Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders
Abstract
1. Introduction
2. Results and Discussion
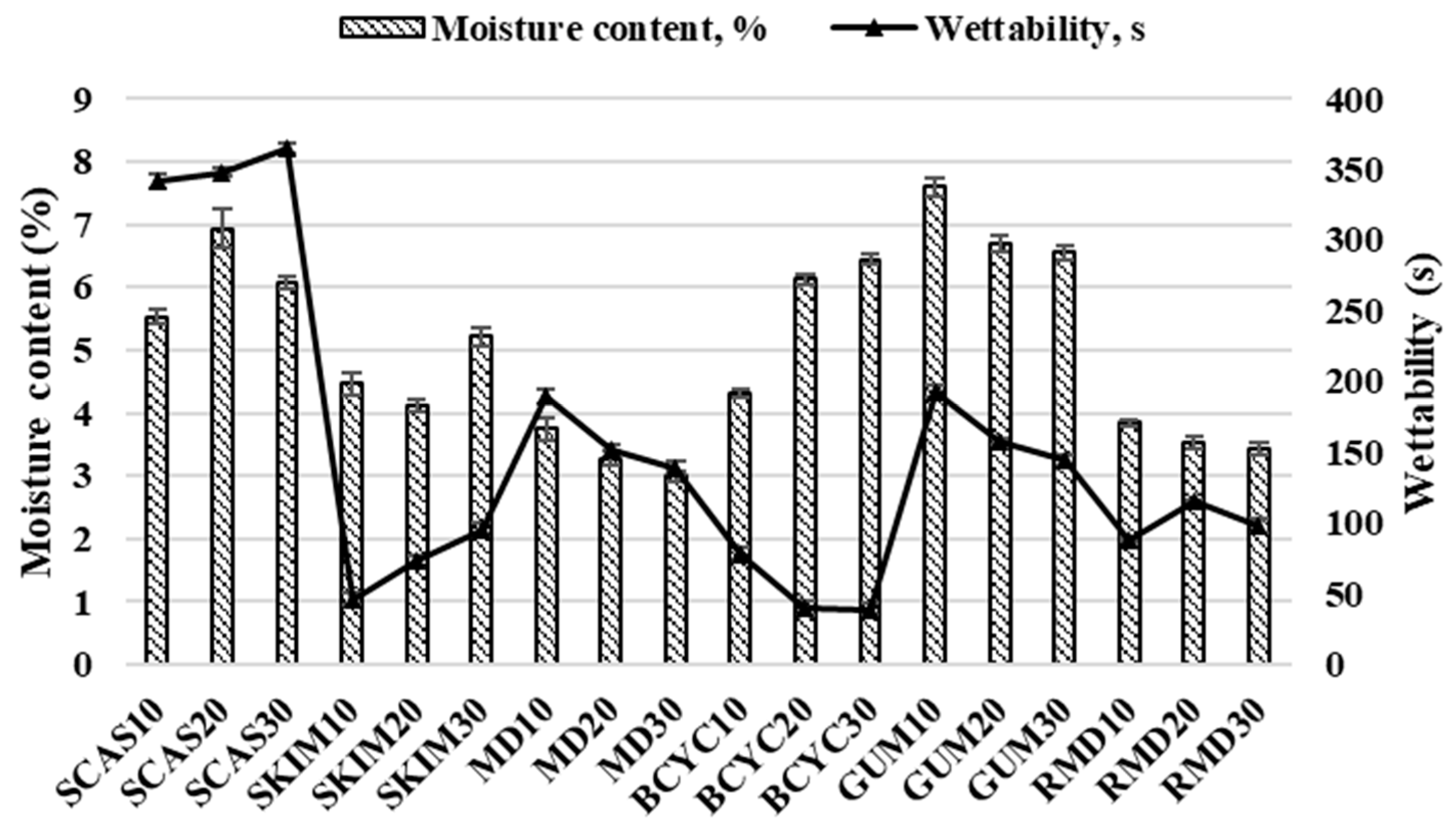
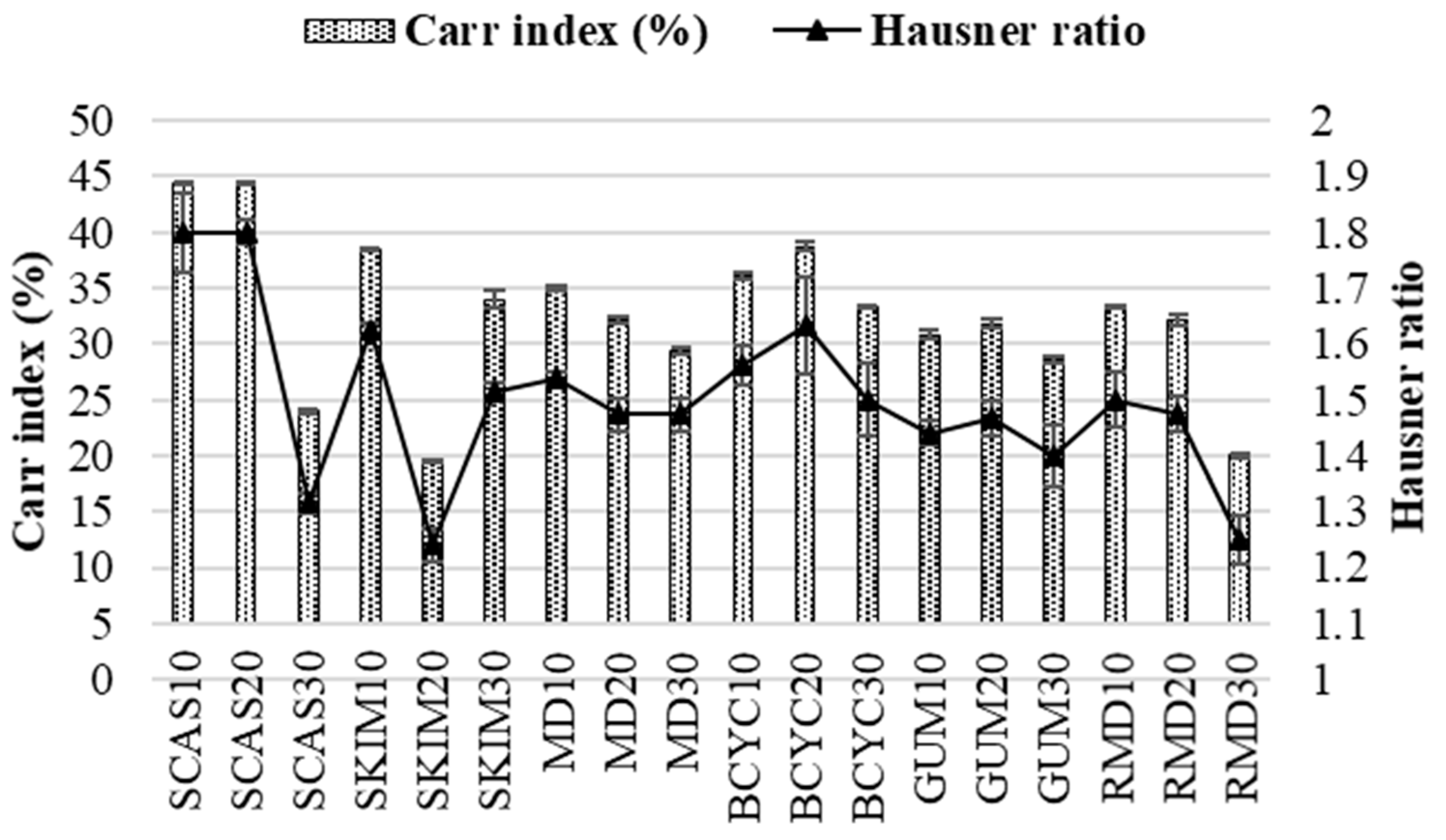
2.1. Influence of Wall Material Components on the Physicochemical Properties
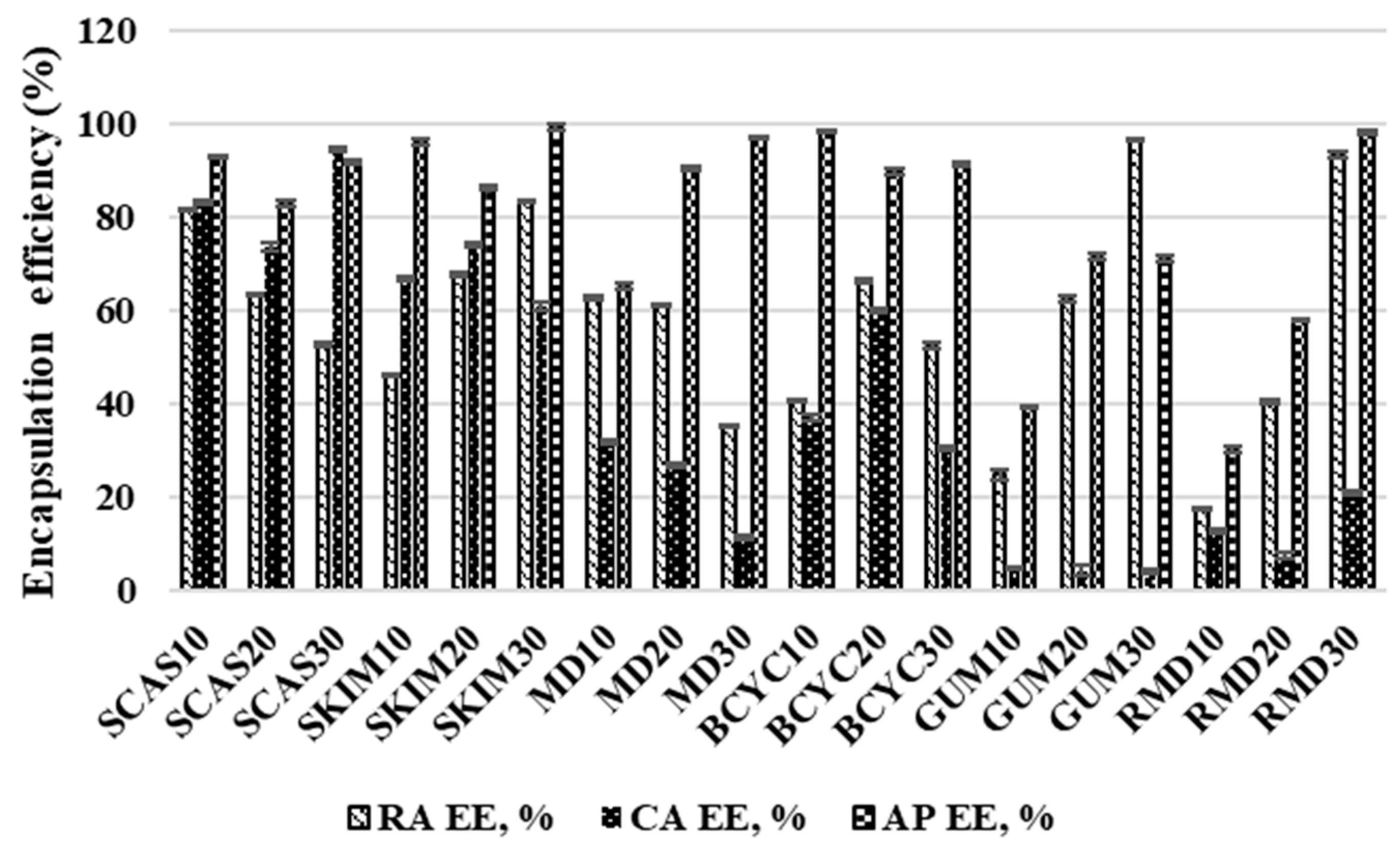
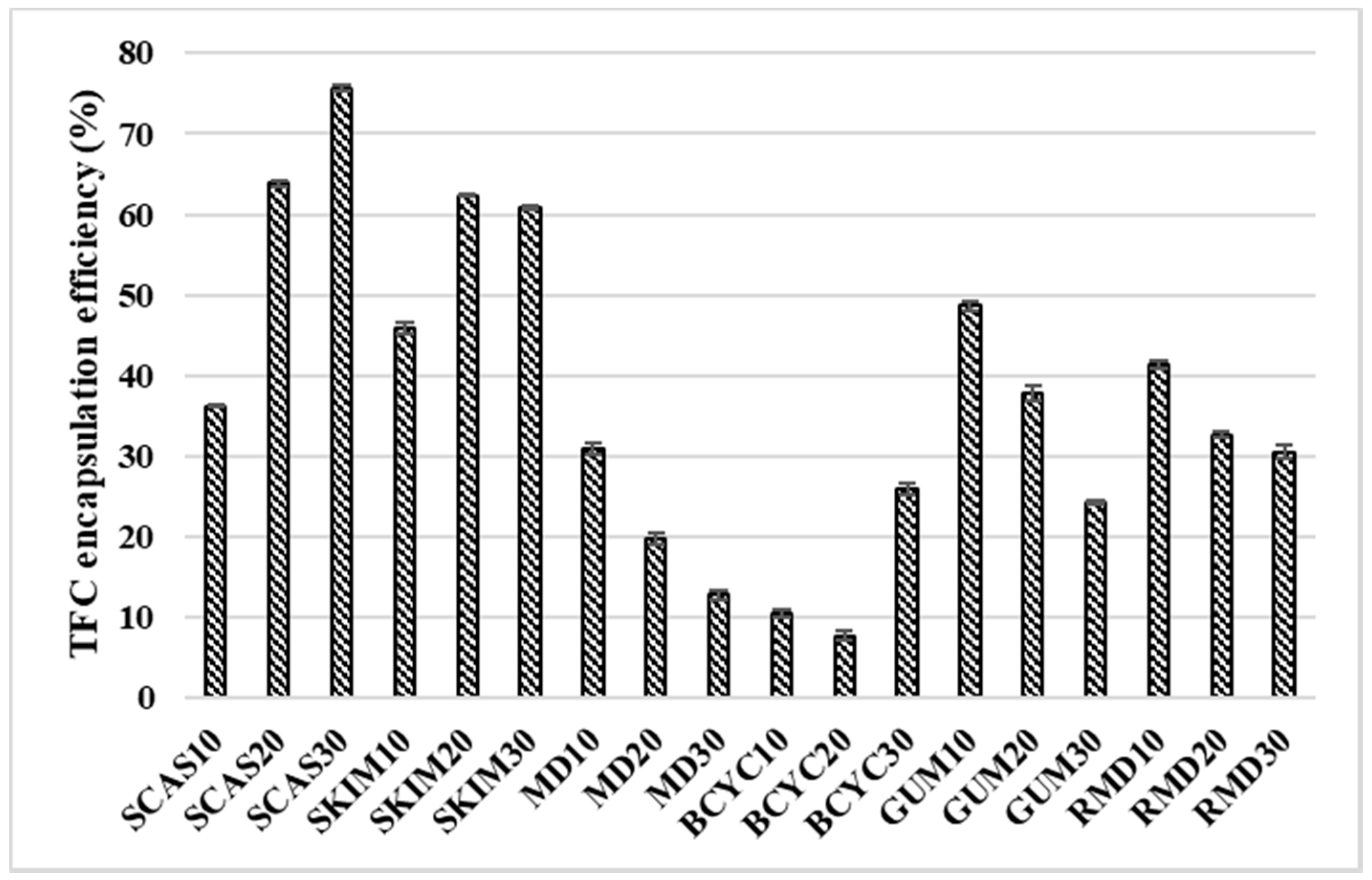
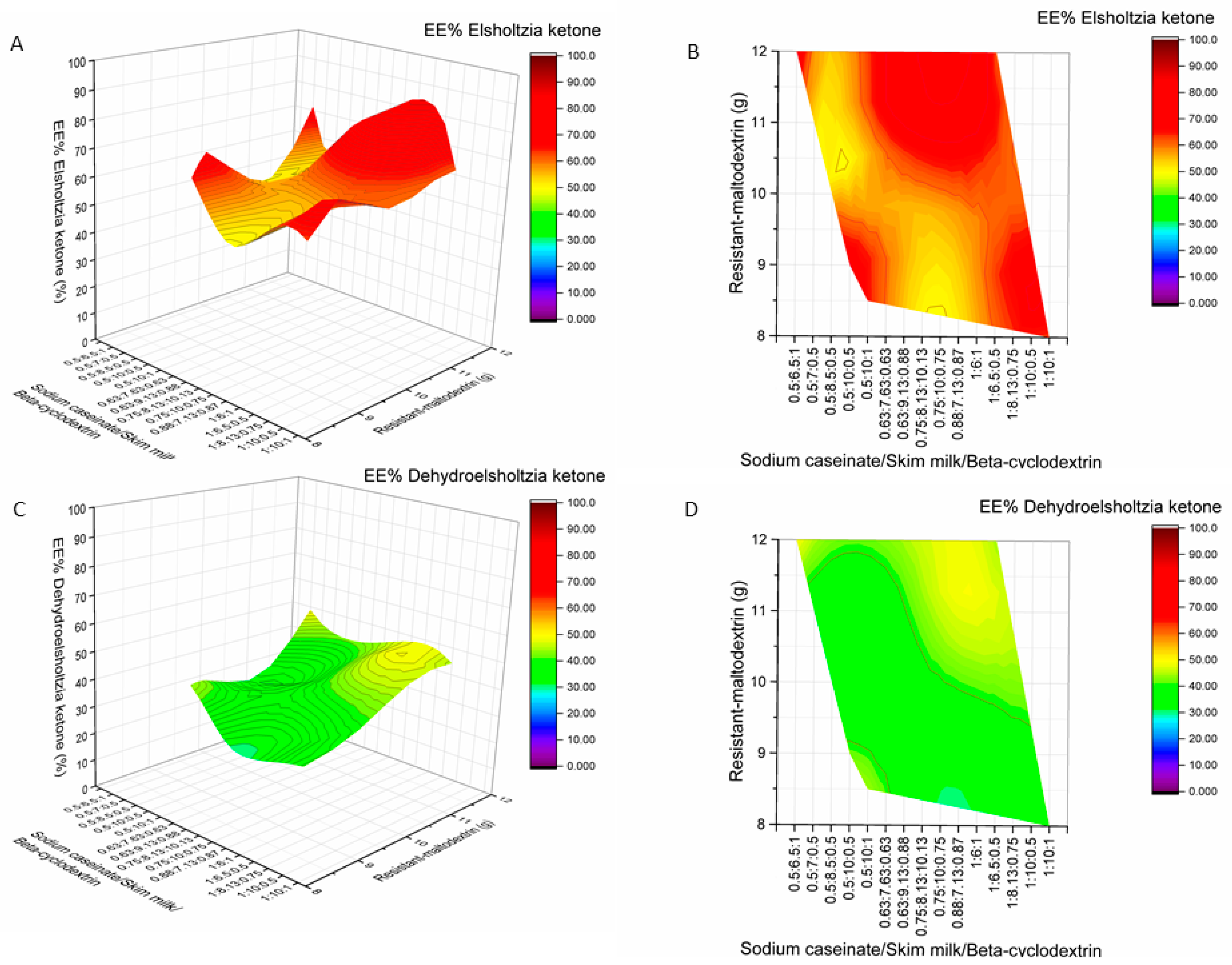
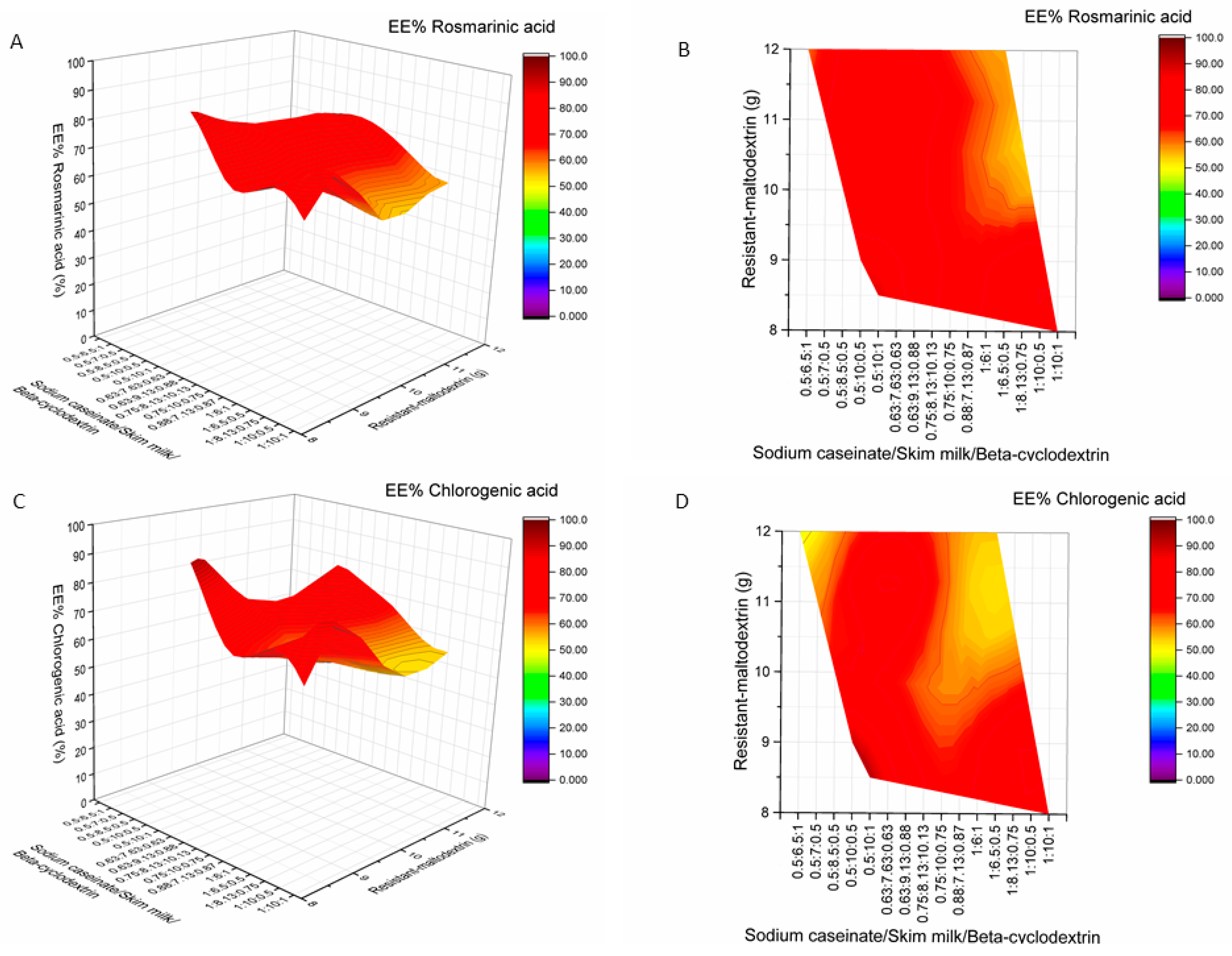
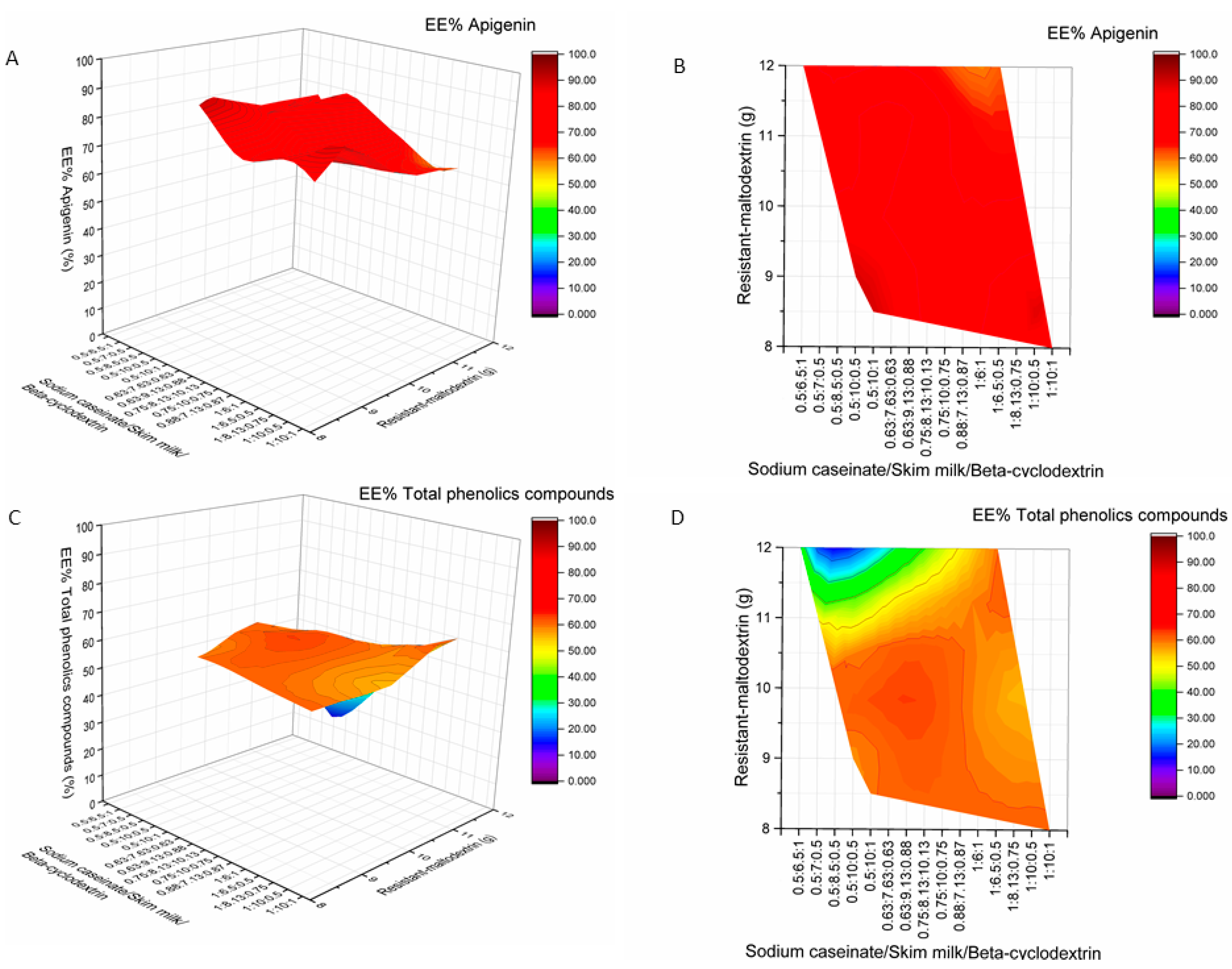
2.2. Influence of Wall Material Components on the Encapsulation Efficiency of Active Compounds
2.3. The Influence of Wall Material Containing Four Encapsulants on the Physicochemical Properties and Encapsulation Efficiency of Active Compounds
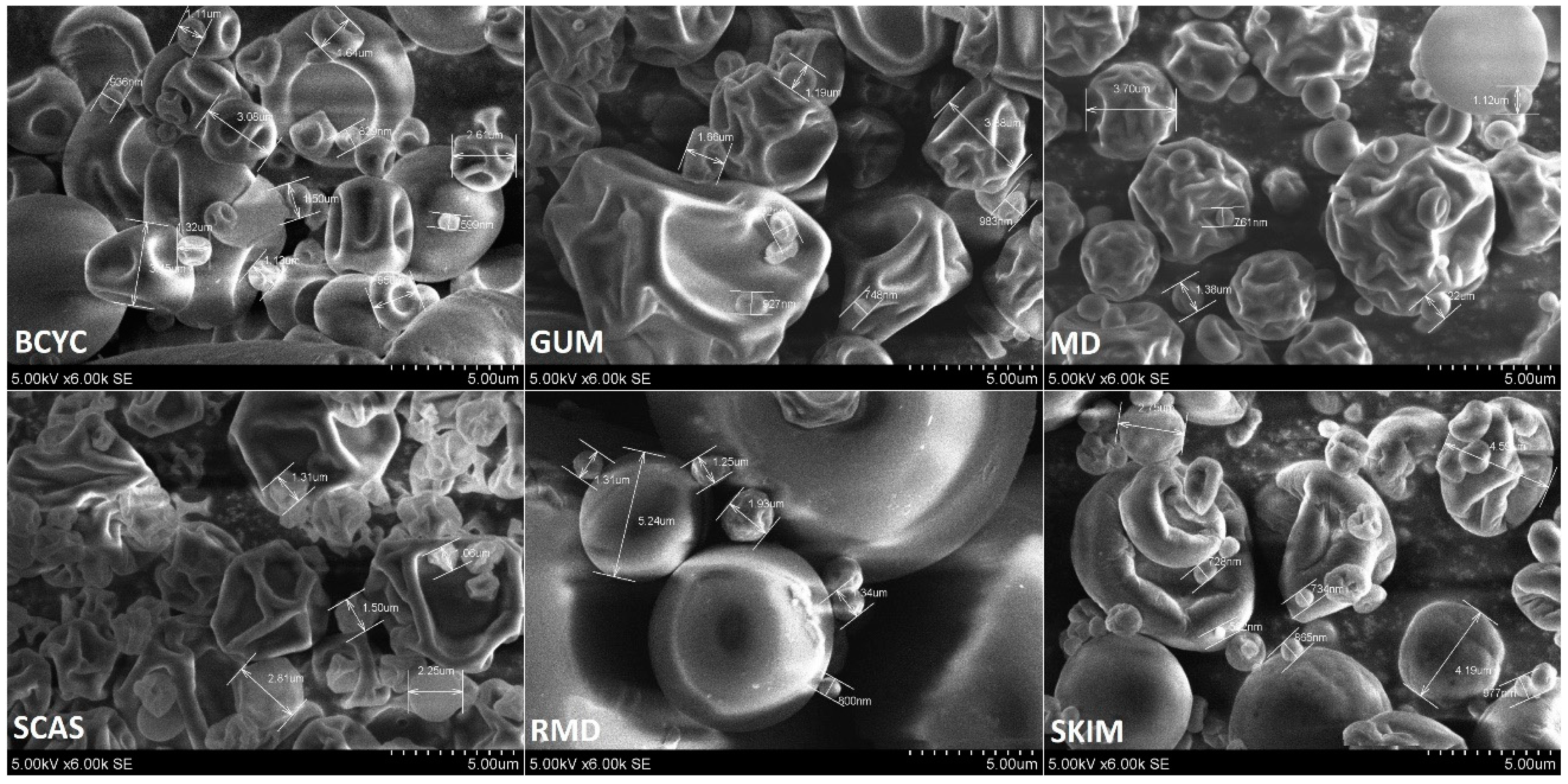
2.4. Scanning Electron Microscopy of Spray-Dried Powders
3. Materials and Methods
3.1. Materials
3.2. Preparation of E. ciliata Ethanolic Extract
3.3. Preparation of E. ciliata Essential Oil
3.4. Liquid Feed Preparation for Spray-Drying
3.5. Spray-Drying Conditions
3.6. Analysis of the Spray-Dried Powder
3.7. Moisture Content
3.8. Wettability
3.9. Solubility
3.10. Bulk and Tapped Volumes
3.11. Spray-Dried Product Yield and Microencapsulation Efficiency
3.12. Powder Preparation for HPLC Analysis
3.13. HPLC Conditions for Determination of Rosmarinic Acid, Chlorogenic Acid, and Apigenin
3.14. GC-FID Conditions for Determination of Elsholtzia Ketone and Dehydroelsholtzia Ketone
3.15. Total Phenolic Content (TPC) and Surface Phenolic Content (SPC) Determination
3.16. Scanning Electron Microscopy
3.17. Statistical Analysis
3.18. Experimental Design
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheng, L.; Linyun, M.; Mingfeng, W.; Ganpeng, L. Comparative Analysis of Chemical Composition of Three Elsholtzia Volatile Oils. Nat. Prod. Ind. J. 2017, 13, 113. [Google Scholar]
- Guo, Z.; Liu, Z.; Wang, X.; Liu, W.; Jiang, R.; Cheng, R.; She, G. Elsholtzia: Phytochemistry and biological activities. Chem. Cent. J. 2012, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Pudziuvelyte, L.; Stankevicius, M.; Maruska, A.; Petrikaite, V.; Ragazinskiene, O.; Draksiene, G.; Bernatoniene, J. Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods. Ind. Crop. Prod. 2017, 107, 90–96. [Google Scholar] [CrossRef]
- Liu, X.; Jia, J.; Yang, L.; Yang, F.; Ge, H.; Zhao, C.; Zhang, L.; Zu, Y. Evaluation of antioxidant activities of aqueous extracts and fractionation of different parts of Elsholtzia ciliate. Molecules 2012, 17, 5430–5441. [Google Scholar] [CrossRef]
- Tresserra-rimbau, A.; Lamuela-raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 1–9. [Google Scholar] [CrossRef]
- Brglez-Mojzer, E.; Knez, H.M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Poomkokrak, J.; Niamnuy, C.; Choicharoen, K.; Devahastin, S. Encapsulation of soybean extract using spray drying. J. Food Sci. Agric. Technol. 2015, 1, 105–110. [Google Scholar]
- Peanparkdee, M.; Iwamoto, S.; Borompichaichartkul, C.; Duangmal, K. Microencapsulation of bioactive compounds from mulberry (Morus alba L.) leaf extracts by protein – polysaccharide interactions. Int. J. Food Sci. Technol. 2016, 51, 649–655. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. Sci. Food Agric. 2014, 94, 825–833. [Google Scholar] [CrossRef]
- Mocanu, M.M.; Nagy, P.; Szöllosi, J. Chemoprevention of breast cancer by dietary polyphenols. Molecules 2015, 20, 22578–22620. [Google Scholar] [CrossRef]
- Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of extraction. Sci. Revs. Chem. Commun. 2015, 5, 1–6. [Google Scholar]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef]
- Dias, D.R.; Botrel, D.A.; Victoria, R.; Fernandes, D.B.; Borges, S.V. Encapsulation as a tool for bioprocessing of functional foods. Curr. Opin. Food Sci. 2017, 13, 31–37. [Google Scholar] [CrossRef]
- Pai, D.A.; Vangala, V.R.; Ng, J.W.; Ng, W.K.; Tan, R.B.H. Resistant maltodextrin as a shell material for encapsulation of naringin: Production and physicochemical characterization. J. Food Eng. 2015, 161, 68–74. [Google Scholar] [CrossRef]
- Wisniewski, R. Spray Drying Technology Review. In Proceedings of the 45th International Conference on Environmental Systems, Bellevue, WA, USA, 12–16 July 2015. [Google Scholar]
- Sun-waterhouse, D.; Wadhwa, S.S.; Waterhouse, G.I.N. Spray-Drying Microencapsulation of Polyphenol Bioactives: A Comparative Study Using Different Natural Fibre Polymers as Encapsulants. Food Bioprocess Technol. 2013, 6, 2376–2388. [Google Scholar] [CrossRef]
- Tan, S.P.; Kha, T.C.; Parks, S.; Stathopoulos, C.; Roach, P.D. Optimising the Encapsulation of an Aqueous Bitter Melon Extract by Spray-Drying. Foods 2015, 4, 400–419. [Google Scholar] [CrossRef]
- Shamaei, S.; Sadegh, S.; Aghbashlo, M.; Tsotsas, E.; Kharaghani, A. Microencapsulation of walnut oil by spray drying: Effects of wall material and drying conditions on physicochemical properties of microcapsules. Innov. Food Sci. Emerg. Technol. 2017, 39, 101–112. [Google Scholar] [CrossRef]
- Eroglu, E.; Tontul, I.; Topuz, A. Optimization of aqueous extraction and spray drying conditions for efficient processing of hibiscus blended rosehip tea powder. J. Food Process. Preserv. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Yogeshini, R.; Naranizam, M.A.; Yus, A.Y.; Kharidah, M. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar]
- Seethadevi, S.J.S.R.I.; Prabha, A.; Muthuprasanna, P. Microencapsulation: A review involved. Int. J. Pharma Biol. Sci. 2012, 3, 509–531. [Google Scholar]
- Le, H.D.; Le, V.V.M. Application of ultrasound to microencapsulation of coconut milk fat by spray drying method. J. Food Sci. Technol. 2015, 52, 2474–2478. [Google Scholar] [CrossRef][Green Version]
- Chawda, P.J.; Shi, J.; Xue, S.; Quek, S.Y. Co-encapsulation of bioactives for food applications. Food Qual. Saf. 2017, 1, 302–309. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D. Effects of spray drying conditions on the physicochemical and antioxidant properties of the Gac (Momordica cochinchinensis) fruit aril powder. J. Food Eng. 2010, 98, 385–392. [Google Scholar] [CrossRef]
- Hoyos-leyva, J.D.; Bello-pérez, L.A.; Hugo, S. Microencapsulation using starch as wall material: A review. Food Rev. Int. 2018, 34, 148–161. [Google Scholar] [CrossRef]
- Di Battista, C.A.; Constenla, D.; Ramirez-Rigo, M.V.; Pina, J. The use of arabic gum, maltodextrin and surfactants in the microencapsulation of phytosterols by spray drying. Powder Technol. 2015, 286, 193–201. [Google Scholar] [CrossRef]
- Vict, R.; Fernandes, B.; Borges, S.V.; Botrel, D.A. Influence of spray drying operating conditions on microencapsulated rosemary essential oil properties. Ciênc. Tecnol. Aliment. 2013, 33, 171–178. [Google Scholar]
- Hundre, S.Y.; Karthik, P.; Anandharamakrishnan, C. Effect of whey protein isolate and b-cyclodextrin wall systems on stability of microencapsulated vanillin by spray – freeze drying method. Food Chem. 2015, 174, 16–24. [Google Scholar] [CrossRef]
- Borghetti, G.S.; Lula, I.S.; Sinisterra, R.D.; Bassani, V.L. Quercetin/beta-cyclodextrin solid complexes prepared in aqueous solution followed by spray-drying or by physical mixture. AAPS Pharm. Sci. Tech. 2009, 10, 235–242. [Google Scholar] [CrossRef]
- Ratchathani, U.; Yai, H. Oxidation stability of sesame oil encapsulated by spray drying. Int. Food Res. J. 2018, 25, 784–792. [Google Scholar]
- Chen, Q.; Zhong, F.; Wen, J.Y.; McGillivray, D.; Quek, S.Y. Properties and Stability of Spray-Dried and Freeze-Dried Microcapsules Co-Encapsulated with Fish Oil, Phytosterol Esters, and Limonene. Dry. Technol. 2013, 31, 707–716. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Jakstas, V.; Ivanauskas, L.; Laukeviciene, A.; Ibe, D.F.C.; Kursvietiene, L.; Bernatoniene, J. Different extraction methods for phenolic and volatile compounds recovery from Elsholtzia ciliata fresh and dried herbal materials. Ind. Crop. Prod. 2018, 120, 286–294. [Google Scholar] [CrossRef]
- Izadi, M.; Eskandari, M.H.; Niakousari, M.; Shekarforoush, S. Optimisation of a pilot-scale spray drying process for probiotic yoghurt, using response surface methodology. Int. J. Dairy Technol. 2014, 67, 211–219. [Google Scholar] [CrossRef]
- Seth, D.; Niwas, H.; Sankar, M.; Deka, C. Effect of spray drying process conditions on bacteria survival and acetaldehyde retention in sweetened yoghurt powder: An optimization study. J. Food Process. Eng. 2017, 40, e12487. [Google Scholar] [CrossRef]
- Ferrari, C.C.; Germer, S.P.M.; de Aguirre, J.M. Effects of Spray-Drying Conditions on the Physicochemical Properties of Blackberry Powder Effects of Spray-Drying Conditions on the Physicochemical Properties of Blackberry Powder. Dry. Technol. 2012, 30, 154–163. [Google Scholar] [CrossRef]
- A-sun, K.; Thumthanaruk, B.; Lekhavat, S.; Jumnongpon, R. Effect of spray drying conditions on physical characteristics of coconut sugar powder. Int. Food Res. J. 2016, 23, 1315–1319. [Google Scholar]
- De, I.; Secado, P. Influence of Maltodextrin and Spray Drying Process Conditions on Sugarcane Juice Powder Quality. Rev. Fac. Nac. Agron. Medellín. 2015, 68, 7509–7520. [Google Scholar]
- Zhou, D.; Zhou, F.; Ma, J.; Ge, F. Microencapsulation of Ganoderma Lucidum spores oil: Evaluation of its fatty acids composition and enhancement of oxidative stability. Ind. Crop. Prod. 2019, 131, 1–7. [Google Scholar] [CrossRef]
- Antonio, A.; Toledo, C.; Silva, E.K.; Cirillo, M.A. Matrix structure selection in the microparticles of essential oil oregano produced by spray dryer. J. Microencapsul. 2013, 30, 717–727. [Google Scholar]
- Gonçalves, B.; Moeenfard, M.; Rocha, F.; Alves, A.; Estevinho, B.N.; Santos, L. Microencapsulation of a Natural Antioxidant from Coffee—Chlorogenic Acid (3-Caffeoylquinic Acid). Food Bioprocess Technol. 2017, 10, 1521–1530. [Google Scholar] [CrossRef]
- Panda, A.; Meena, J.; Katara, R.; Majumdar, D.K. Formulation and characterization of clozapine and risperidone co-entrapped spray-dried PLGA nanoparticles. Pharm. Dev. Technol. 2016, 21, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of grape polyphenols using maltodextrin and gum Arabic as two alternative coating materials: Development and characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Saenz, C.; Tapia, S.; Chavez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
Sample Availability: Samples of the E. ciliata ethanolic extracts are available from the authors. |
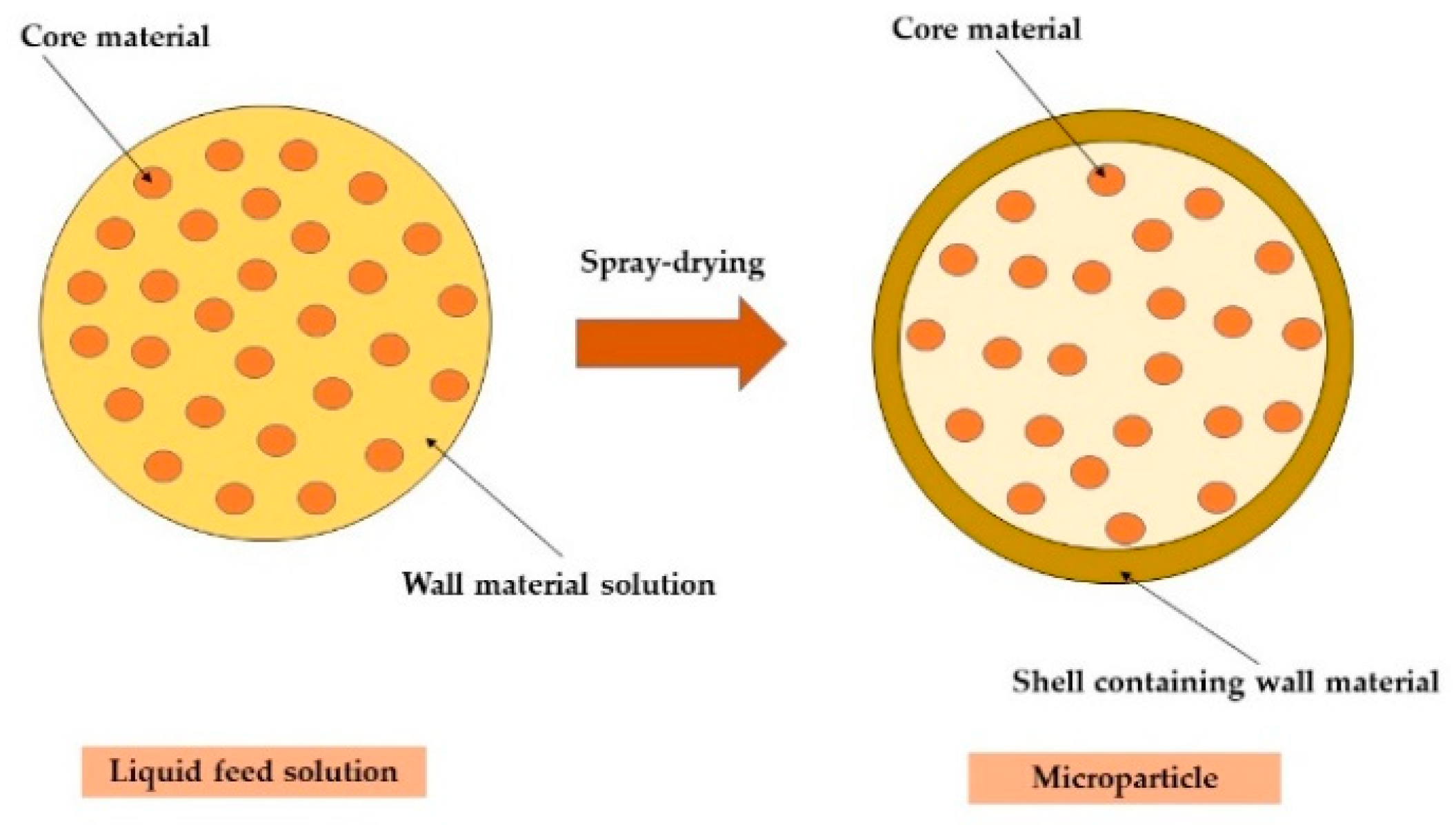




| Code | Wall Material | Low Level (g) | High Level (g) |
|---|---|---|---|
| A | Sodium caseinate | 0.5 | 1 |
| B | Skim milk | 6 | 10 |
| C | Resistant maltodextrin | 8 | 12 |
| D | Beta-cyclodextrin | 0.5 | 1 |
| Response (%) | Final Equation | p Value | R2 | R2Adjusted |
|---|---|---|---|---|
| RA EE | = −10.00 A + 91.41 B + 64.50 C − 31.00 D | 0.002 | 0.91 | 0.81 |
| CA EE | = +10.41 A + 93.00 B + 55.29 C − 35.88 D | 0.003 | 0.86 | 0.74 |
| AP EE | = −5.89 A + 96.38 B + 67.51 C + 34.64 D | 0.001 | 0.92 | 0.86 |
| EK EE | = +17.89 A + 81.19 B + 50.49 C − 6.43 D | 0.001 | 0.93 | 0.85 |
| DK EE | = +52.44 A + 31.74 B + 42.73 C + 94.35 D | 0.015 | 0.87 | 0.74 |
| TPC EE | = −55.73 A + 22.37 B − 65.52 C + 24.38 D | 0.002 | 0.97 | 0.9 |
| Run | ID | Yield (%) | Moisture Content (%) | Solubility (%) | Wettability (s) | Carr Index (%) | Hausner Ratio |
|---|---|---|---|---|---|---|---|
| 1 | 01M | 66.97 ± 0.98 | 5.46 ± 0.21 | 99.8 ± 0.03 | 86 ± 0.03 | 25.45 ± 0.04 | 1.341 ± 0.04 |
| 2 | 02M | 59.17 ± 0.23 | 5.01 ± 0.09 | 97.5 ± 0.05 | 77 ± 0.05 | 28.36 ± 0.06 | 1.396 ± 0.06 |
| 3 | 03M | 61.04 ± 0.26 | 5.27 ± 0.16 | 99.8 ± 0.02 | 80 ± 0.06 | 30.91 ± 0.08 | 1.447 ± 0.08 |
| 4 | 04M | 44.19 ± 0.33 | 5.01 ± 0.11 | 97.5 ± 0.04 | 73 ± 0.05 | 34.29 ± 0.06 | 1.522 ± 0.06 |
| 5 | 05M | 56.86 ± 0.15 | 5.41 ± 0.07 | 85 ± 0.06 | 72 ± 0.04 | 24.44 ± 0.07 | 1.324 ± 0.07 |
| 6 | 06M | 54.32 ± 0.43 | 4.88 ± 0.05 | 95 ± 0.08 | 90 ± 0.08 | 26.19 ± 0.03 | 1.355 ± 0.03 |
| 7 | 07M | 66.25 ± 0.11 | 4.09 ± 0.04 | 90 ± 0.07 | 70 ± 0.09 | 26.09 ± 0.05 | 1.353 ± 0.05 |
| 8 | 08M | 58.76 ± 0.09 | 4.58 ± 0.07 | 77.5 ± 0.09 | 72 ± 0.03 | 31.82 ± 0.07 | 1.467 ± 0.07 |
| 9 | 09M | 47.09 ± 0.12 | 4.95 ± 0.06 | 99.25 ± 0.02 | 85 ± 0.04 | 37.14 ± 0.03 | 1.591 ± 0.03 |
| 10 | 10M | 47.81 ± 0.36 | 3.72 ± 0.08 | 92.5 ± 0.04 | 105 ± 0.09 | 35.48 ± 0.02 | 1.55 ± 0.02 |
| 11 | 11M | 56.04 ± 0.22 | 4.68 ± 0.04 | 95 ± 0.06 | 93 ± 0.07 | 28.57 ± 0.03 | 1.4 ± 0.03 |
| 12 | 12M | 46.93 ± 0.17 | 4.54 ± 0.03 | 99.8 ± 0.01 | 66 ± 0.12 | 25.71 ± 0.06 | 1.346 ± 0.06 |
| 13 | 13M | 46.92 ± 0.15 | 4.53 ± 0.05 | 99.7 ± 0.03 | 66 ± 0.14 | 25.70 ± 0.04 | 1.347 ± 0.09 |
| 14 | 14M | 45.22 ± 0.17 | 4.55 ± 0.23 | 97.5 ± 0.09 | 62 ± 0.35 | 25.23 ± 0.06 | 1.342 ± 0.06 |
| 15 | 15M | 47.82 ± 0.23 | 3.71 ± 0.05 | 92.5 ± 0.06 | 105 ± 0.32 | 35.47 ± 0.07 | 1.55 ± 0.06 |
| 16 | 16M | 44.20 ± 0.13 | 5.00 ± 0.07 | 97.5 ± 0.05 | 73 ± 0.12 | 34.28 ± 0.09 | 1.522 ± 0.07 |
| 17 | 17M | 46.92 ± 0.41 | 5.92 ± 0.08 | 82.5 ± 0.03 | 76 ± 0.06 | 27.45 ± 0.04 | 1.383 ± 0.04 |
| 18 | 18M | 59.45 ± 0.09 | 4.77 ± 0.06 | 87.5 ± 0.05 | 97 ± 0.05 | 28.89 ± 0.06 | 1.406 ± 0.06 |
| 19 | 19M | 59.7 ± 0.08 | 4.73 ± 0.04 | 88.75 ± 0.06 | 83 ± 0.04 | 31.82 ± 0.07 | 1.468 ± 0.07 |
| 20 | 20M | 52.27 ± 0.24 | 5.32 ± 0.08 | 88.75 ± 0.08 | 68 ± 0.11 | 28.21 ± 0.02 | 1.393 ± 0.02 |
| Independent Variables | |||
| Amount Limits (g) | Predicted Optimized Amount (g) | ||
| Sodium caseinate (A) | 0.5–1 | 0.54 | |
| Skim milk (B) | 6–10 | 10 | |
| Resistant-maltodextrin (C) | 8–12 | 8.96 | |
| Beta-cyclodextrin (D) | 0.5–1 | 0.5 | |
| Response Variables | |||
| Encapsulation Efficiency | Criteria | Predicted Mean Value (%) | Obtained Mean Value (%) |
| Rosmarinic acid (RA) | Maximize | 85.37 | 85.27 |
| Chlorogenic acid (CA) | Maximize | 85.06 | 85.36 |
| Apigenin (AP) | Maximize | 89.96 | 90.51 |
| Elsholtzia ketone (EK) | Maximize | 66.44 | 67.38 |
| Dehydroelsholtzia ketone (DK) | Maximize | 34.02 | 35.12 |
| Total phenolics content (TPC) | Maximize | 62.05 | 62.78 |
| Run | ID | Sodium Caseinate | Skim Milk | Resistant Maltodextrin | Beta-Cyclodextrin |
|---|---|---|---|---|---|
| 1 | 01M | 0.50 | 7.00 | 12.00 | 0.50 |
| 2 | 02M | 0.50 | 6.50 | 12.00 | 1.00 |
| 3 | 03M | 1.00 | 6.50 | 12.00 | 0.50 |
| 4 | 04M | 1.00 | 8.13 | 10.13 | 0.75 |
| 5 | 05M | 1.00 | 6.00 | 12.00 | 1.00 |
| 6 | 06M | 0.88 | 7.13 | 11.13 | 0.87 |
| 7 | 07M | 0.63 | 9.13 | 9.38 | 0.88 |
| 8 | 08M | 0.50 | 8.50 | 10.50 | 0.50 |
| 9 | 09M | 0.75 | 10.00 | 8.50 | 0.75 |
| 10 | 10M | 1.00 | 10.00 | 8.00 | 1.00 |
| 11 | 11M | 0.50 | 10.00 | 8.50 | 1.00 |
| 12 | 12M | 1.00 | 10.00 | 8.50 | 0.50 |
| 13 | 13M | 1.00 | 10.00 | 8.50 | 0.50 |
| 14 | 14M | 0.63 | 7.63 | 11.13 | 0.63 |
| 15 | 15M | 1.00 | 10.00 | 8.00 | 1.00 |
| 16 | 16M | 1.00 | 8.13 | 10.13 | 0.75 |
| 17 | 17M | 0.75 | 8.13 | 10.13 | 1.00 |
| 18 | 18M | 1.00 | 6.50 | 12.00 | 0.50 |
| 19 | 19M | 0.50 | 6.50 | 12.00 | 1.00 |
| 20 | 20M | 0.50 | 10.00 | 9.00 | 0.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders. Molecules 2019, 24, 1461. https://doi.org/10.3390/molecules24081461
Pudziuvelyte L, Marksa M, Jakstas V, Ivanauskas L, Kopustinskiene DM, Bernatoniene J. Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders. Molecules. 2019; 24(8):1461. https://doi.org/10.3390/molecules24081461
Chicago/Turabian StylePudziuvelyte, Lauryna, Mindaugas Marksa, Valdas Jakstas, Liudas Ivanauskas, Dalia M. Kopustinskiene, and Jurga Bernatoniene. 2019. "Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders" Molecules 24, no. 8: 1461. https://doi.org/10.3390/molecules24081461
APA StylePudziuvelyte, L., Marksa, M., Jakstas, V., Ivanauskas, L., Kopustinskiene, D. M., & Bernatoniene, J. (2019). Microencapsulation of Elsholtzia ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders. Molecules, 24(8), 1461. https://doi.org/10.3390/molecules24081461









