Blackcurrant Extract with Phytoestrogen Activity Alleviates Hair Loss in Ovariectomized Rats
Abstract
:1. Introduction
2. Results and Discussion
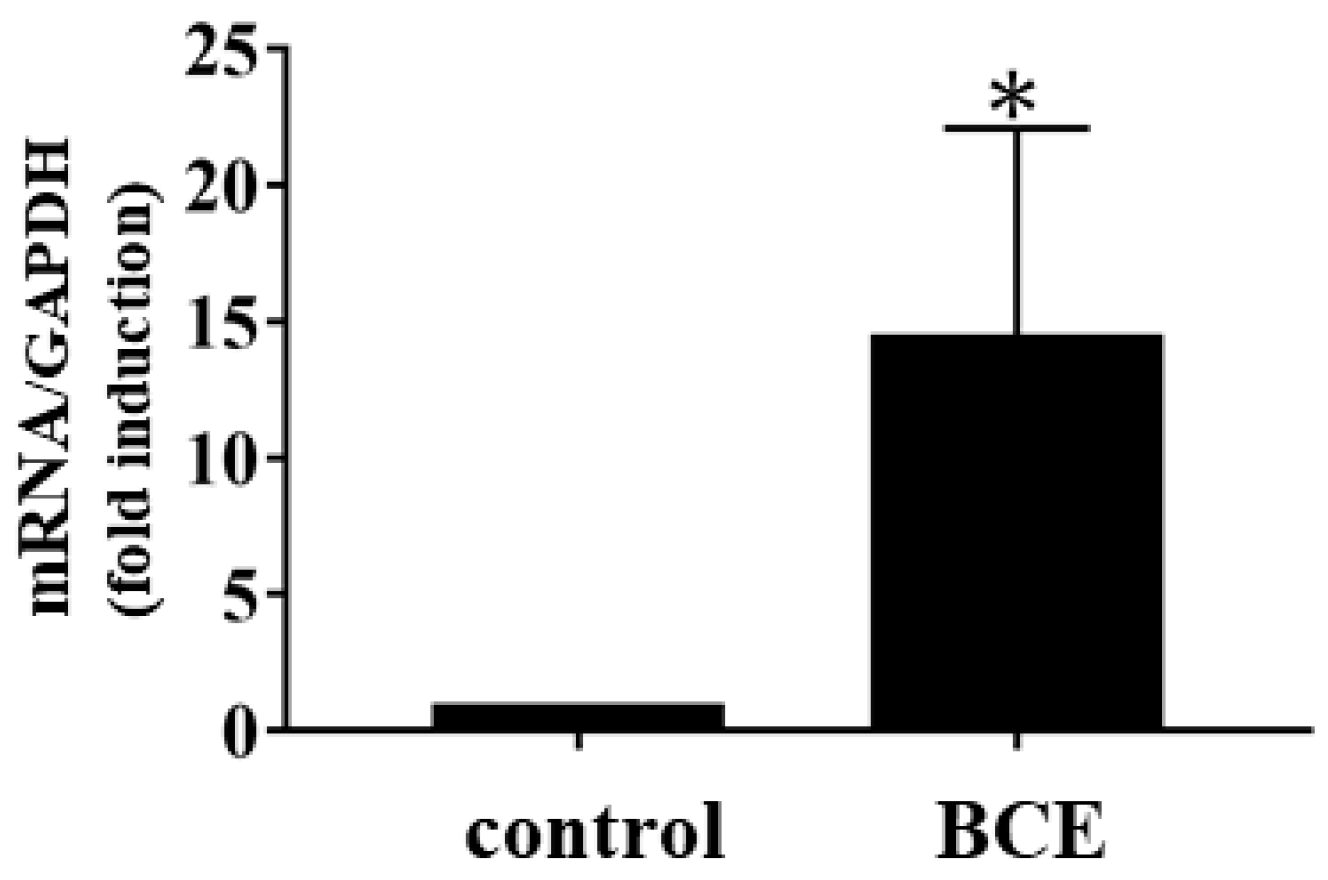
2.1. Gene Expression Profiling of HFDPCs Exposed to BCE
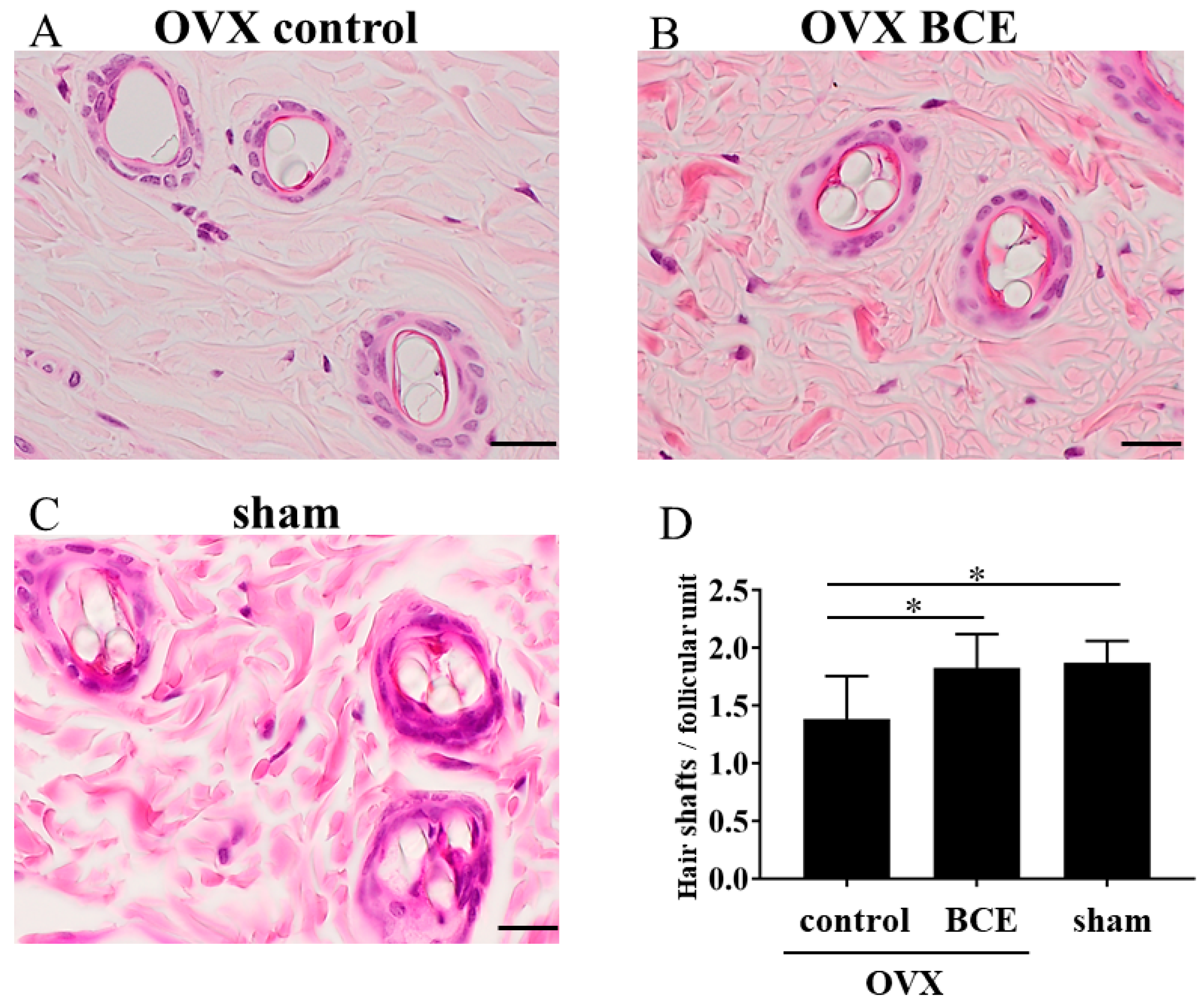
2.2. BCE Alleviated Hair Loss in OVX Rats
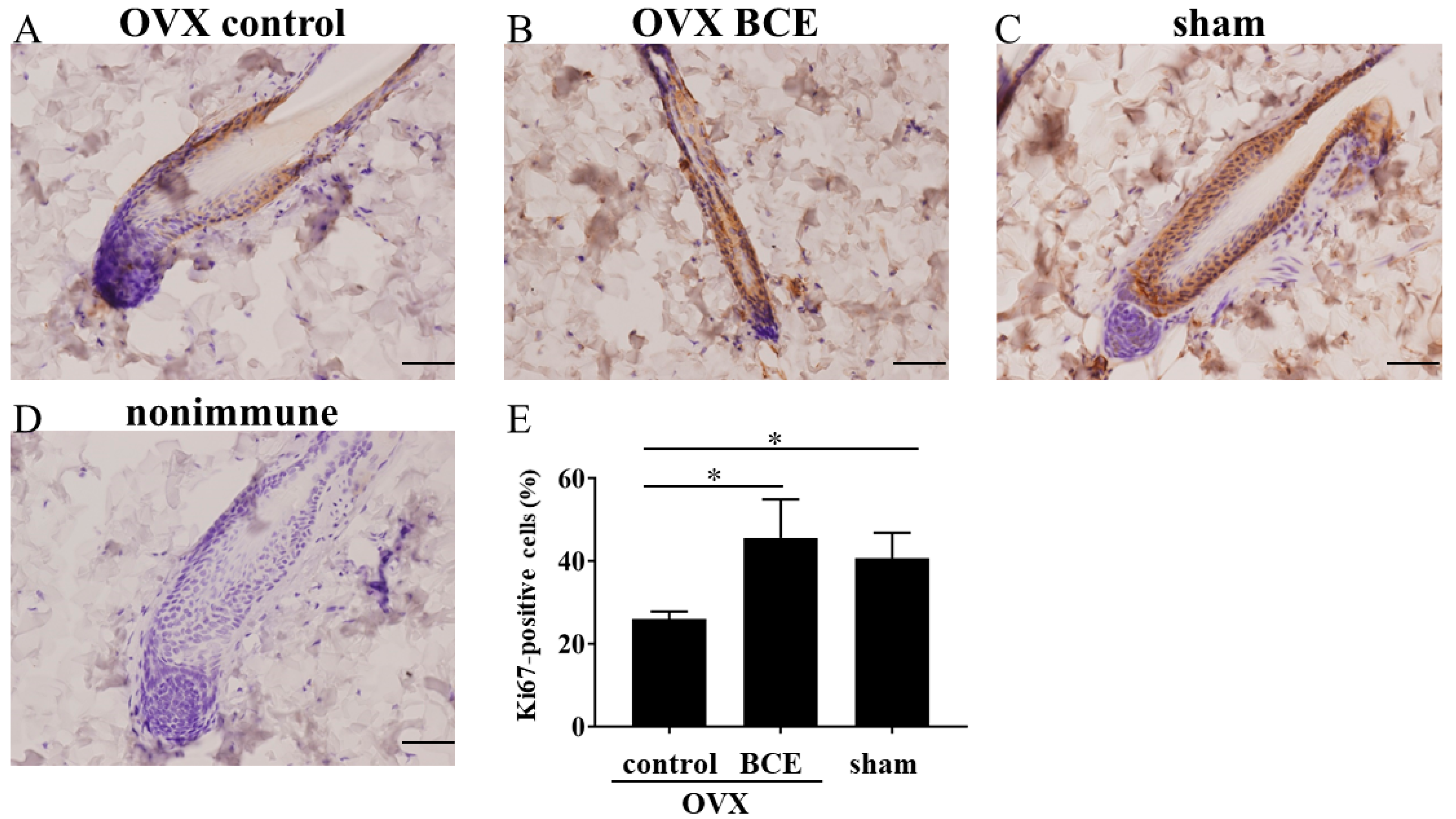
2.3. BCE Enhanced the Proliferation of Hair Follicle Cells
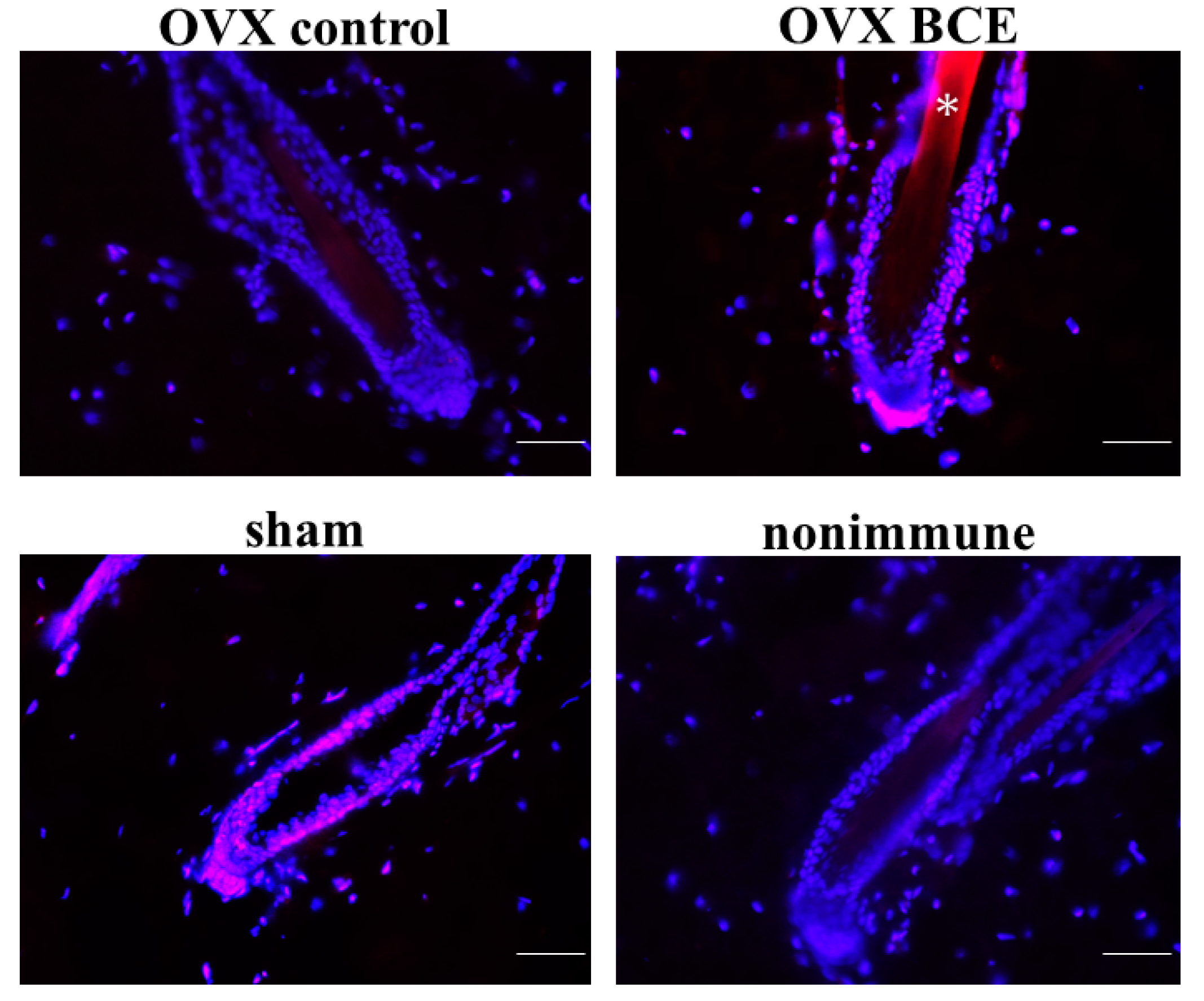
2.4. Effects of BCE on the Expression of the Hair Follicle Stem Cell Marker K19 in OVX Rats
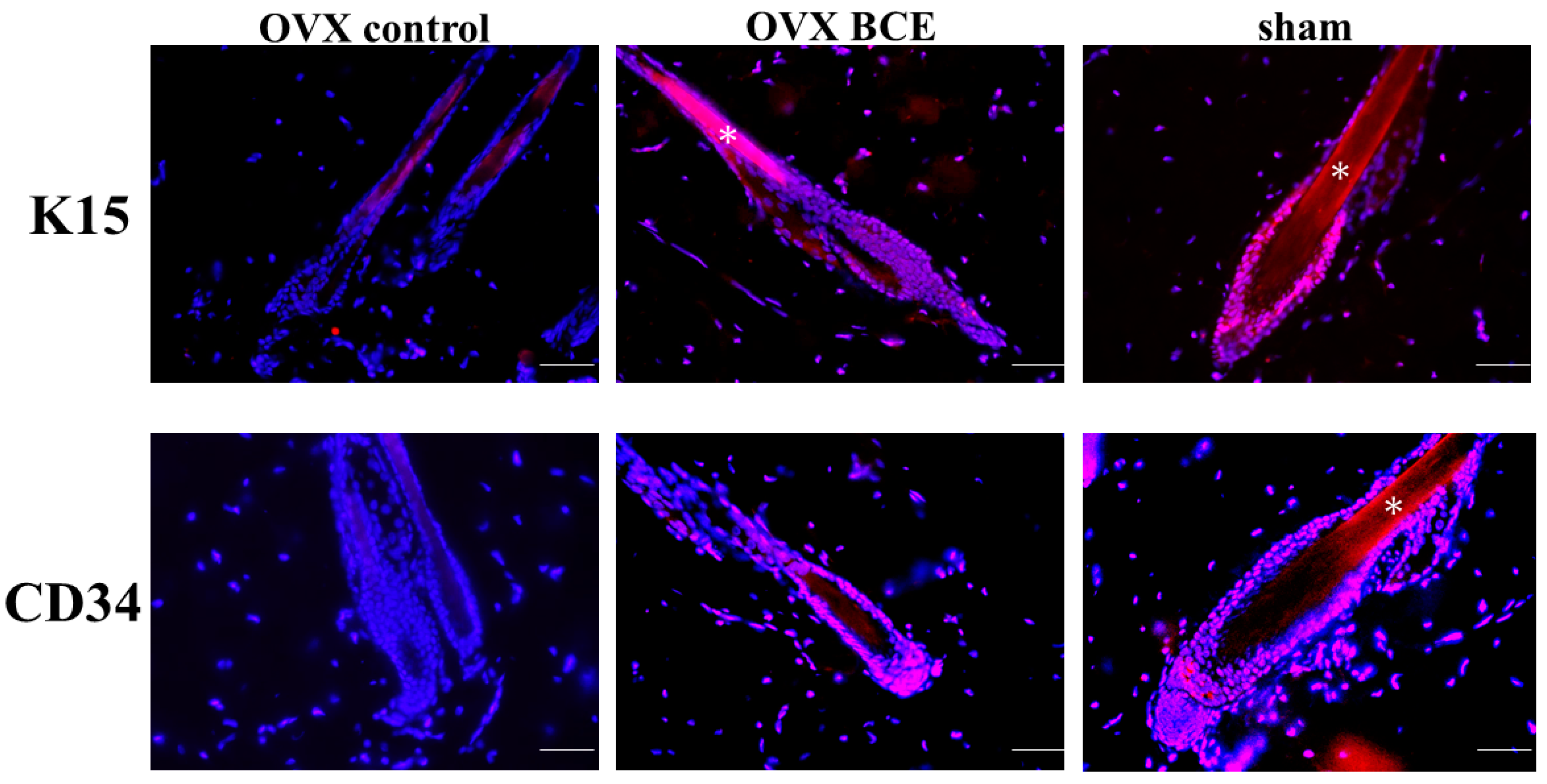
2.5. Effects of BCE on the Expression of the Hair Follicle Stem Cell Markers K15 and CD34 in OVX Rats
3. Materials and Methods
3.1. Materials and Cell Culture
3.2. Microarray Gene Expression Profiling
3.3. IPA
3.4. qPCR
3.5. Animals and Treatments
3.6. Enumeration of Hair Shafts per Follicular Unit and Analysis of Immunohistochemical Staining
3.7. Immunofluorescence Staining
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary anthocyanins against obesity and inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Chiba, M.; Nakano, M.; Maeda, H.; Nakamura, T. Anthocyaninrich blackcurrant extract inhibits proliferation of the MCF10A healthy human breast epithelial cell line through induction of G0/G1 arrest and apoptosis. Mol. Med. Rep. 2017, 16, 6134–6141. [Google Scholar] [CrossRef] [PubMed]
- Shaw, O.M.; Nyanhanda, T.; McGhie, T.K.; Harper, J.L.; Hurst, R.D. Blackcurrant anthocyanins modulate CCL11 secretion and suppress allergic airway inflammation. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Ros, G.; Nieto, G. Bioactive compounds and extracts from traditional herbs and their potential anti-inflammatory health effects. Medicines 2018, 5, 76. [Google Scholar] [CrossRef]
- Guo, D.; Wang, J.; Wang, X.; Luo, H.; Zhang, H.; Cao, D.; Chen, L.; Huang, N. Double directional adjusting estrogenic effect of naringin from Rhizoma drynariae (Gusuibu). J. Ethnopharmacol. 2011, 138, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, J.B.; Bae, J.H.; Lee, J.S.; Kim, P.S.; Jang, H.H.; Kim, H.R. Estrogen-like activity of aqueous extract from Agrimonia pilosa Ledeb. in MCF-7 cells. BMC Complement. Altern. Med. 2012, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Limer, J.L.; Speirs, V. Phyto-oestrogens and breast cancer chemoprevention. Breast Cancer Res. 2004, 6, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Nanashima, N.; Horie, K.; Maeda, H. Phytoestrogenic activity of blackcurrant anthocyanins is partially mediated through estrogen receptor beta. Molecules 2017, 23, 74. [Google Scholar] [CrossRef]
- Nanashima, N.; Horie, K.; Tomisawa, T.; Chiba, M.; Nakano, M.; Fujita, T.; Maeda, H.; Kitajima, M.; Takamagi, S.; Uchiyama, D.; et al. Phytoestrogenic activity of blackcurrant (Ribes nigrum) anthocyanins is mediated through estrogen receptor alpha. Mol. Nutr. Food Res. 2015, 59, 2419–2431. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008, 60, 10–18. [Google Scholar] [CrossRef]
- Prisby, R.D. Mechanical, hormonal and metabolic influences on blood vessels, blood flow and bone. J. Endocrinol. 2017, 235, R77–R100. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.J. Estrogens and aging skin. Dermatoendocrinology 2013, 5, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.C.; Silva, A.M.; Santos, M.S.; Sardao, V.A. Phytoestrogens as alternative hormone replacement therapy in menopause: What is real, what is unknown. J. Steroid Biochem. Mol. Biol. 2014, 143, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Wuttke, W.; Jarry, H.; Westphalen, S.; Christoffel, V.; Seidlova-Wuttke, D. Phytoestrogens for hormone replacement therapy? J. Steroid Biochem. Mol. Biol. 2002, 83, 133–147. [Google Scholar] [CrossRef]
- Herskovitz, I.; Tosti, A. Female pattern hair loss. Int. J. Endocrinol. Metab. 2013, 11, e9860. [Google Scholar] [CrossRef]
- Koebele, S.V.; Bimonte-Nelson, H.A. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas 2016, 87, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Obayashi, Y.; Ono, T.; Serizawa, T.; Murakoshi, M.; Ohyama, M. Reversal of the hair loss phenotype by modulating the estradiol-ANGPT2 axis in the mouse model of female pattern hair loss. J. Dermatol. Sci. 2018, 91, 43–51. [Google Scholar] [CrossRef]
- Endo, Y.; Takahashi, M.; Obayashi, Y.; Serizawa, T.; Murakoshi, M.; Ohyama, M. The ovariectomized mouse simulates the pathophysiology of postmenopausal female pattern hair loss. J. Dermatol. Sci. 2017, 87, 79–82. [Google Scholar] [CrossRef]
- Michel, M.; Torok, N.; Godbout, M.J.; Lussier, M.; Gaudreau, P.; Royal, A.; Germain, L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: Keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J. Cell Sci. 1996, 109, 1017–1028. [Google Scholar] [PubMed]
- Larouche, D.; Kim, D.H.; Ratte, G.; Beaumont, C.; Germain, L. Effect of intense pulsed light treatment on human skin in vitro: Analysis of immediate effects on dermal papillae and hair follicle stem cells. Br. J. Dermatol. 2013, 169, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Rompolas, P.; Greco, V. Stem cell dynamics in the hair follicle niche. Semin. Cell Dev. Biol. 2014, 25–26, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Narisawa, Y.; Hashimoto, K.; Kohda, H. Immunohistochemical demonstration of keratin 19 expression in isolated human hair follicles. J. Investig. Dermatol. 1994, 103, 191–195. [Google Scholar] [CrossRef]
- Larouche, D.; Hayward, C.; Cuffley, K.; Germain, L. Keratin 19 as a stem cell marker in vivo and in vitro. Methods Mol. Biol. 2005, 289, 103–110. [Google Scholar] [PubMed]
- Youssef, K.K.; Van Keymeulen, A.; Lapouge, G.; Beck, B.; Michaux, C.; Achouri, Y.; Sotiropoulou, P.A.; Blanpain, C. Identification of the cell lineage at the origin of basal cell carcinoma. Nat. Cell Biol. 2010, 12, 299–305. [Google Scholar] [CrossRef]
- Trempus, C.S.; Morris, R.J.; Bortner, C.D.; Cotsarelis, G.; Faircloth, R.S.; Reece, J.M.; Tennant, R.W. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Investig. Dermatol. 2003, 120, 501–511. [Google Scholar]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef]
- Burclaff, J.; Mills, J.C. Plasticity of differentiated cells in wound repair and tumorigenesis, part II: Skin and intestine. Dis. Models Mech. 2018, 11, dmm035071. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef]
- Martel, J.L.; Badri, T. Anatomy, Hair Follicle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Nanashima, N.; Horie, K.; Maeda, H.; Tomisawa, T.; Kitajima, M.; Nakamura, T. Blackcurrant anthocyanins increase the levels of collagen, elastin, and hyaluronic acid in human skin fibroblasts and ovariectomized rats. Nutrients 2018, 10, 495. [Google Scholar] [CrossRef]
- Ramot, Y.; Mastrofrancesco, A.; Camera, E.; Desreumaux, P.; Paus, R.; Picardo, M. The role of PPARgamma-mediated signalling in skin biology and pathology: New targets and opportunities for clinical dermatology. Exp. Dermatol. 2015, 24, 245–251. [Google Scholar] [CrossRef]
- Karnik, P.; Tekeste, Z.; McCormick, T.S.; Gilliam, A.C.; Price, V.H.; Cooper, K.D.; Mirmirani, P. Hair follicle stem cell-specific PPARgamma deletion causes scarring alopecia. J. Investig. Dermatol. 2009, 129, 1243–1257. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R.; Dutton, T.; Sarba, B. Glucocorticoid effect on hair growth initiation: A reconsideration. Skin Pharmacol. 1993, 6, 125–134. [Google Scholar] [CrossRef]
- Perez, P. Glucocorticoid receptors, epidermal homeostasis and hair follicle differentiation. Dermatoendocrinology 2011, 3, 166–174. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, K.; Xu, Z.; Huang, H.; Qian, N.; Lu, Z.; Chen, D.; Di, R.; Yuan, T.; Du, Z.; et al. Hoxc-dependent mesenchymal niche heterogeneity drives regional hair follicle regeneration. Cell Stem Cell 2018, 23, 487–500. [Google Scholar] [CrossRef]
- Park, G.H.; Park, K.Y.; Cho, H.I.; Lee, S.M.; Han, J.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C.; et al. Red ginseng extract promotes the hair growth in cultured human hair follicles. J. Med. Food 2015, 18, 354–362. [Google Scholar] [CrossRef]
- Chen, S.; Takahara, M.; Kido, M.; Takeuchi, S.; Uchi, H.; Tu, Y.; Moroi, Y.; Furue, M. Increased expression of an epidermal stem cell marker, cytokeratin 19, in cutaneous squamous cell carcinoma. Br. J. Dermatol. 2008, 159, 952–955. [Google Scholar] [CrossRef]
- Kiratipaiboon, C.; Tengamnuay, P.; Chanvorachote, P. Ciprofloxacin improves the stemness of human dermal papilla cells. Stem Cells Int. 2016, 2016, 5831276. [Google Scholar] [CrossRef]
- Ramot, Y.; Mastrofrancesco, A.; Herczeg-Lisztes, E.; Biro, T.; Picardo, M.; Kloepper, J.E.; Paus, R. Advanced inhibition of undesired human hair growth by PPARgamma modulation? J. Investig. Dermatol. 2014, 134, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Chermnykh, E.; Kalabusheva, E.; Vorotelyak, E. Extracellular matrix as a regulator of epidermal stem cell fate. Int. J. Mol. Sci. 2018, 19, 1003. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Han, H.J.; Lee, S.; Kim, G.M.; Park, S.; Choi, Y.A.; Lee, J.D.; Kim, G.M.; Sohn, J.; Kim, S.I. Detection of circulating tumor cells in breast cancer patients using cytokeratin-19 real-time RT-PCR. Yonsei Med. J. 2017, 58, 19–26. [Google Scholar] [CrossRef] [PubMed]
| Predicted Upstream Regulator | Z-Score |
|---|---|
| Beta-estradiol | 3.07 |
| PPARG | 2.2 |
| Glucocorticoid | 2.2 |
| Estrogen receptor | 2.14 |
| Gene Symbol | Gene Name | Fold Change |
|---|---|---|
| KRT19 | Keratin 19 | 58.8 |
| HOXC6 | Homeobox C6 | 18.1 |
| NR4A3 | Nuclear receptor subfamily 4 group A member 3 | 9.5 |
| HOXC4 | Homeobox C4 | 8.7 |
| CDH2 | Cadherin 2 | 5.8 |
| HSPB8 | Heat shock protein family B (small) member 8 | 4.4 |
| HSD17B7 | Hydroxysteroid 17-beta dehydrogenase 7 | 2.7 |
| PPARGC1B | PPARG coactivator 1 beta | 2.6 |
| AGT | Angiotensinogen | 2.4 |
| NKX3-1 | NK3 homeobox 1 | 2.3 |
| SEMA3F | Semaphorin 3F | 2.2 |
| HTRA3 | HtrA serine peptidase 3 | 2.2 |
| SLC52A1 | Solute carrier family 52 member 1 | 2.2 |
| FASN | Fatty acid synthase | 2.1 |
| CYP1B1 | Cytochrome P450 family 1 subfamily B member 1 | 2.1 |
| SLC7A5 | Solute carrier family 7 member 5 | 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanashima, N.; Horie, K. Blackcurrant Extract with Phytoestrogen Activity Alleviates Hair Loss in Ovariectomized Rats. Molecules 2019, 24, 1272. https://doi.org/10.3390/molecules24071272
Nanashima N, Horie K. Blackcurrant Extract with Phytoestrogen Activity Alleviates Hair Loss in Ovariectomized Rats. Molecules. 2019; 24(7):1272. https://doi.org/10.3390/molecules24071272
Chicago/Turabian StyleNanashima, Naoki, and Kayo Horie. 2019. "Blackcurrant Extract with Phytoestrogen Activity Alleviates Hair Loss in Ovariectomized Rats" Molecules 24, no. 7: 1272. https://doi.org/10.3390/molecules24071272
APA StyleNanashima, N., & Horie, K. (2019). Blackcurrant Extract with Phytoestrogen Activity Alleviates Hair Loss in Ovariectomized Rats. Molecules, 24(7), 1272. https://doi.org/10.3390/molecules24071272






