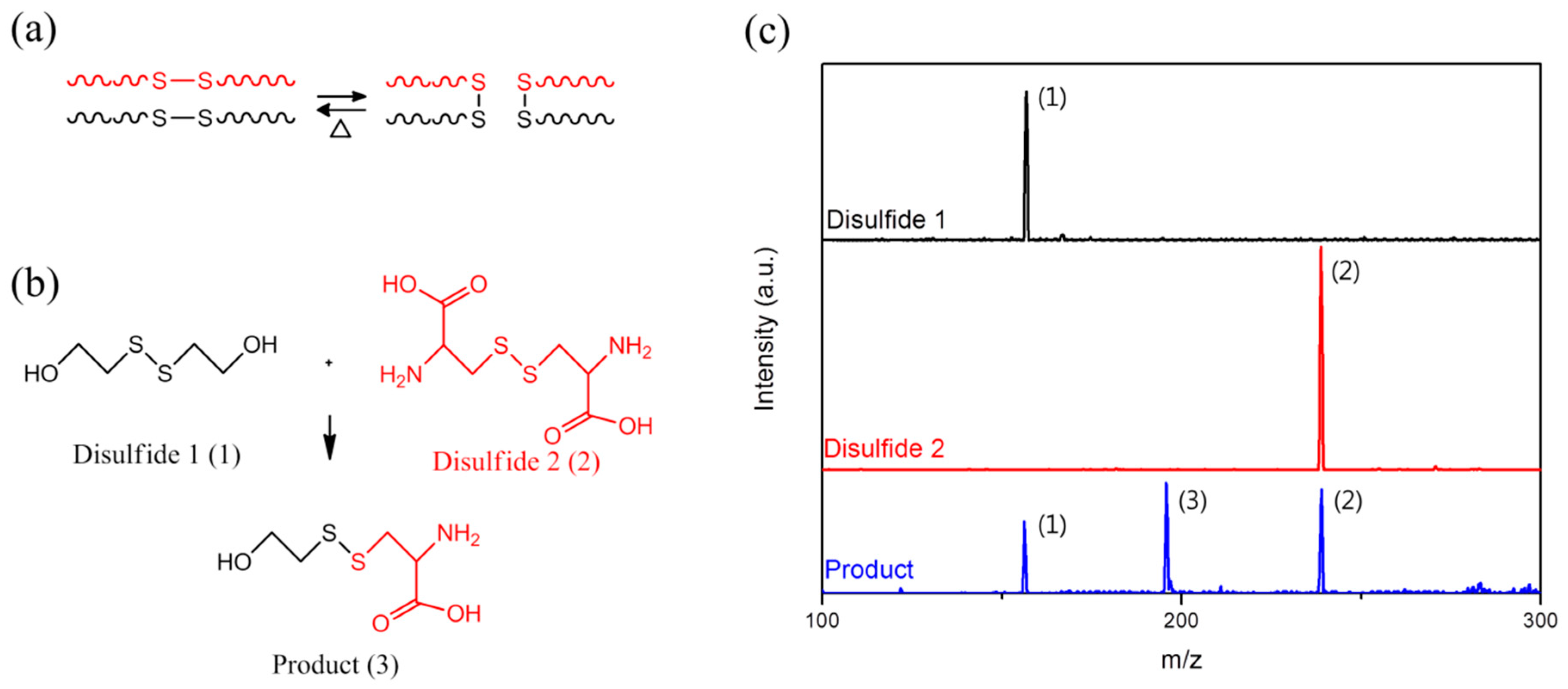
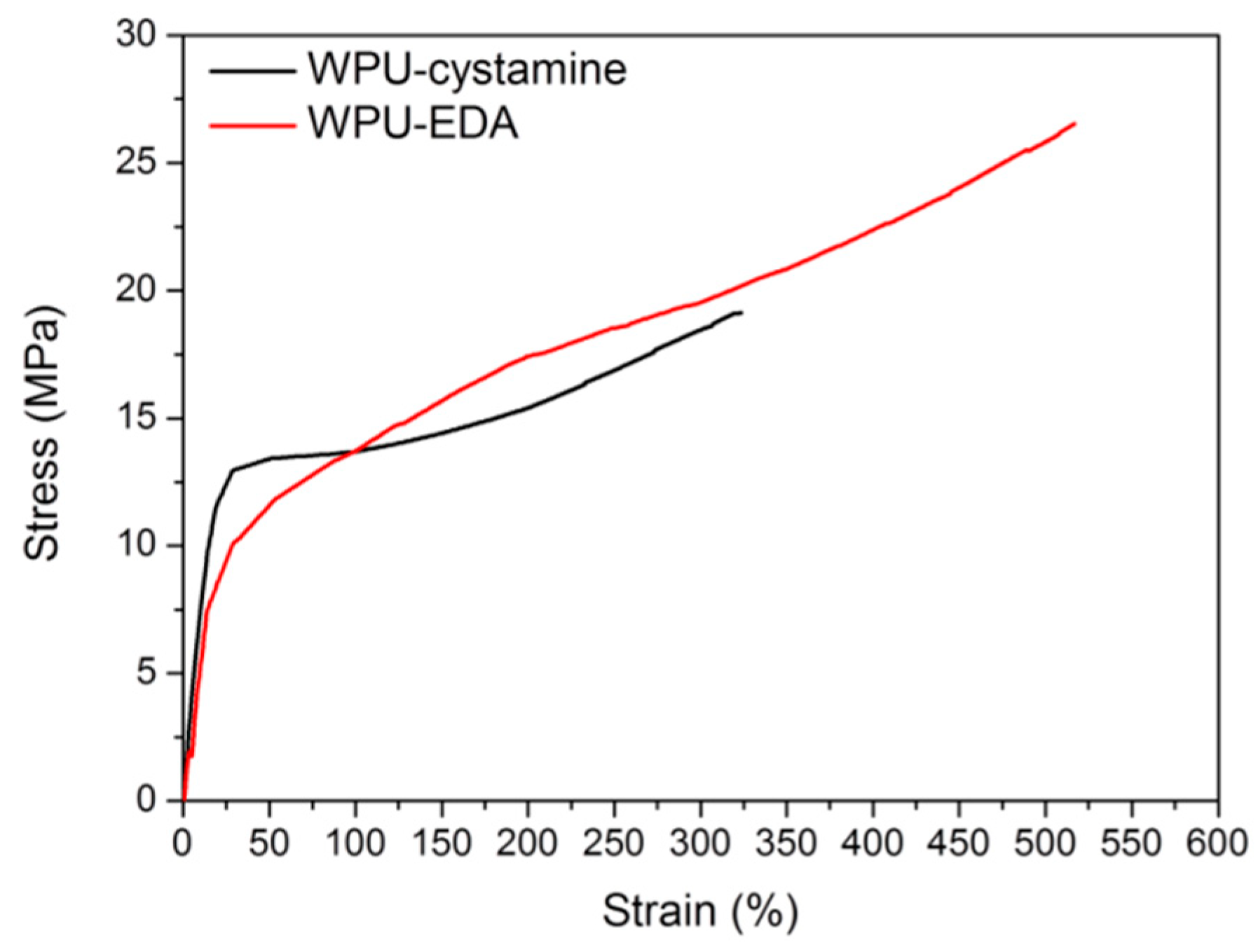
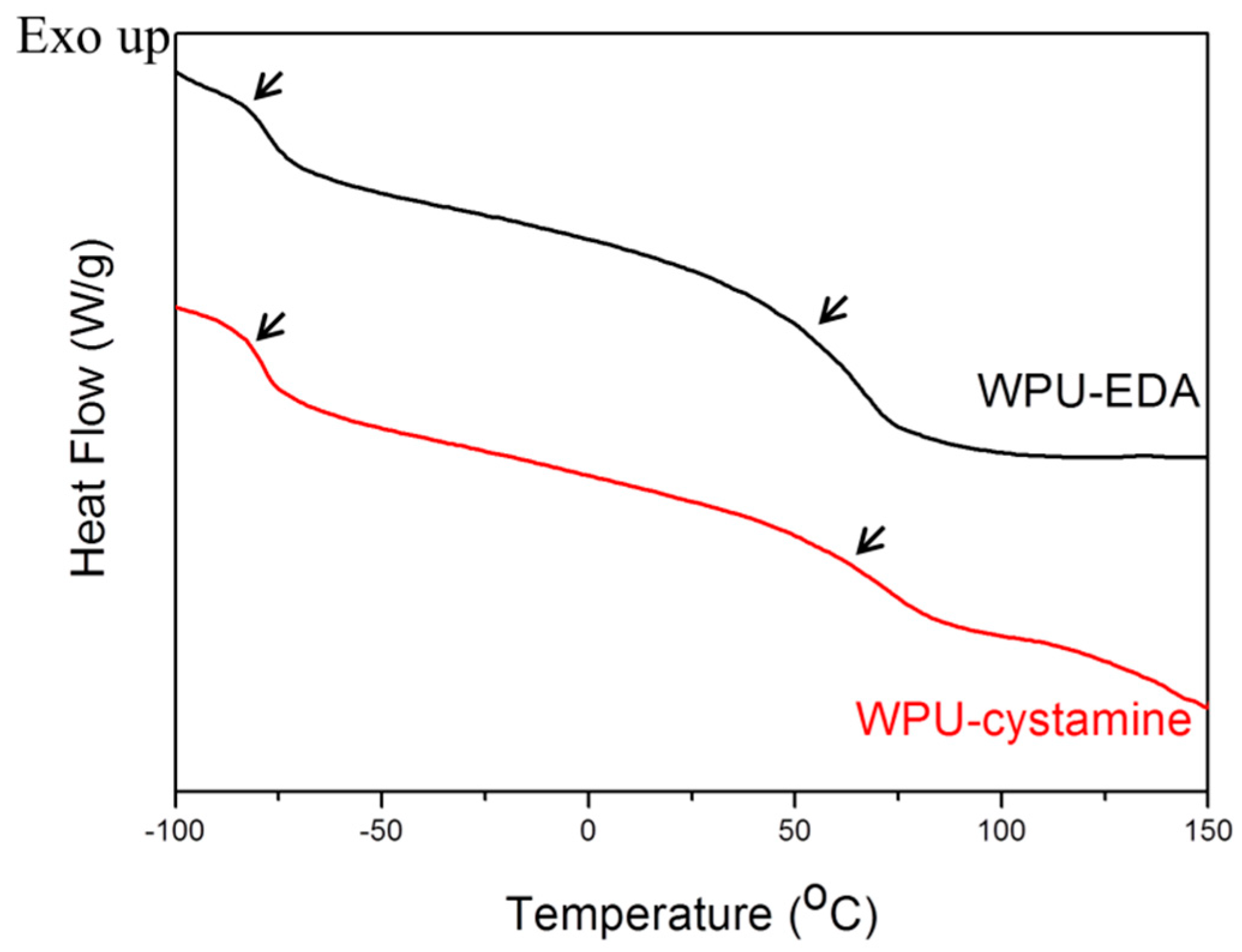
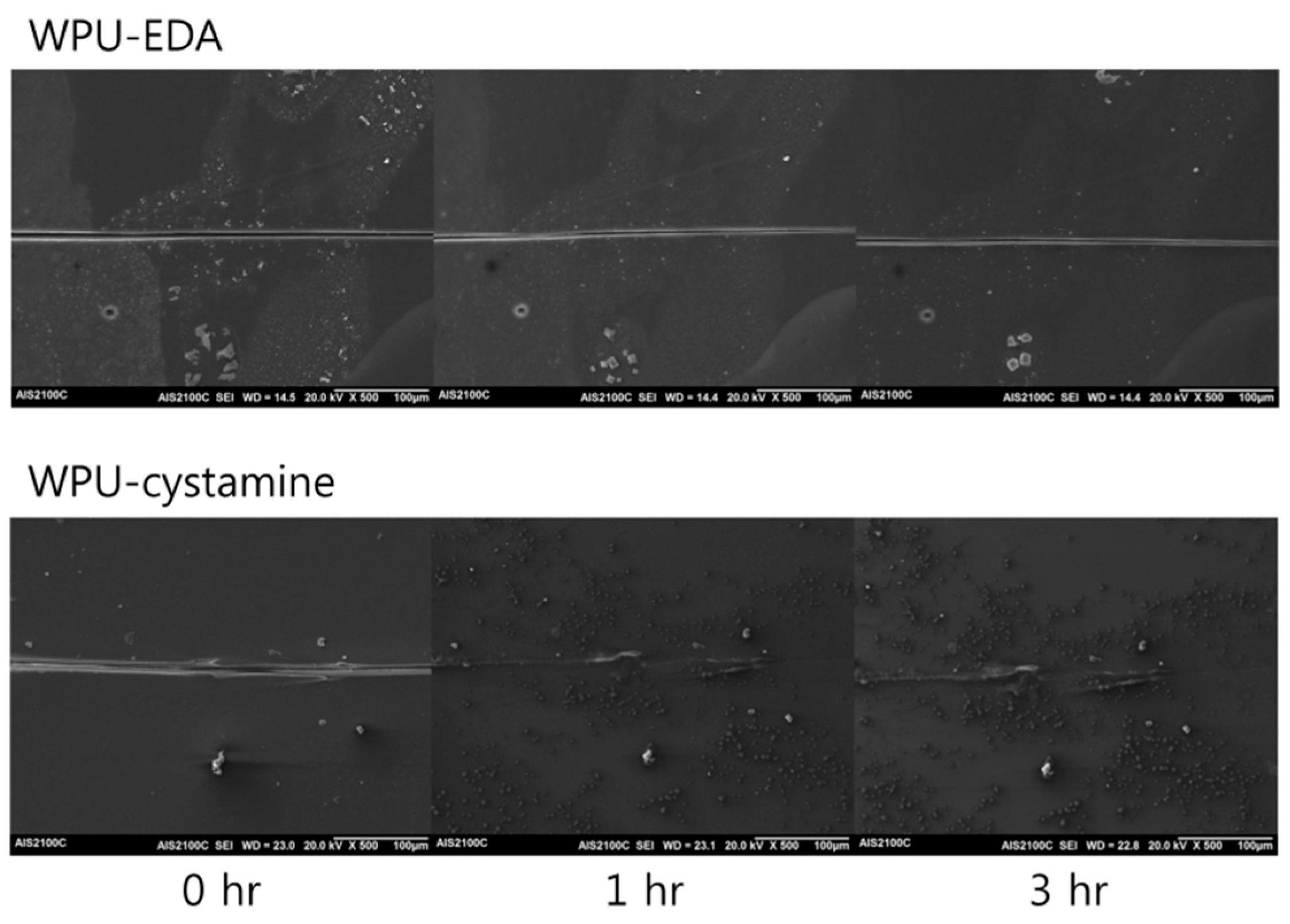
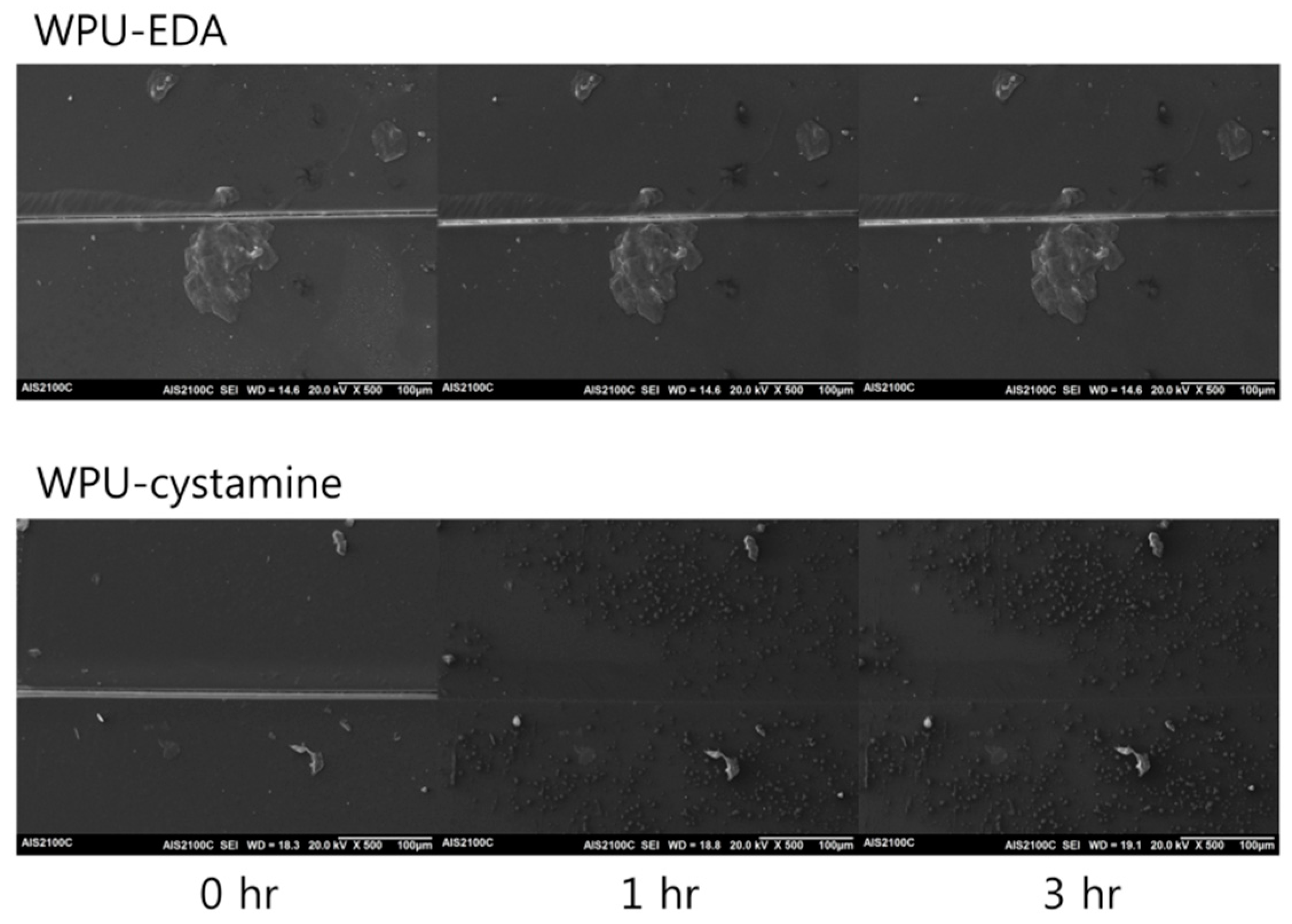
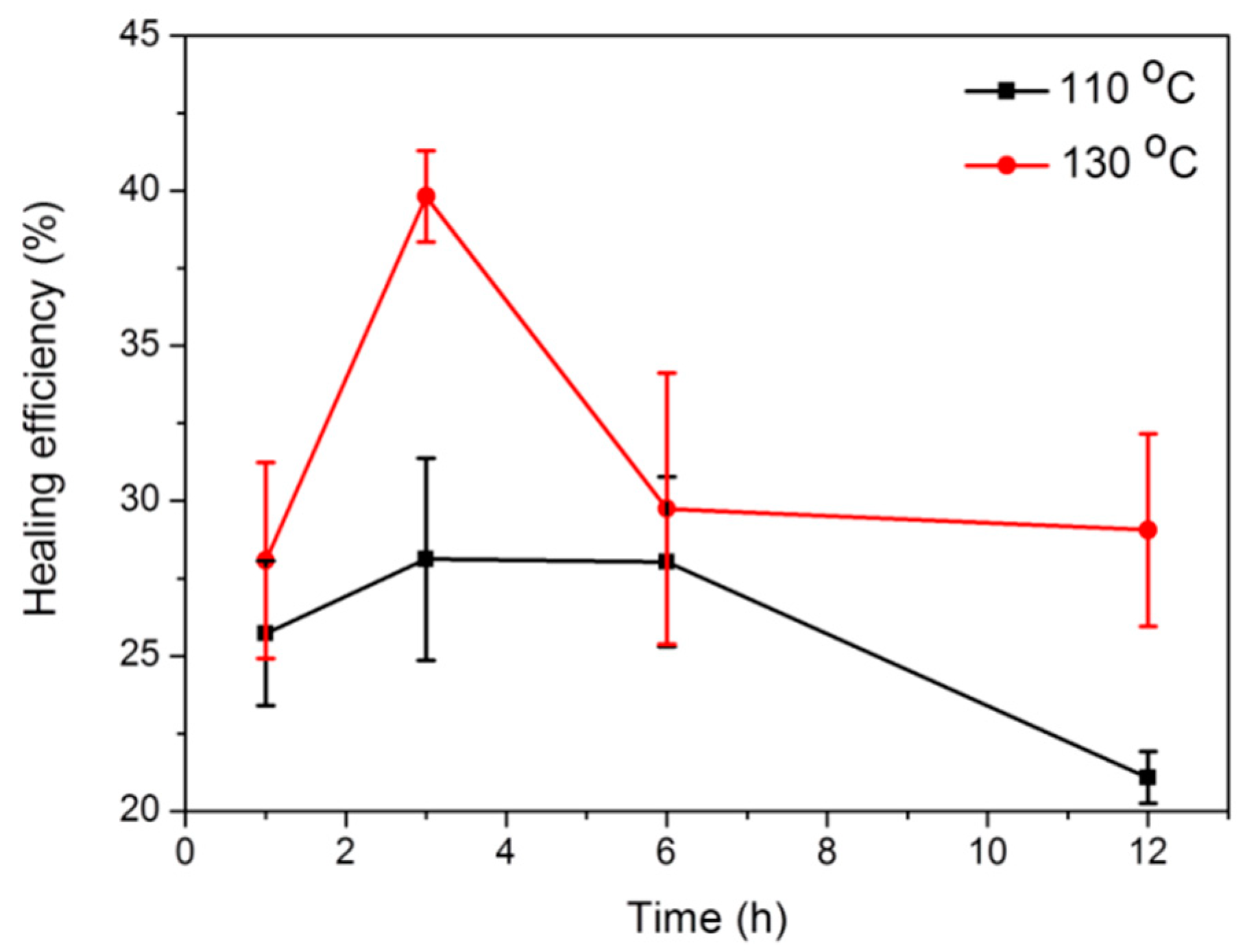
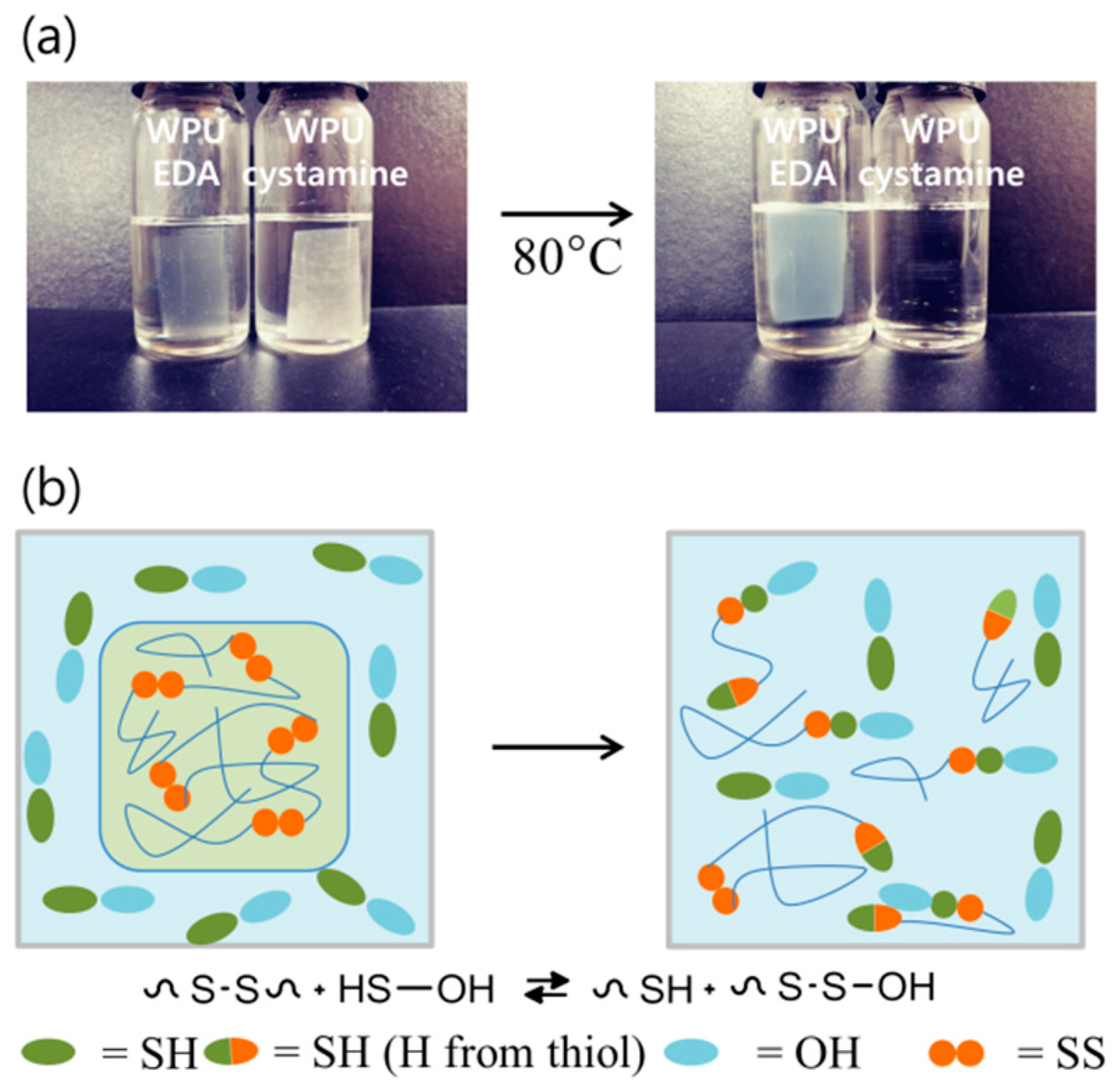
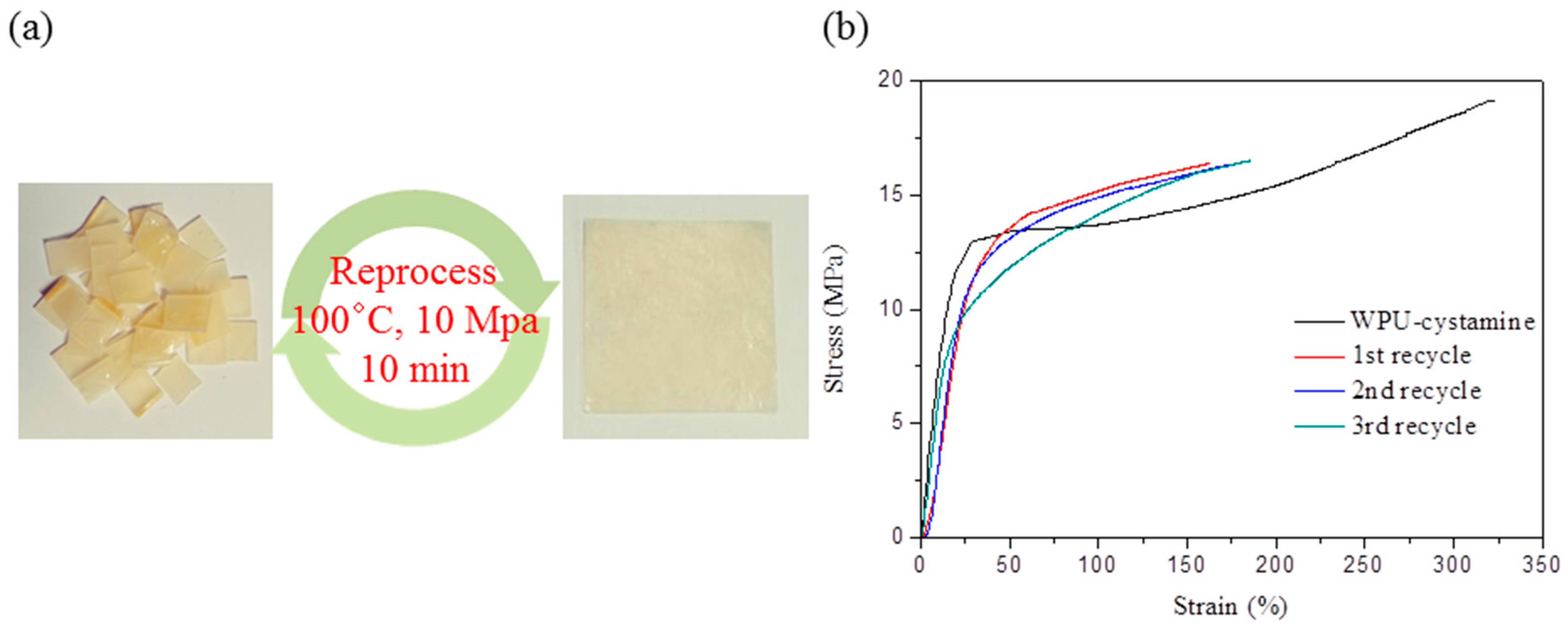
2. Results and Discussion
3. Experimental
3.1. Materials
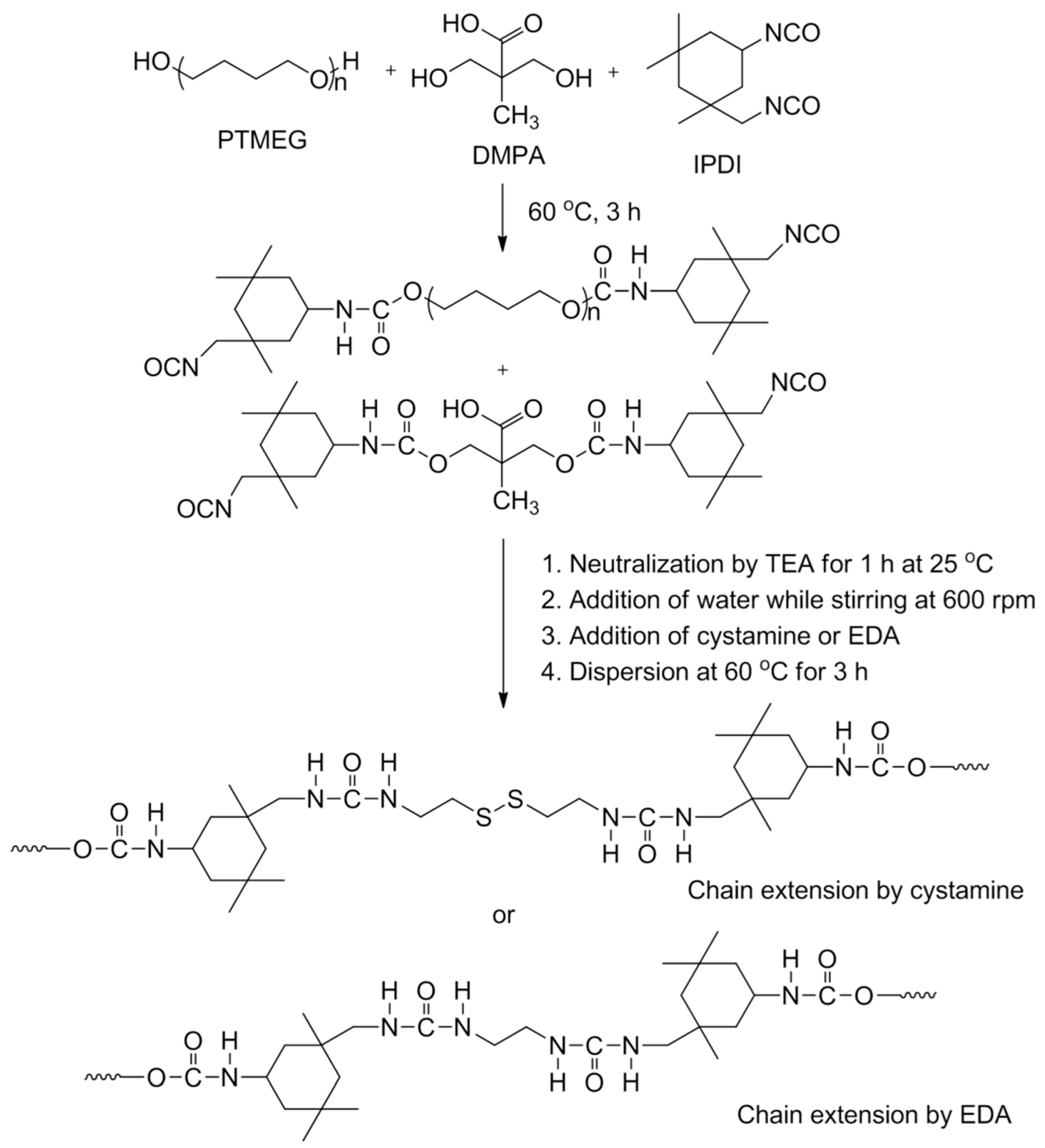
3.2. Synthesis of Waterborne Polyurethane
3.3. Confirmation of the Disulfide Metathesis Reaction
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rahman, M.M.; Kim, H.D. Characterization of waterborne polyurethane adhesives containing different soft segments. J. Adhes. Sci. Technol. 2007, 21, 81–96. [Google Scholar] [CrossRef]
- Bai, C.Y.; Zhang, X.Y.; Dai, J.B.; Zhang, C.Y. Water resistance of the membranes for UV curable waterborne polyurethane dispersions. Prog. Org. Coat. 2007, 59, 331–336. [Google Scholar] [CrossRef]
- Yu, Y.; Liao, B.; Li, G.N.; Jiang, S.L.; Sun, F. Synthesis and Properties of Photosensitive Silicone-Containing Polyurethane Acrylate for Leather Finishing Agent. Ind. Eng. Chem. Res. 2014, 53, 564–571. [Google Scholar] [CrossRef]
- Perez-Liminana, M.A.; Aran-Ais, F.; Torro-Palau, A.M.; Orgiles-Barcelo, A.C.; Martin-Martinez, J.M. Characterization of waterborne polyurethane adhesives containing different amounts of ionic groups. Int. J. Adhes. Adhes. 2005, 25, 507–517. [Google Scholar] [CrossRef]
- Fang, Y.L.; Du, X.S.; Jiang, Y.X.; Du, Z.L.; Pan, P.T.; Cheng, X.; Wang, H.B. Thermal-Driven Self-Healing and Recyclable Waterborne Polyurethane Films Based on Reversible Covalent Interaction. ACS Sustain. Chem. Eng. 2018, 6, 14490–14500. [Google Scholar] [CrossRef]
- Wan, T.; Chen, D.J. Mechanical enhancement of self-healing waterborne polyurethane by graphene oxide. Prog. Org. Coat. 2018, 121, 73–79. [Google Scholar] [CrossRef]
- Shahabadi, S.I.S.; Kong, J.H.; Lu, X.H. Aqueous-Only, Green Route to Self-Healable, UV-Resistant, and Electrically Conductive Polyurethane/Graphene/Lignin Nanocomposite Coatings. ACS Sustain. Chem. Eng. 2017, 5, 3148–3157. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, H.H.; Peng, X.H. Synthesis of self-healing waterborne polyurethanes containing sulphonate groups. RSC Adv. 2017, 7, 20093–20100. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Martin, L.; Aramburu, N.; Irusta, L.; Fernandez-Berridi, M.J. Coumarin based light responsive healable waterborne polyurethanes. Prog. Org. Coat. 2016, 99, 314–321. [Google Scholar] [CrossRef]
- Wan, T.; Chen, D. Preparation of β-cyclodextrin reinforced waterborne polyurethane nanocomposites with excellent mechanical and self-healing property. Compos. Sci. Technol. 2018, 168, 55–62. [Google Scholar] [CrossRef]
- Erice, A.; de Luzuriaga, A.R.; Matxain, J.M.; Ruipérez, F.; Asua, J.M.; Grande, H.-J.; Rekondo, A. Reprocessable and recyclable crosslinked poly (urea-urethane) s based on dynamic amine/urea exchange. Polymer 2018, 145, 127–136. [Google Scholar] [CrossRef]
- Li, J.; Feng, Q.; Cui, J.; Yuan, Q.; Qiu, H.; Gao, S.; Yang, J. Self-assembled graphene oxide microcapsules in Pickering emulsions for self-healing waterborne polyurethane coatings. Compos. Sci. Technol. 2017, 151, 282–290. [Google Scholar] [CrossRef]
- Zhao, S.; Abu-Omar, M.M. Recyclable and Malleable Epoxy Thermoset Bearing Aromatic Imine Bonds. Macromolecules 2018, 51, 9816–9824. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Luo, X.; Zheng, N.; Ma, T.; Tan, J.; Li, C.; Zhang, Q.; Gu, J. Self-healing, recoverable epoxy elastomers and their composites with desirable thermal conductivities by incorporating BN fillers via in-situ polymerization. Compos. Sci. Technol. 2018, 164, 59–64. [Google Scholar] [CrossRef]
- Li, Y.; Gao, F.; Xue, Z.; Luan, Y.; Yan, X.; Guo, Z.; Wang, Z. Synergistic effect of different graphene-CNT heterostructures on mechanical and self-healing properties of thermoplastic polyurethane composites. Mater. Des. 2018, 137, 438–445. [Google Scholar] [CrossRef]
- Yang, J.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of isocyanates for self-healing polymers. Macromolecules 2008, 41, 9650–9655. [Google Scholar] [CrossRef]
- Hernández, M.; Grande, A.M.; Dierkes, W.; Bijleveld, J.; Van Der Zwaag, S.; García, S.J. Turning vulcanized natural rubber into a self-healing polymer: Effect of the disulfide/polysulfide ratio. ACS Sustain. Chem. Eng. 2016, 4, 5776–5784. [Google Scholar] [CrossRef]
- Amamoto, Y.; Otsuka, H.; Takahara, A.; Matyjaszewski, K. Self-healing of covalently cross-linked polymers by reshuffling thiuram disulfide moieties in air under visible light. Adv. Mater. 2012, 24, 3975–3980. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, H.; Nagano, S.; Kobashi, Y.; Maeda, T.; Takahara, A. A dynamic covalent polymer driven by disulfide metathesis under photoirradiation. Chem. Commun. 2010, 46, 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Shin, S.R.; Lee, D.S. Sorbitol as a Chain Extender of Polyurethane Prepolymers to Prepare Self-Healable and Robust Polyhydroxyurethane Elastomers. Molecules 2018, 23, 2515. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.Q.; Xie, P.; Rong, M.Z.; Zhang, M.Q. Catalyst-free dynamic exchange of aromatic Schiff base bonds and its application to self-healing and remolding of crosslinked polymers. J. Mater. Chem. A 2015, 3, 19662–19668. [Google Scholar] [CrossRef]
- Kim, C.; Ejima, H.; Yoshie, N. Polymers with autonomous self-healing ability and remarkable reprocessability under ambient humidity conditions. J. Mater. Chem. A 2018, 6, 19643–19652. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, H.; Hart, K.R.; Wu, Y.; Hsu, A.J.; Coppola, A.M.; Kim, T.A.; Yang, K.; Sottos, N.R.; White, S.R.; et al. Malleable and Recyclable Poly(urea-urethane) Thermosets bearing Hindered Urea Bonds. Adv. Mater. 2016, 28, 7646–7651. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Qi, T.; Li, G.L. Towards Dynamic but Supertough Healable Polymers through Biomimetic Hierarchical Hydrogen-Bonding Interactions. Angew. Chem. Int. Ed. 2018, 57, 13838–13842. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, C.; Yu, C.; Fei, G.; Wang, Z.; Xia, H. A Facile Strategy for Self-Healing Polyurethanes Containing Multiple Metal-Ligand Bonds. Macromol. Rapid Commun. 2018, 39, e1700678. [Google Scholar] [CrossRef]
- Xiang, H.; Yin, J.; Lin, G.; Liu, X.; Rong, M.; Zhang, M. Photo-crosslinkable, self-healable and reprocessable rubbers. Chem. Eng. J. 2019, 358, 878–890. [Google Scholar] [CrossRef]
- Nevejans, S.; Ballard, N.; Miranda, J.I.; Reck, B.; Asua, J.M. The underlying mechanisms for self-healing of poly(disulfide)s. PCCP 2016, 18, 27577–27583. [Google Scholar] [CrossRef]
- Wan, T.; Chen, D.J. Synthesis and properties of self-healing waterborne polyurethanes containing disulfide bonds in the main chain. J. Mater. Sci. 2017, 52, 197–207. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Martin, L.; Fernandez-Berridi, M.J.; Irusta, L. Autonomic healable waterborne organic-inorganic polyurethane hybrids based on aromatic disulfide moieties. Express Polym. Lett. 2017, 11, 266–277. [Google Scholar] [CrossRef]
- Fernandes, P.A.; Ramos, M.J. Theoretical insights into the mechanism for thiol/disulfide exchange. Chem. Eur. J. 2004, 10, 257–266. [Google Scholar] [CrossRef]
- Nevejans, S.; Ballard, N.; Rivilla, I.; Fernández, M.; Santamaria, A.; Reck, B.; Asua, J.M. Synthesis of mechanically strong waterborne poly (urethane-urea) s capable of self-healing at elevated temperatures. Eur. Polym. J. 2019, 112, 411–422. [Google Scholar] [CrossRef]
- Nevejans, S.; Ballard, N.; Fernández, M.; Reck, B.; Asua, J.M. Flexible aromatic disulfide monomers for high-performance self-healable linear and cross-linked poly (urethane-urea) coatings. Polymer 2019. [Google Scholar] [CrossRef]
- Lai, Y.; Kuang, X.; Zhu, P.; Huang, M.M.; Dong, X.; Wang, D.J. Colorless, Transparent, Robust, and Fast Scratch-Self-Healing Elastomers via a Phase-Locked Dynamic Bonds Design. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.H.; Chen, L.F.; Rowan, S.J. Trapping Dynamic Disulfide Bonds in the Hard Segments of Thermoplastic Polyurethane Elastomers. Macromol. Chem. Phys. 2017, 218. [Google Scholar] [CrossRef]
- Shi, Y.; Zhan, X.; Luo, Z.; Zhang, Q.; Chen, F. Quantitative IR characterization of urea groups in waterborne polyurethanes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2433–2444. [Google Scholar] [CrossRef]
- Mishra, A.K.; Chattopadhyay, D.; Sreedhar, B.; Raju, K. FT-IR and XPS studies of polyurethane-urea-imide coatings. Prog. Org. Coat. 2006, 55, 231–243. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Sample Code | DSC | DMA | ||
|---|---|---|---|---|
| Tgs (°C) | Tgh (°C) | Tgs (°C) | Tgh (°C) | |
| WPU-EDA | −82.3 | 52.7 | −70.5 | 40.0 |
| WPU-cystamine | −78.6 | 54.5 | −68.1 | 49.3 |
| Sample Code | Tensile Strength (MPa) | Strain (%) | Healing Temperature (°C) | Self-Healing Efficiency (%) | |||
|---|---|---|---|---|---|---|---|
| 1 h | 3 h | 6 h | 12 h | ||||
| WPU-EDA | 26.5 | 516 | 110 | - | - | - | - |
| 130 | - | - | - | - | |||
| WPU-cystamine | 19.1 | 323 | 110 | 26 | 28 | 28 | 21 |
| 130 | 28 | 40 | 30 | 29 | |||
| Sample Code | Composition (by wt) | Hard Segment Contents (%) | ||||
|---|---|---|---|---|---|---|
| DMPA | PTMEG | IPDI | EDA | Cystamine | ||
| WPU-EDA | 6.00 | 57.02 | 32.57 | 4.40 | - | 42.98 |
| WPU-cystamine | 6.00 | 51.83 | 31.41 | - | 10.76 | 48.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-I.; Kim, S.-H.; Lee, D.-S. Synthesis and Characterization of Healable Waterborne Polyurethanes with Cystamine Chain Extenders. Molecules 2019, 24, 1492. https://doi.org/10.3390/molecules24081492
Lee D-I, Kim S-H, Lee D-S. Synthesis and Characterization of Healable Waterborne Polyurethanes with Cystamine Chain Extenders. Molecules. 2019; 24(8):1492. https://doi.org/10.3390/molecules24081492
Chicago/Turabian StyleLee, Dae-Il, Seung-Hyun Kim, and Dai-Soo Lee. 2019. "Synthesis and Characterization of Healable Waterborne Polyurethanes with Cystamine Chain Extenders" Molecules 24, no. 8: 1492. https://doi.org/10.3390/molecules24081492
APA StyleLee, D.-I., Kim, S.-H., & Lee, D.-S. (2019). Synthesis and Characterization of Healable Waterborne Polyurethanes with Cystamine Chain Extenders. Molecules, 24(8), 1492. https://doi.org/10.3390/molecules24081492