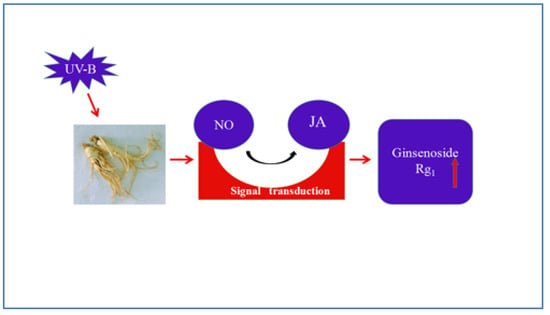
Postharvest UV-B Irradiation Stimulated Ginsenoside Rg1 Biosynthesis through Nitric Oxide (NO) and Jasmonic Acid (JA) in Panax quinquefolius Roots
Abstract
:1. Introduction
2. Results and Discussion
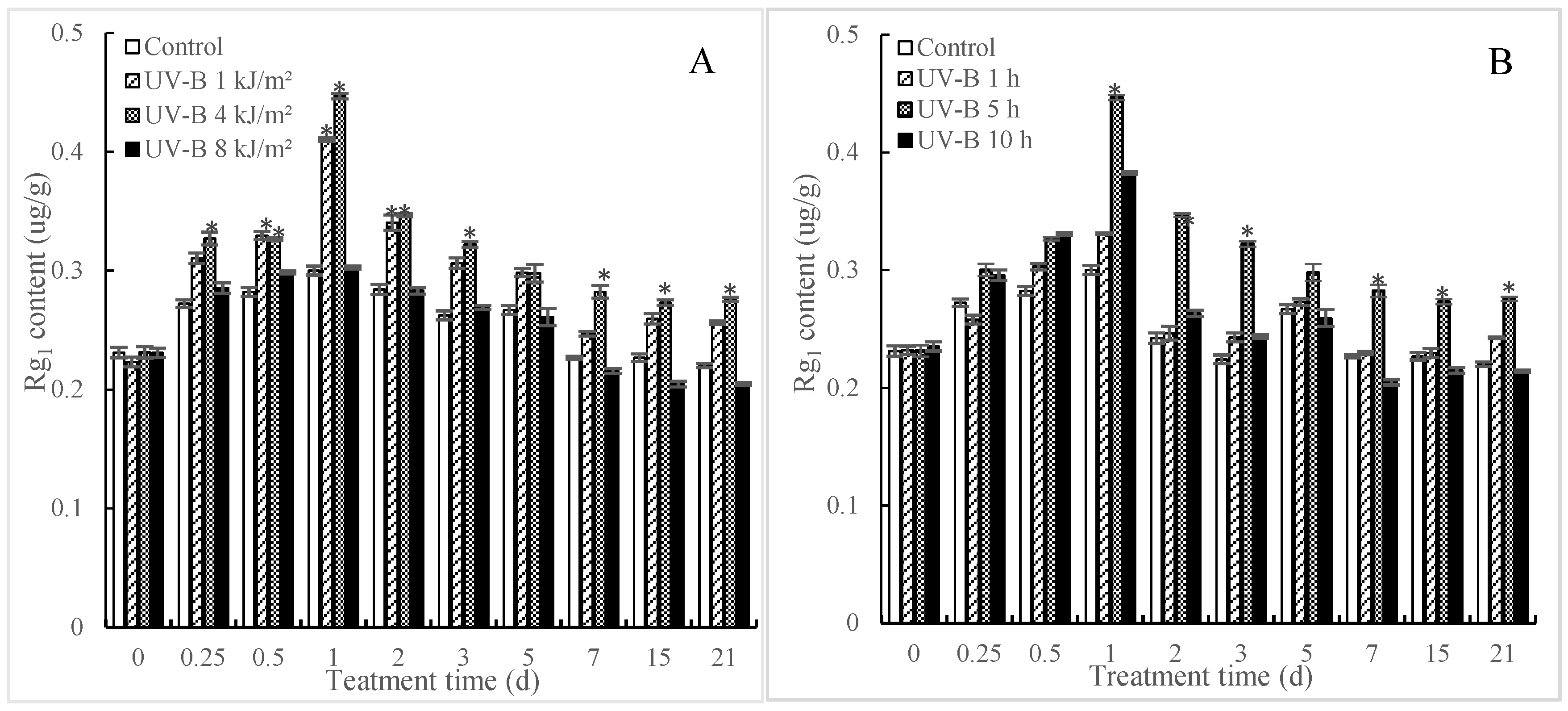
2.1. Effects of UV-B Irradiation on Accumulation of Rg1
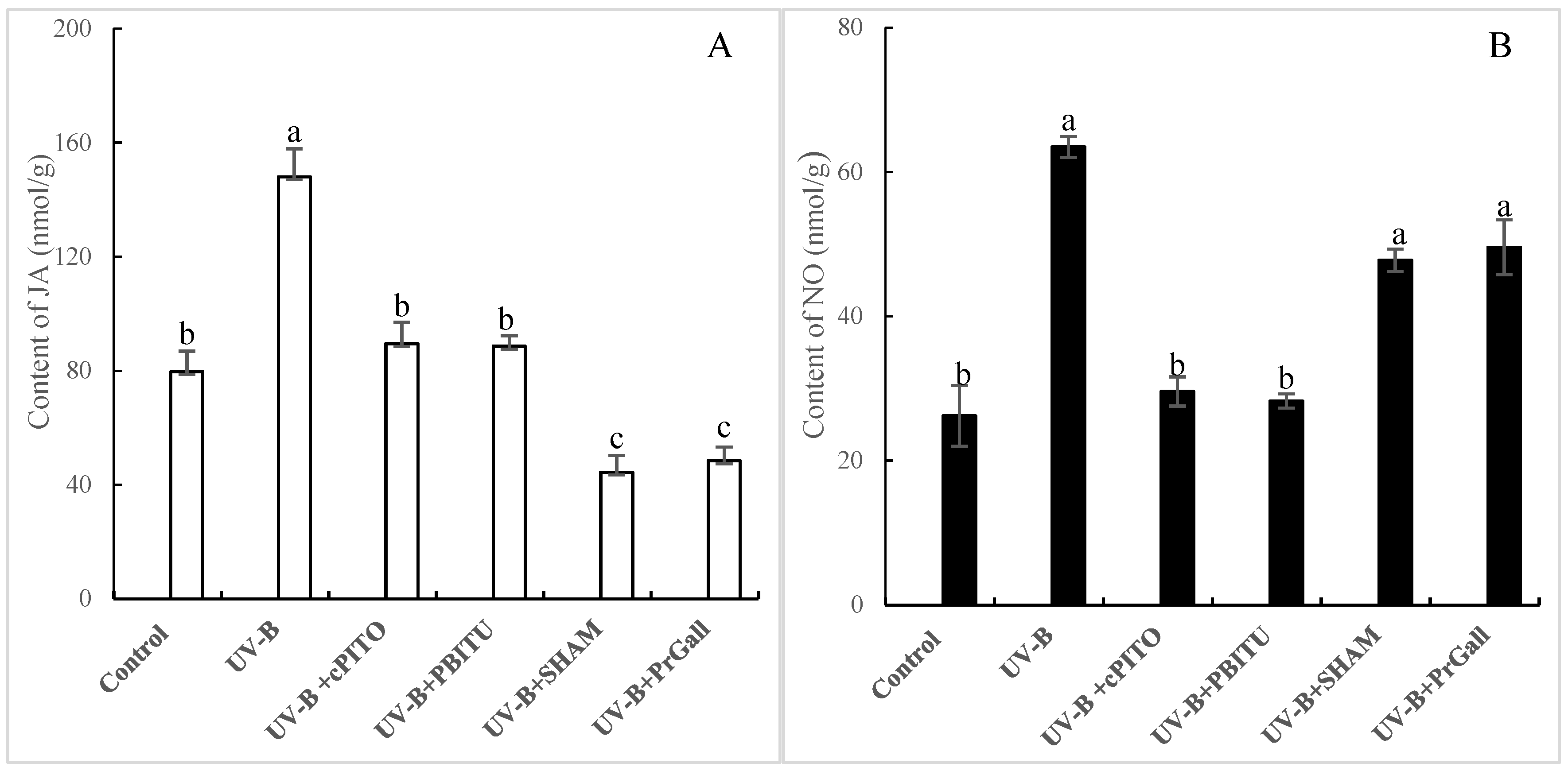
2.2. Effects of UV-B Irradiation on NO Generation
2.3. Effects of UV-B Irradiation on JA Generation
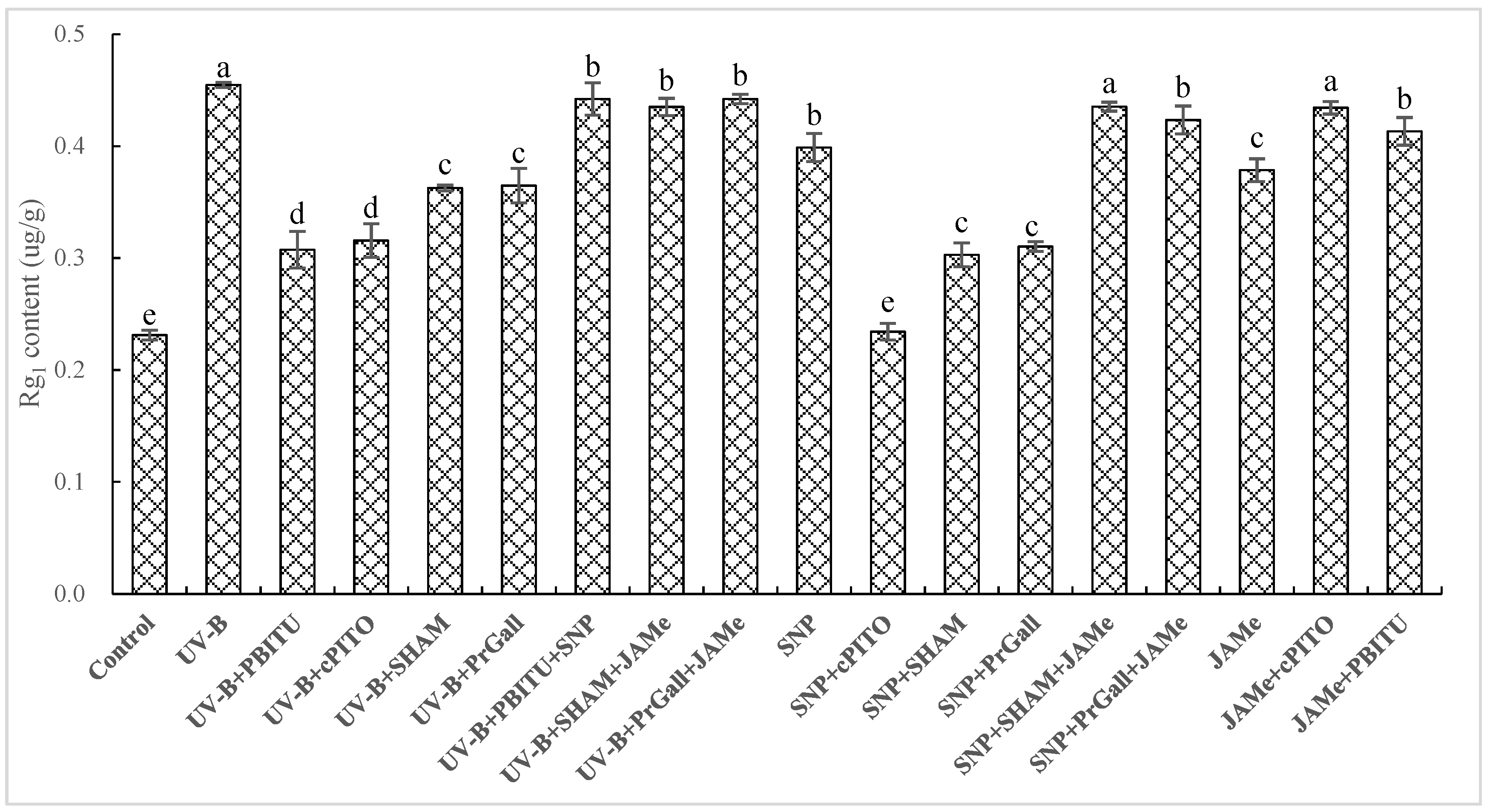
2.4. Dependence of UV-B-Induced Increase of Rg1 on NO and JA
2.5. Dependence of UV-B-Induced JA Biosynthesis on NO
2.6. Dependence of NO-Induced Rg1 Production on JA
3. Materials and Methods
3.1. Plant Materials and Experimental Design
3.2. Measurement of JA
3.3. Measurement of NO
3.4. HPLC Analysis of Rg1
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- China Pharmacopoeia Committee. Pharmacopoeia of Peoples Republic of China; China Medical Science and Technology Press: Beijing, China, 2015; p. 76. [Google Scholar]
- Wang, Z.H.; Huang, L.F. Panax quinquefolius: An overview of the contaminants. Phytochem. Lett. 2015, 11, 89–94. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, W.B.; Li, X.Y.; Piao, X.C.; Jiang, J.; Lian, M.L. Pathogenic fungal elicitors enhance ginsenoside biosynthesis of adventitious roots in Panax quinquefolius, during bioreactor culture. Ind. Crops Prod. 2016, 94, 729–735. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.X.; Li, J.L.; Liu, S.J.; Wu, X.L.; Li, J.; Gao, W.Y. Transcriptome profiling shows gene regulation patterns in ginsenoside pathway in response to methyl jasmonate in Panax Quinquefolium adventitious root. Sci. Rep. 2016, 6, 37263. [Google Scholar] [PubMed]
- Britta, H.P.; Kalpana, P.; Karin, S. Influence of postharvest UV-B treatment and fermentation on secondary plant compounds in white cabbage leaves. Food Chem. 2016, 197, 47–56. [Google Scholar]
- And, L.F.R.; Cisneros-Zevallos, L. Wounding stress increases the phenolic content and antioxidant capacity of purple-flesh potatoes (Solanum tuberosum L.). J. Agric. Food Chem. 2003, 51, 5296–5300. [Google Scholar]
- Alegria, C. Heat Shock and UV-C Abiotic Stress Treatments as Alternative Tools to Promote Fresh-Cut Carrot Quality and Shelf-Life. Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 2015. [Google Scholar]
- Darré, M.; Valerga, L.; Ortiz Araque, L.C.; Lemoine, M.L.; Demkura, P.V.; Vicente, A.R.; Concellón, A. Role of UV-B irradiation dose and intensity on color retention and antioxidant elicitation in broccoli florets (Brassica oleracea var. Italica). Postharvest Biol. Technol. 2017, 128, 76–82. [Google Scholar]
- Formica-Oliveira, A.C.; Martínez-Hernández, G.B.; Díaz-López, V.; Artés, F.; Artés-Hernández, F. Effects of UV-B and UV-C combination on phenolic compounds biosynthesis in fresh-cut carrots. Postharvest Biol. Technol. 2017, 127, 99–104. [Google Scholar] [CrossRef]
- Scattino, C.; Castagna, A.; Neugart, S.; Chan, H.M.; Schreiner, M.; Crisosto, C.H.; Tonutti, P.; Ranieri, A. Post-harvest UV-B irradiation induces changes of phenol contents and corresponding biosynthetic gene expression in peaches and nectarines. Food Chem. 2014, 163, 51–60. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, J.F.; Jin, H.H.; Sun, L.N.; Xu, M.J. Ultraviolet-B-induced flavonoid accumulation in Betula pendula leaves is dependent upon nitrate reductase-mediated nitric oxide signaling. Tree Physiol. 2011, 31, 798. [Google Scholar] [CrossRef]
- Jiao, C.F.; Yang, R.Q.; Zhou, Y.L.; Gu, Z.X. Nitric oxide mediates isoflavone accumulation and the antioxidant system enhancement in soybean sprouts. Food Chem. 2016, 204, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Dillon, F.M.; Chludil, H.D.; Reichelt, M.; Mithöfer, A.; Pagano, E.A.; Zavala, J.A. Solar UV-B radiation and ethylene play a key role in modulating effective defenses against Anticarsia gemmatalis larvae in field-grown soybean. Plant Cell Environ. 2018, 41, 383–394. [Google Scholar] [CrossRef]
- Wei, Z.F.; Luo, M.; Zhao, C.J.; Li, C.Y.; Gu, C.B.; Wang, W.; Zu, Y.G.; Efferth, T.; Fu, Y.J. UV-induced changes of active components and antioxidant activity in postharvest pigeon pea [Cajanus cajan (L.) millsp.] leaves. J. Agric. Food Chem. 2013, 61, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.; Krumbein, A.; Mewis, I.; Ulrichs, C.; Huyskens-Keil, S. Short-term and moderate UV-B radiation effects on secondary plant metabolism in different organs of nasturtium (Tropaeolum majus L.). Innov. Food Sci. Emerg. Technol. 2009, 10, 93–96. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenneret, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Radyukina, N.L.; Shashukova, A.V.; Makarova, S.S.; Kuznetsov, V.V. Exogenous proline modifies differential expression of superoxide dismutase genes in UV-B-irradiated Salvia officinalis plants. Russ. J. Plant Physiol. 2011, 58, 51–59. [Google Scholar] [CrossRef]
- Hu, X.Y.; Neill, S.J.; Cai, W.M.; Tang, Z.C. Nitric oxide mediates elicitor-induced saponin synthesis in cell cultures of Panax ginseng. Funct. Plant Physiol. 2003, 30, 901–907. [Google Scholar] [CrossRef]
- Yu, K.W.; Gao, W.Y.; Son, S.H.; Paek, K.Y. Improvement of ginsenoside production by jasmonic acid and some other elicitors in hairy root culture of ginseng (Panax ginseng C. A. Meyer). In Vitro Cell. Dev. Biol. Plant 2000, 36, 424–428. [Google Scholar] [CrossRef]
- Xu, X. Fungal elicitor induces singlet oxygen generation, ethylene release and saponin synthesis in cultured cells of Panax ginseng C. A. Meyer. Plant Cell Physiol. 2005, 46, 947–954. [Google Scholar] [CrossRef]
- Yendo, A.C.A.; Costa, F.D.; Costa, C.T.D.; Colling, L.C.; Gosmann, G.; Fett-Neto, A.G. Biosynthesis of plant triterpenoid saponins: Genes, enzymes and their regulation. Mini-Rev. Org. Chem. 2014, 11, 292. [Google Scholar] [CrossRef]
- Ramos, P.; Rivas, N.; Pollmann, S.; Casati, P.; Molina-Montenegro, M. Hormonal and physiological changes driven by fungal endophytes increase antarctic plant performance under UV-B radiation. Fungal Ecol. 2018, 34, 76–82. [Google Scholar] [CrossRef]
- Menke, F.L.H.; Parchmann, S.; Mueller, M.J.; Kijne, J.W.; Memelink, J. Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in Catharanthus roseus. Plant Physiol. 1999, 119, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Han, X.X.; Cai, L.Y.; Lu, X.Y.; Ying, T.J.; Jiang, Z.H. Postharvest UV-B irradiation maintains sensory qualities and enhances antioxidant capacity in tomato fruit during storage. Postharvest Biol. Technol. 2011, 59, 232–237. [Google Scholar] [CrossRef]
- Zhou, J.; Jia, F.F.; Shao, S.J.; Zhang, H.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q.; Shi, K. Involvement of nitric oxide in the jasmonate-dependent basal defense against root-knot nematode in tomato plants. Front. Plant Sci. 2015, 6, 193. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, X.; Zhu, S.; Wang, Y. Postharvest application of MeJA and NO reduced chilling injury in cucumber (cucumis sativus) through inhibition of H2O2 accumulation. Postharvest Biol. Technol. 2016, 119, 77–83. [Google Scholar] [CrossRef]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Zhou, J.; Ran, Z.F.; Xu, Z.X.; Liu, Q.; Huang, M.; Fang, L.; Guo, L.P. Effects of smoke-water and smoke-isolated karrikinolide on tanshinones production in salvia miltiorrhiza hairy roots. S. Afr. J. Bot. 2018, 119, 265–270. [Google Scholar] [CrossRef]
- Li, J.X.; Liu, S.J.; Wang, J.; Li, J.; Liu, D.H.; Li, J.; Gao, W.Y. Fungal elicitors enhance ginsenosides biosynthesis, expression of functional genes as well as signal molecules accumulation in adventitious roots of Panax ginseng C. A. Mey. J. Biotechnol. 2016, 239, 106–114. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Ran, Z.-f.; Yang, X.-t.; Li, J. Postharvest UV-B Irradiation Stimulated Ginsenoside Rg1 Biosynthesis through Nitric Oxide (NO) and Jasmonic Acid (JA) in Panax quinquefolius Roots. Molecules 2019, 24, 1462. https://doi.org/10.3390/molecules24081462
Zhou J, Ran Z-f, Yang X-t, Li J. Postharvest UV-B Irradiation Stimulated Ginsenoside Rg1 Biosynthesis through Nitric Oxide (NO) and Jasmonic Acid (JA) in Panax quinquefolius Roots. Molecules. 2019; 24(8):1462. https://doi.org/10.3390/molecules24081462
Chicago/Turabian StyleZhou, Jie, Zhi-fang Ran, Xiao-tong Yang, and Jia Li. 2019. "Postharvest UV-B Irradiation Stimulated Ginsenoside Rg1 Biosynthesis through Nitric Oxide (NO) and Jasmonic Acid (JA) in Panax quinquefolius Roots" Molecules 24, no. 8: 1462. https://doi.org/10.3390/molecules24081462
APA StyleZhou, J., Ran, Z.-f., Yang, X.-t., & Li, J. (2019). Postharvest UV-B Irradiation Stimulated Ginsenoside Rg1 Biosynthesis through Nitric Oxide (NO) and Jasmonic Acid (JA) in Panax quinquefolius Roots. Molecules, 24(8), 1462. https://doi.org/10.3390/molecules24081462