Non-Isothermal Sublimation Kinetics of 2,4,6-Trinitrotoluene (TNT) Nanofilms
Abstract
1. Introduction
2. Experimental Section
3. Theory
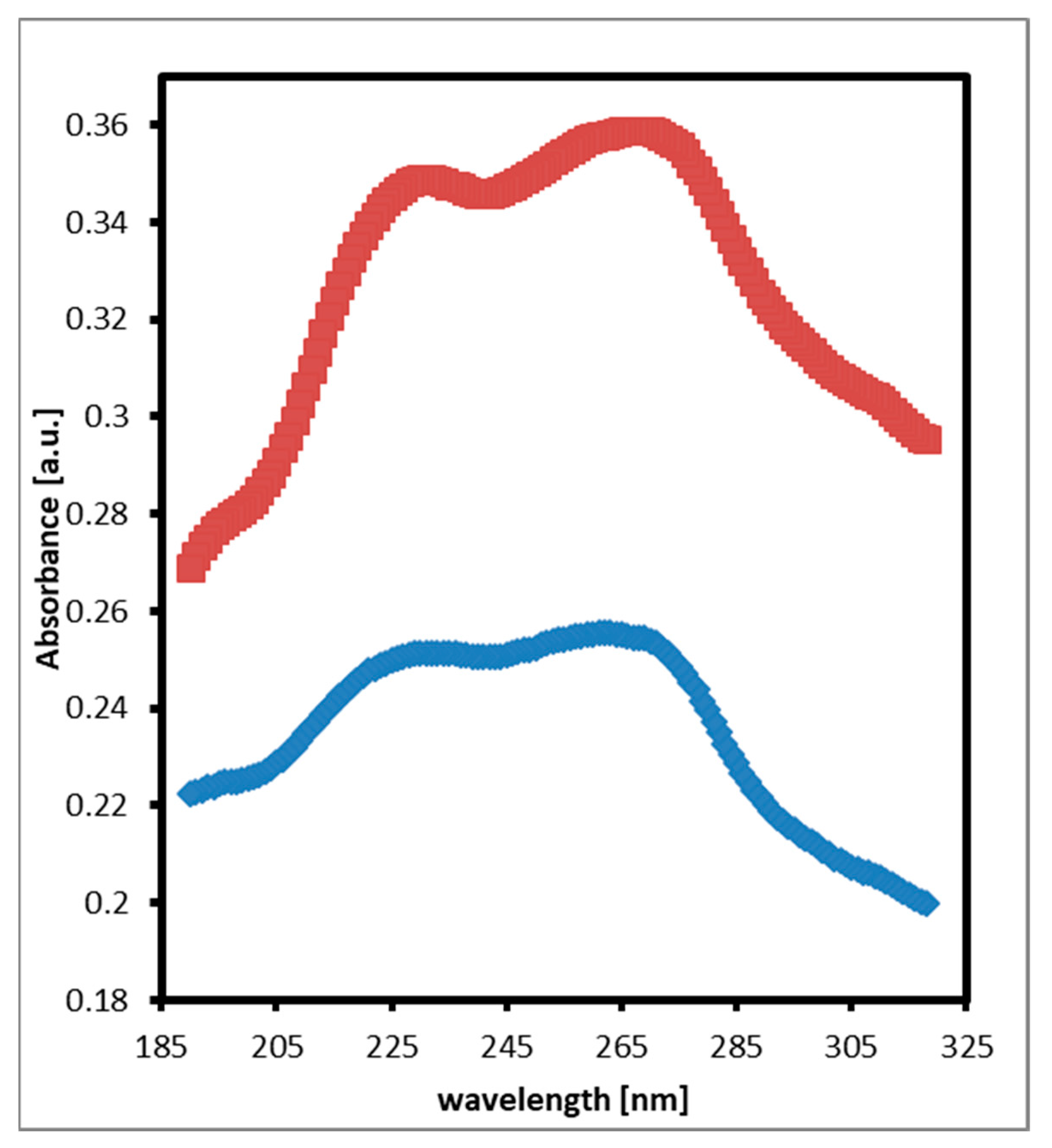
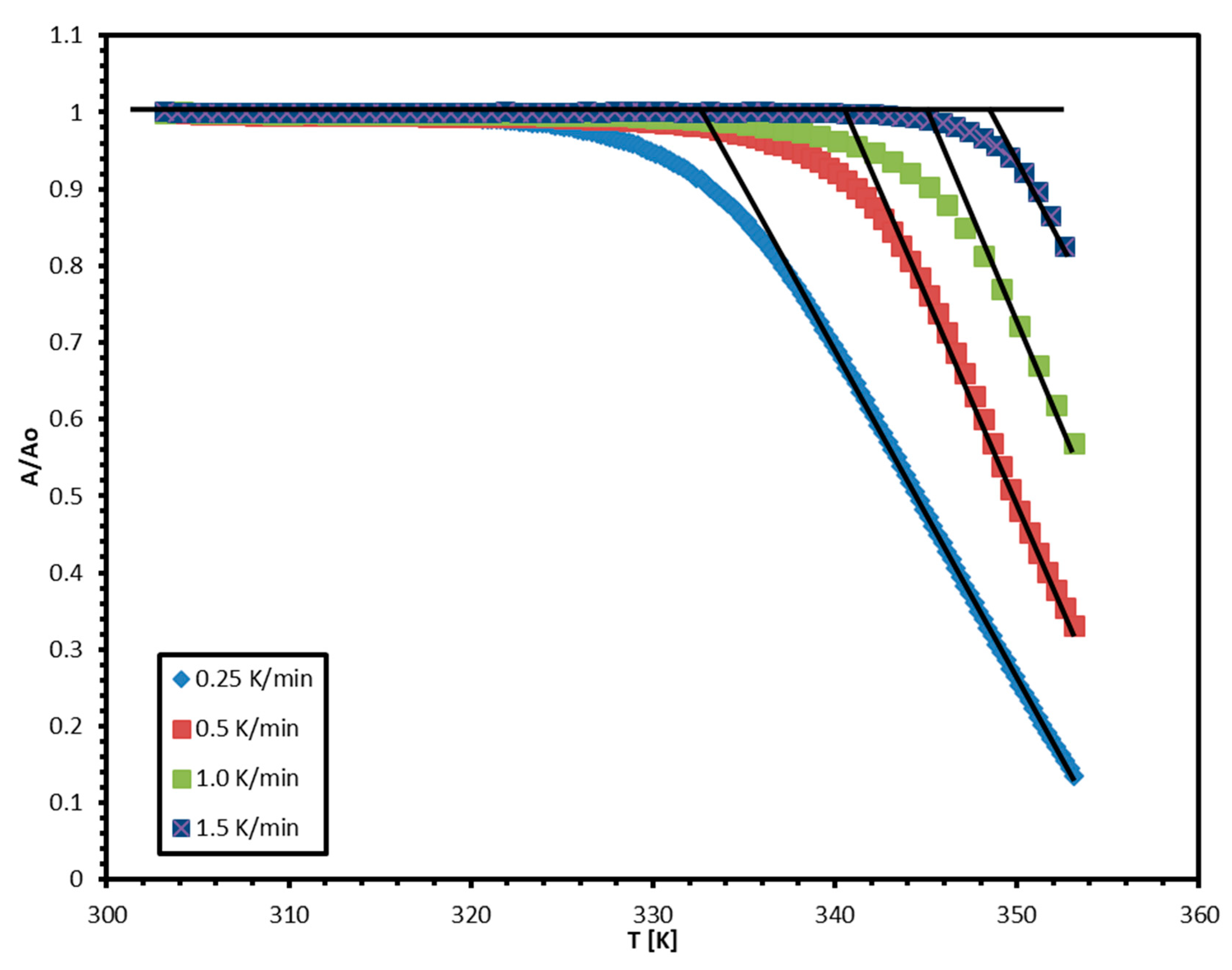
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Czarnecki, J.; Sestak, J. Practical Thermogravimetry. J. Therm. Anal. Calorim. 2000, 60, 759–778. [Google Scholar] [CrossRef]
- Hajimirsadeghis, S.S.; Teimouri, M.R.; Rahimi-Nasrabadi, M.; Dehghanpour, S. Non-isothermal kinetic study of the thermal decomposition of N-{bis[benzyl(methyl)amino]phosphoryl}-2,2-dichloroacetamide and N-{bis[dibenzylamino]phosphoryl}-2,2-dichloroacetamide. J. Therm. Anal. Calorim. 2009, 98, 463–468. [Google Scholar] [CrossRef]
- Hobbs, M.L.; Nakos, J.T.; Brady, P.D. Response of a glass/phenolic composite to high temperatures. J. Therm. Anal. Calorim. 2011, 103, 543–553. [Google Scholar] [CrossRef]
- Gershanik, A.P.; Zeiri, Y. Sublimation Rate of TNT Microcrystals in Air. J. Phys. Chem. A 2010, 114, 12403–12410. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Ueda, A.; Liu, Y.C.; Wu, M.; Henderson, D.O.; Lareau, R.T.; Chamberlain, R.T. Effects of interfacial interaction potential on the sublimation rates of TNT films on a silica surface examined by QCM and AFM techniques. Surf. Sci. 2003, 530, L293–L296. [Google Scholar] [CrossRef]
- Pitchimani, R.; Burnham, A.K.; Weeks, B.L. Quantitative Thermodynamic Analysis of Sublimation Rates Using an Atomic Force Microscope. J. Phys. Chem. B 2007, 11, 9182–9185. [Google Scholar] [CrossRef] [PubMed]
- Burnham, A.K.; Qiu, S.R.; Pitchimani, R.; Weeks, B.L. Comparison of kinetic paprameters of single crystal pentaerythritol tetranitrate using atomic force microscopy and thermogravimetric analysis: Implications on coarsening mechanisms. J. Appl. Phys. 2009, 105, 104312. [Google Scholar] [CrossRef]
- Hikal, W.M.; Weeks, B.L. Determination of sublimation rate of 2,4,6-trinitrotoluene (TNT) nano thin films using UV-absorbance spectroscopy. J. Therm. Anal. Calorim. 2010, 110, 955–960. [Google Scholar] [CrossRef]
- Hikal, W.M.; Paden, J.T.; Weeks, B.L. Simple method for determining the vapor pressure of materials using UV-absorbance spectroscopy. J. Phys. Chem. B 2011, 115, 13287–13291. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Paden, J.T.; Weeks, B.L. Thermo-optical determination of vapor pressures of TNT and RDX nanofilms. Talanta 2011, 87, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Hikal, W.M.; Paden, J.T.; Weeks, B.L. Rapid estimation of the thermodynamic parameters and vapor pressures of volatile materials at the nanoscale. ChemPhysChem 2012, 13, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- Antony Premkumar, P.; Nagaraja, K.S.; Pankajavalli, R.; Mallika, C.; Sreedharan, O.M. DTA studies on the liquidus temperatures of Cr complex with the addition of an anhydrous Ni complex. Mater. Lett. 2004, 58, 474–477. [Google Scholar] [CrossRef]
- Gao, X.; Chen, D.; Dollimore, D. The correlation between the value of a at the maximum reaction rate and the reaction mechanisms: A theoretical study. Thermochim. Acta 1993, 223, 75–82. [Google Scholar] [CrossRef]
- Burnham, L.; Dollimore, D.; Alexander, K. Calculation of the vapor pressure–temperature relationship using thermogravimetry for the drug allopurinol. Thermochim. Acta 2001, 367–368, 15–22. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Budrugeac, P.; Segal, E. Applicability of the Kissinger equation in thermal analysis. J. Therm. Anal. Calorim. 2007, 88, 703–707. [Google Scholar] [CrossRef]
- Jankovic, B.; Mantus, S. Model-fitting and model-free analysis of thermal decomposition of palladium acetylacetonate [Pd(acac)2]. J. Therm. Anal. Calorim. 2008, 94, 395–403. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ingle, J.D.J.; Crouch, S.R. Spectrochemical Analysis; Prentice Hall PTR: Upper Saddle River, NJ, USA, 1988. [Google Scholar]
- Schubert, E.F. Light-Emitting Diodes, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Pella, P.E. Measurement of the vapor pressures of TNT, 2,4-DNT, 2,6-DNT, and EGDN. J. Chem. Thermodyn. 1977, 9, 301–305. [Google Scholar] [CrossRef]
- Leggett, D.C. Vapor pressure of 2,4,6-trinitrotoluene by a gas chromatographic headspace Technique. J. Chromatogr. A 1977, 133, 83–90. [Google Scholar] [CrossRef]
- Cundall, R.B.; Palmer, T.F.; Wood, C.E.C. Vapour pressure measurements on some organic high explosives. J. Chem. Soc. Faraday Trans. 1 1978, 74, 1339–1345. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Shinde, K.; Moran, J. Determination of the Vapor Density of Triacetone Triperoxide (TATP) Using a Gas Chromatography Headspace Technique. Propell. Explos. Pyrot. 2005, 30, 127–130. [Google Scholar] [CrossRef]
- Lenchitz, C.; Velicky, R.W. Vapor pressure and heat of sublimation of three nitrotoluenes. J. Chem. Eng. Data 1970, 15, 401–403. [Google Scholar] [CrossRef]
- Edwards, G.T. The vapor pressure of 2,4,6-trinitrotoluene. Trans. Faraday Soc. 1950, 46, 423–427. [Google Scholar] [CrossRef]
- Phelan, J.M.; Patton, R.T. Sublimation Rates of Explosive Materials-Method Development and Initial Results, Sandia Report SAND2004-4525; Sandia national laboratories: Albuquerque, CA, USA, 2004. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hikal, W.M.; Weeks, B.L. Non-Isothermal Sublimation Kinetics of 2,4,6-Trinitrotoluene (TNT) Nanofilms. Molecules 2019, 24, 1163. https://doi.org/10.3390/molecules24061163
Hikal WM, Weeks BL. Non-Isothermal Sublimation Kinetics of 2,4,6-Trinitrotoluene (TNT) Nanofilms. Molecules. 2019; 24(6):1163. https://doi.org/10.3390/molecules24061163
Chicago/Turabian StyleHikal, Walid M., and Brandon L. Weeks. 2019. "Non-Isothermal Sublimation Kinetics of 2,4,6-Trinitrotoluene (TNT) Nanofilms" Molecules 24, no. 6: 1163. https://doi.org/10.3390/molecules24061163
APA StyleHikal, W. M., & Weeks, B. L. (2019). Non-Isothermal Sublimation Kinetics of 2,4,6-Trinitrotoluene (TNT) Nanofilms. Molecules, 24(6), 1163. https://doi.org/10.3390/molecules24061163




