Discovery of 2-Substituted 3-Arylquinoline Derivatives as Potential Anti-Inflammatory Agents Through Inhibition of LPS-Induced Inflammatory Responses in Macrophages
Abstract
1. Introduction
2. Results
2.1. Chemistry
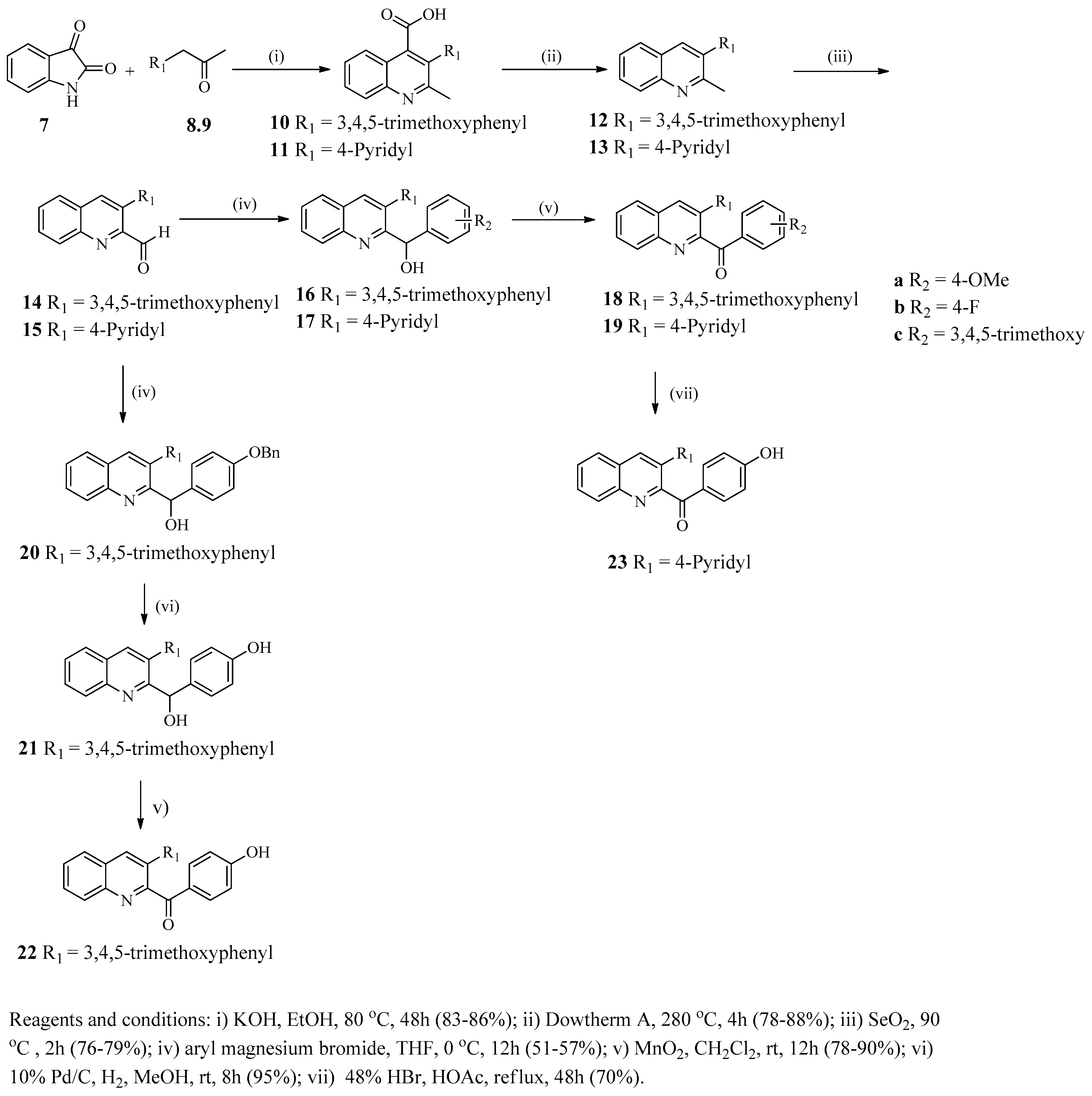
Preparation of 2-Substituted 3-Arylquinoline Derivatives
2.2. Biological Activities
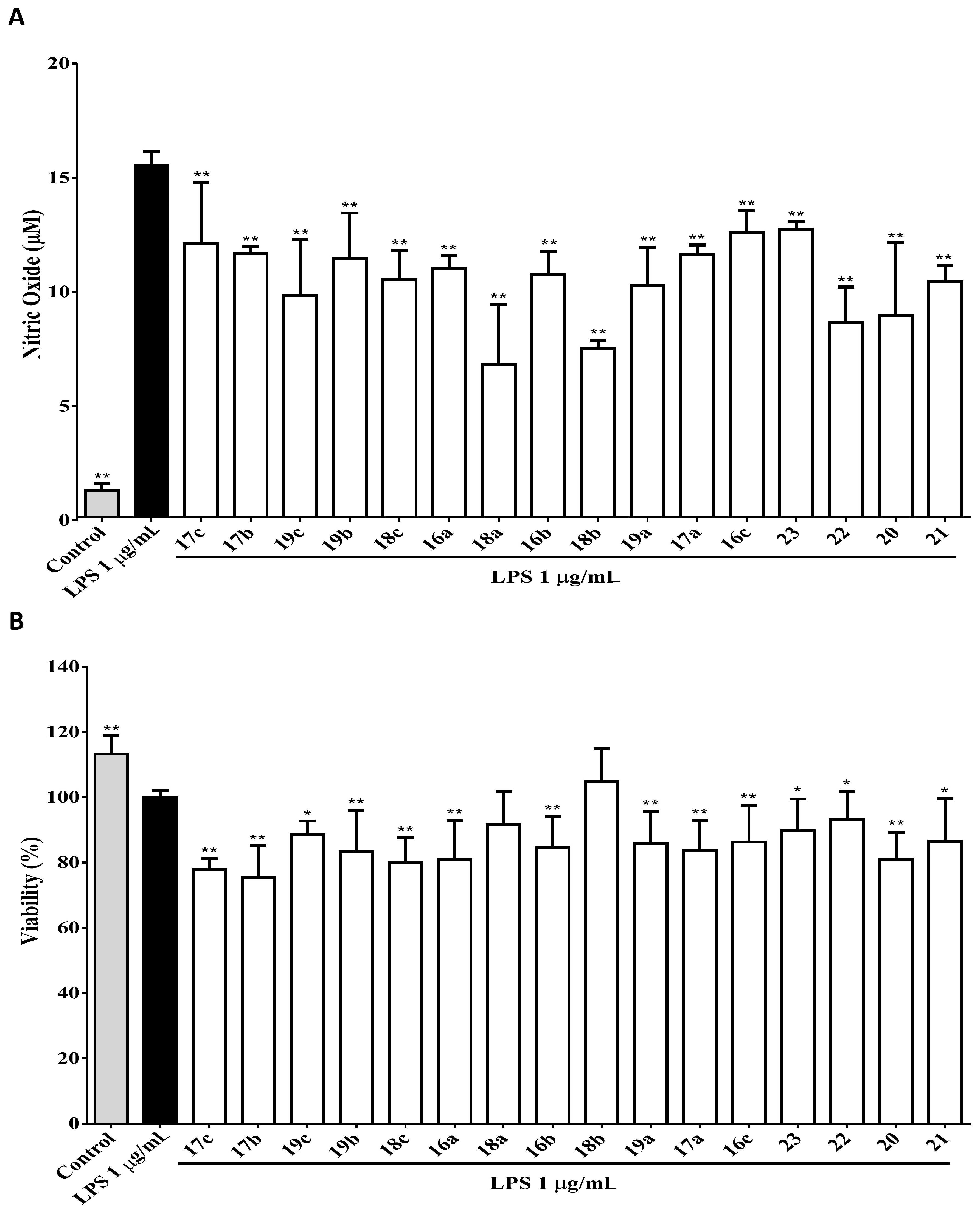
2.2.1. Effect of 2-substituted 3-arylquinoline Derivatives on NO Production and Cell Survival in Macrophages
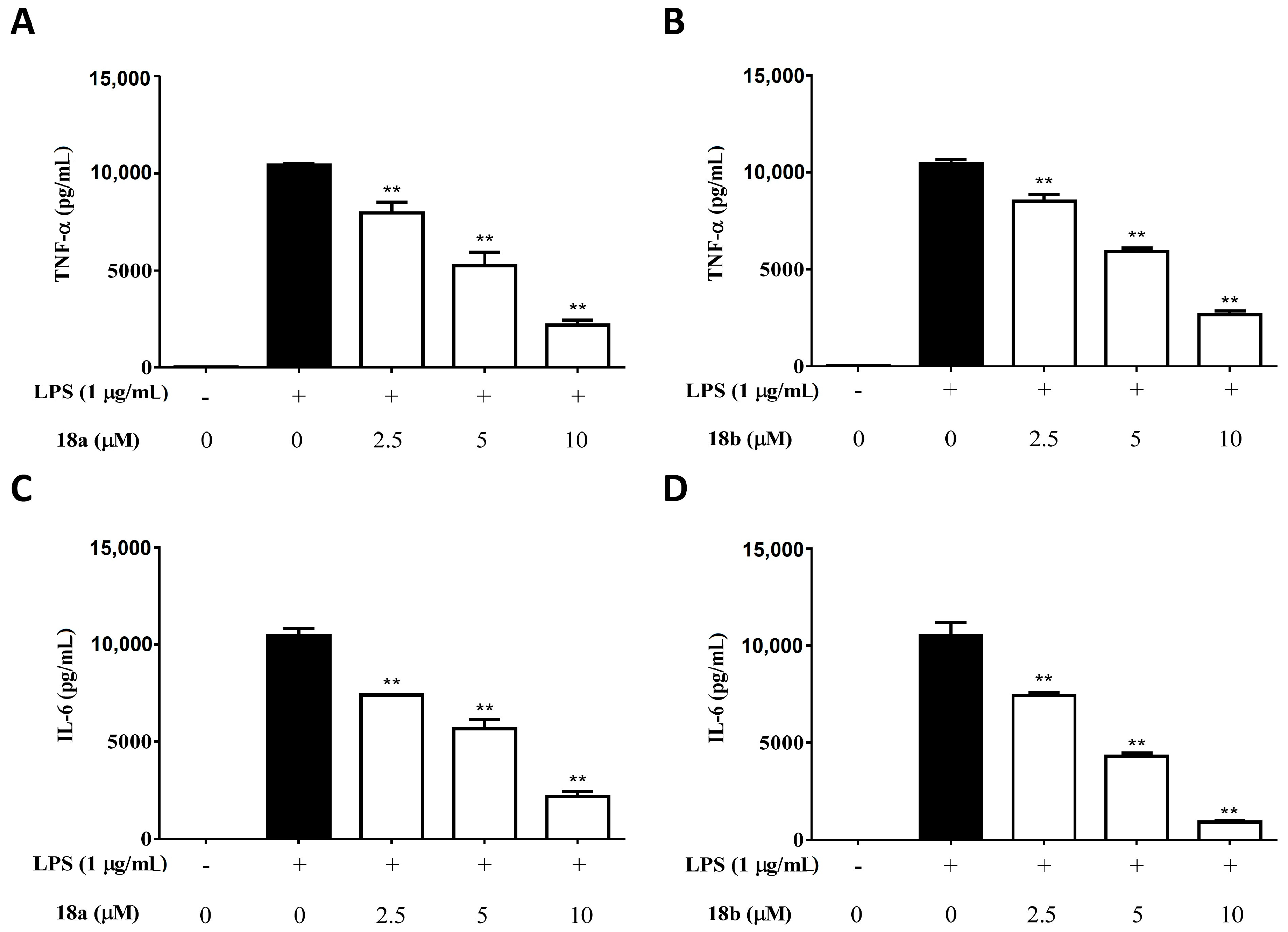
2.2.2. Compounds 18a and 18b Significantly Suppresses the Production of TNF-α and IL-6 by LPS-Activated Macrophages
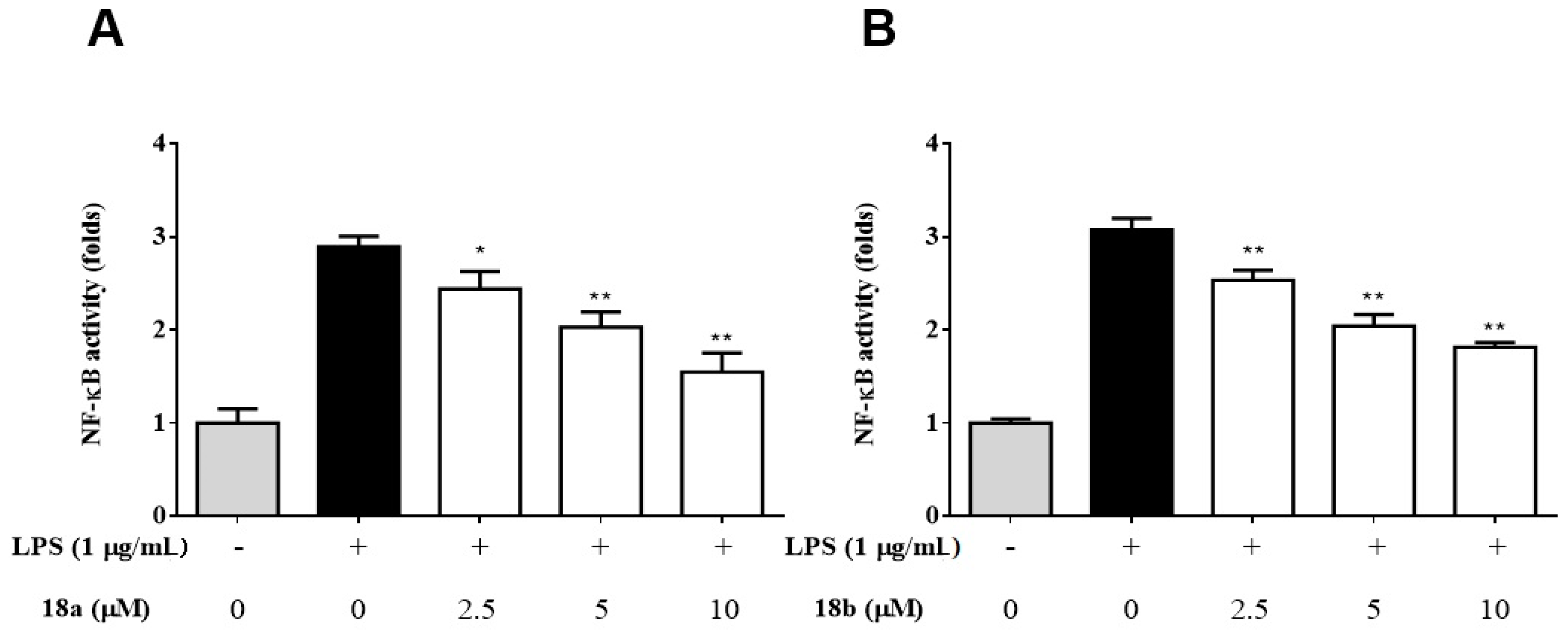
2.2.3. Compounds 18a and 18b Significantly Attenuate the Activity of NF-êB by LPS-Activated Macrophages
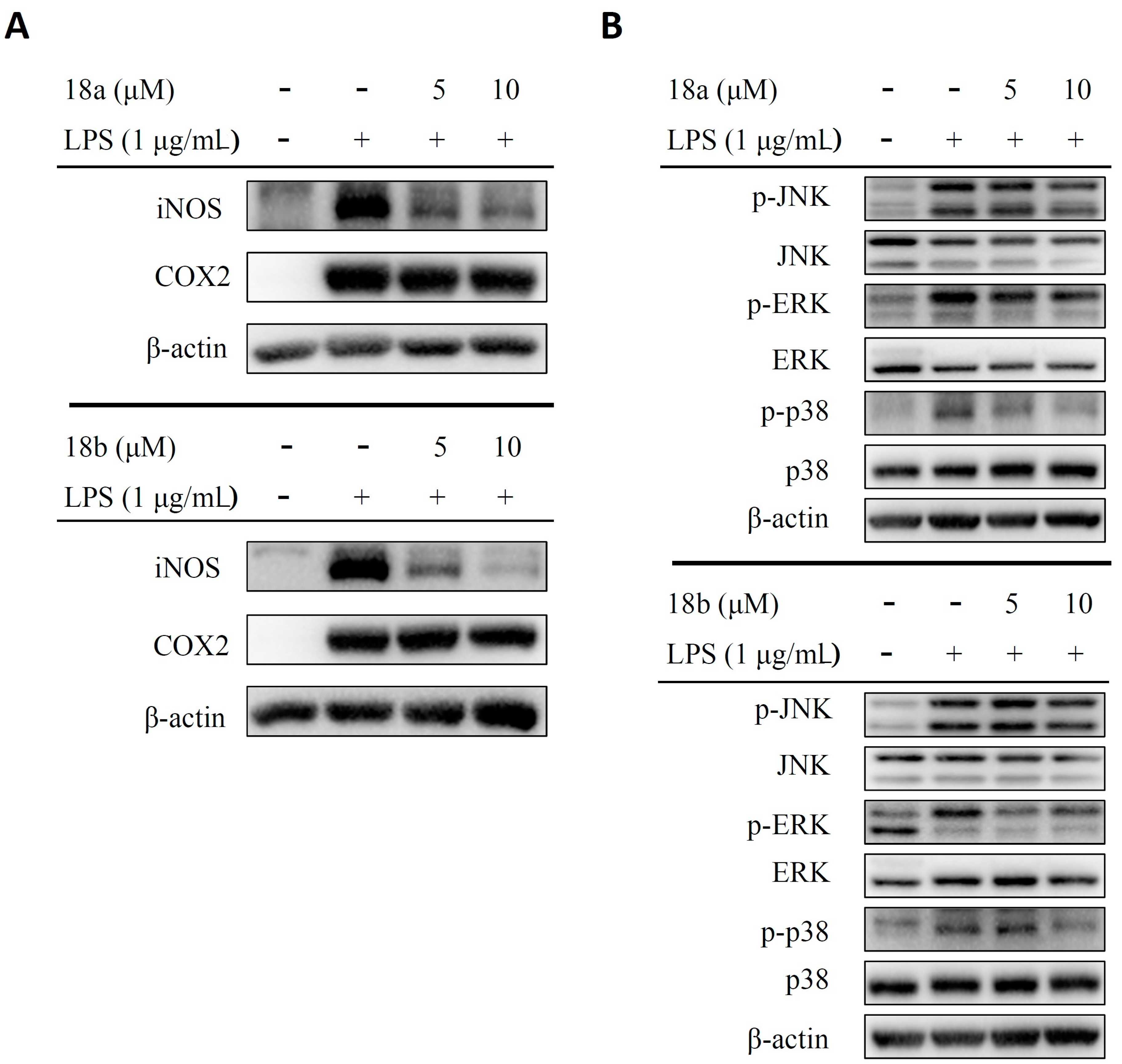
2.2.4. Compounds 18a and 18b Inhibit the Expression of iNOS and Suppress the Phosphorylation of MAPKs
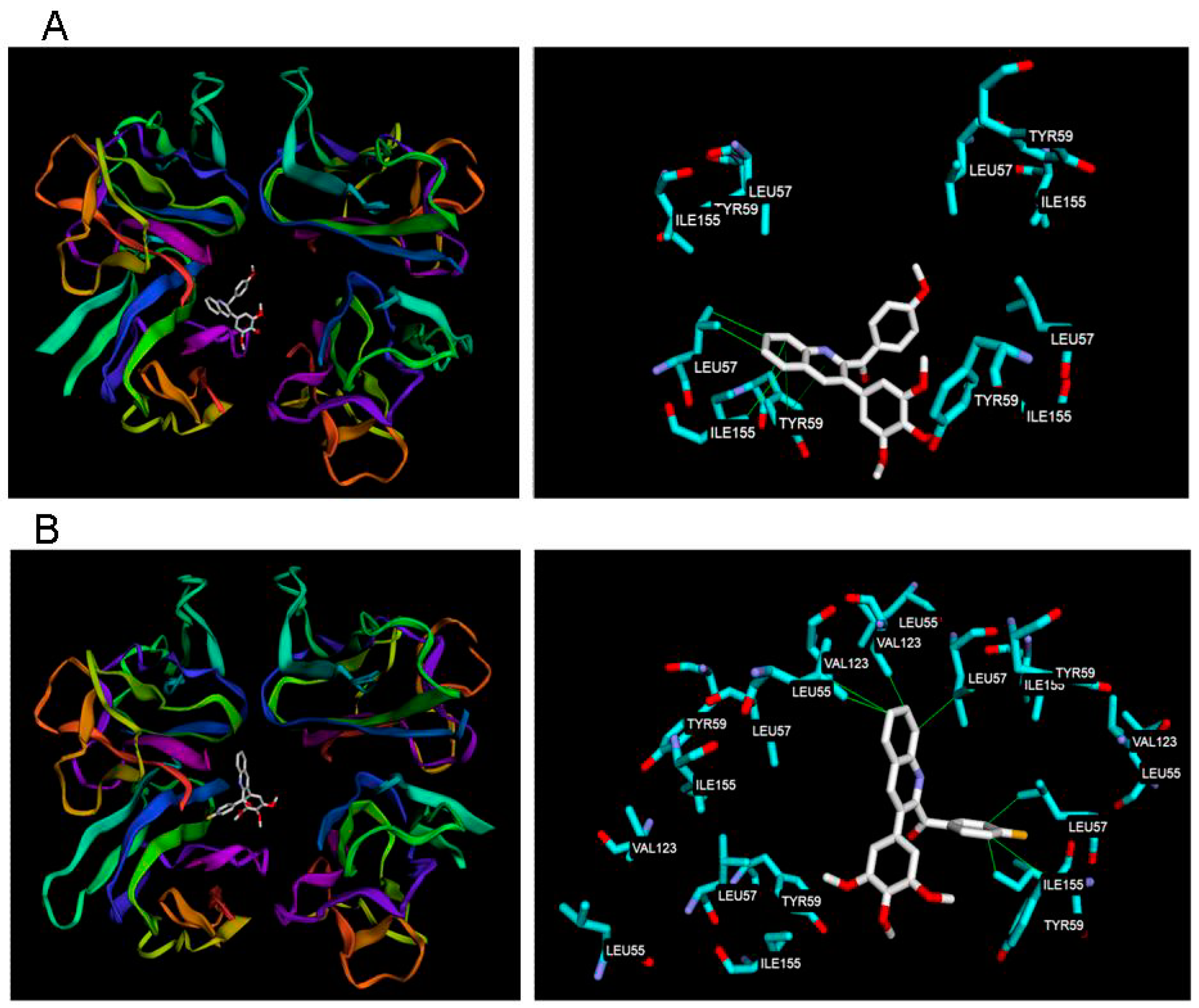
2.2.5. Molecular Docking Results of 18a, 18b and TNF-á
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Chemistry
4.2.1. General Procedure for the Preparation of 3-aryl-2-methylquinoline-4-carboxylic Acids 10 and 11
4.2.2. General Procedure for the Decarboxylation of Acids 12 and 13
4.2.3. General Procedure for the Preparation of quinoline-2-carbaldehydes 14 and 15
4.2.4. General Procedure for the Preparation of 2-(hydroxyphenylmethyl)-3-phenylquinolines 16a–c and 17a–c
4.2.5. General Procedure for Preparation of 2-benzoyl-3-phenylquinolines 18a–c and 19a–c
4.2.6. Preparation of 2-{1-[(4-Benzyloxy)phenyl]-1-hydroxylmethyl}-3-(3,4,5trimethoxyphenyl)- quinoline (20)
4.2.7. Preparation of 2-(4-Hydroxyphenyl-1-hydroxylmethyl)-3-(3,4,5-trimethoxyphenyl)quinoline (21)
4.2.8. Preparation of 2-(4-Hydroxybenzoyl)-3-(3,4,5-trimethoxyphenyl)quinoline (22)
4.2.9. Preparation of 2-(4-Hydroxybenzoyl)-3-(pyridin-4-yl)quinoline (23)
4.3. Cell Viability and Anti-Inflammatory Activity Assays
4.3.1. Reagents
4.3.2. Cell Culture
4.3.3. NO Determination
4.3.4. Cell Viability
4.3.5. Cytokine Measurement
4.3.6. NF-êB Promoter Reporter Assay
4.3.7. Western Blot
4.3.8. Molecular Docking Study
4.3.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Hung, Y.L.; Fang, S.H.; Wang, S.C.; Cheng, W.C.; Liu, P.L.; Su, C.C.; Chen, C.S.; Huang, M.Y.; Hua, K.F.; Shen, K.H.; et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef] [PubMed]
- Raymond, S.L.; Holden, D.C.; Mira, J.C.; Stortz, J.A.; Loftus, T.J.; Mohr, A.M.; Moldawer, L.L.; Moore, F.A.; Larson, S.D.; Efron, P.A. Microbial recognition and danger signals in sepsis and trauma. Biochim. Biophys. Acta 2017, 1863, 2564–2573. [Google Scholar] [CrossRef]
- Boulange, C.L.; Neves, A.L.; Chilloux, J.; Nicholson, J.K.; Dumas, M.E. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Szabo, C.; Modis, K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock 2010, 34, 4–14. [Google Scholar] [CrossRef]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef] [PubMed]
- El-Feky, S.A.H.; Abd El-Samii, Z.K.; Osman, N.A.; Lashine, J.; Kamel, M.A.; Thabet, H.K. Synthesis, molecular docking and anti-inflammatory screening of novel quinoline incorporated pyrazole derivatives using the Pfitzinger reaction II. Bioorg. Chem. 2015, 58, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Ghodsi, R.; Zarghi, A.; Daraei, B.; Hedayati, M. Design, synthesis and biological evaluation of new 2,3-diarylquinoline derivatives as selective cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. 2010, 18, 1029–1033. [Google Scholar] [CrossRef]
- Suthar, S.K.; Jaiswal, V.; Lohan, S.; Bansal, S.; Chaudhary, A.; Tiwari, A.; Alex, A.T.; Joesph, A. Novel quinolone substituted thiazolidin-4-ones as anti-inflammatory, anticancer agents: design, synthesis and biological screening. Eur. J. Med. Chem. 2013, 63, 589–602. [Google Scholar] [CrossRef]
- Chaaban, I.; Rizk, O.H.; Ibrahim, T.M.; Henen, S.S.; El-Khawass, E.M.; Bayad, A.E.; El-Ashmawy, I.M.; Nematalla, H.A. Synthesis, anti-inflammatory screening, molecular docking, and COX-1,2/-5-LOX inhibition profile of some novel quinoline derivatives. Bioorg. Chem. 2018, 78, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Zarghi, A.; Ghodsi, R. Design, synthesis, and biological evaluation of ketoprofen analogs as potent cyclooxygenase-2 inhibitors. Bioorg. Med. Chem. 2010, 18, 5855–5860. [Google Scholar] [CrossRef]
- Mazzoni, O.; Esposito, G.; Diurno, M.V.; Brancaccio, D.; Carotenuto, A.; Grieco, P.; Novellino, E.; Filippelli, W. Synthesis and pharmacological evaluation of some 4-oxo-quinoline-2-carboxylic acid derivatives as anti-inflammatory and analgesic agents. Arch. Pharm. Chem. Life Sci. 2010, 10, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Lin, C.S.; Shih, P.K.; Tsao, L.T.; Wang, J.P.; Cheng, C.M.; Tzeng, C.C.; Chen, Y.L. Furo[3, 2:3,4]naphtho[1,2-d]imidazole derivatives as potential inhibitors of inflammatory factors in sepsis. Bioorg. Med. Chem. 2009, 17, 6773–6779. [Google Scholar] [CrossRef]
- Tseng, C.H.; Tzeng, C.C.; Shih, P.K.; Yang, C.N.; Chuang, Y.C.; Peng, S.I.; Lin, C.S.; Wang, J.P.; Cheng, C.M.; Chen, Y.L. Identification of furo[3’,2’:3,4]naphtho[1,2-d]imidazole derivatives as orally active and selective inhibitors of microsomal prostaglandin E(2) synthase-1 (mPGES-1). Mol. Divers. 2012, 16, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Tseng, C.H.; Chen, Y.L.; Hwang, T.L.; Tzeng, C.C. Discovery of benzo[f]indole-4,9-dione derivatives as new type of anti-inflammatory agents. Int. J. Mol. Sci. 2015, 16, 6532–6544. [Google Scholar] [CrossRef]
- Tseng, C.H.; Tung, C.W.; Wu, C.H.; Tzeng, C.C.; Chen, Y.H.; Hwang, T.L.; Chen, Y.L. Discovery of indeno[1,2-c]quinoline derivatives as potent dual antituberculosis and anti-Inflammatory agents. Molecules 2017, 22, 1001. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Tung, C.W.; Peng, S.I.; Chen, Y.L.; Tzeng, C.C.; Cheng, C.M. Discovery of pyrazolo[4,3-c]quinolines derivatives as potential anti-inflammatory agents through inhibiting of NO production. Molecules 2018, 23, 1036. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H.; Lin, C.K.; Chen, Y.L.; Hsu, C.Y.; Wu, H.N.; Tseng, C.K.; Lee, J.C. Synthesis, antiproliferative and anti-dengue virus evaluations of 2-aroyl-3-arylquinoline derivatives. Eur. J. Med. Chem. 2014, 79, 66–76. [Google Scholar] [CrossRef]
- Tseng, C.H.; Chen, Y.L.; Hsu, C.Y.; Chen, T.C.; Cheng, C.M.; Tso, H.C.; Lu, Y.J.; Tzeng, C.C. Synthesis and antiproliferative evaluation of 3-phenylquinolinylchalcone derivatives against non-small cell lung cancers and breast cancers. Eur. J. Med. Chem. 2013, 59, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.H.; McIntyre, P.S. The Pfitzinger reaction with unsymmetrical ketones. J. Chem. Soc. B 1969, 5, 539–543. [Google Scholar] [CrossRef]
- Wallace, J.L. Nitric oxide as a regulator of inflammatory processes. Mem. Inst. Oswaldo. Cruz. 2005, 100, 5–9. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 851, 51–70. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Hua, K.F.; Yang, F.L.; Chiu, H.W.; Chou, J.C.; Dong, W.C.; Lin, C.N.; Lin, C.Y.; Wang, J.T.; Li, L.H.; Chiu, H.W.; et al. Capsular Polysaccharide Is Involved in NLRP3 Inflammasome Activation by Klebsiella pneumoniae Serotype K1. Infect. Immun. 2015, 83, 3396–3409. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kaur, S.; Sharma, A.; Kaur, G.; Bhatti, R. TNF-α and IL-6 inhibitors: Conjugates of N-substituted indole and aminophenylmorpholin-3-one as anti-inflammatory agents. Eur. J. Med. Chem. 2017, 140, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.; Lin, Z.C.; Chen, Y.L.; Tzeng, C.C.; Fang, J.Y.; Tseng, C.H. Synthesis and biological evaluation of thalidomide derivatives as potential anti-psoriasis agents. Int. J. Mol. Sci. 2018, 19, 3061. [Google Scholar] [CrossRef] [PubMed]
- Riera Romo, M.; Perez-Martinez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.X.; Donnelly, J.P.; Griffin, R.; Howard, G.; Safford, M.M.; Wang, H.E. Defining sepsis mortality clusters in the United States. Crit. Care Med. 2016, 44, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in sepsis: Potent immunoregulators and potential therapeutic targets--an updated view. Mediat. Inflamm. 2013, 2013, 165974. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- He, M.M.; Smith, A.S.; Oslob, J.D.; Flanagan, W.M.; Braisted, A.C.; Whitty, A.; Cancilla, M.T.; Wang, J.; Lugovskoy, A.A.; Yoburn, J.C.; et al. Small-molecule inhibition of TNF-α. Science 2005, 310, 1022–1025. [Google Scholar] [CrossRef]
- Sánchez-Linares, I.; Pérez-Sánchez, H.; Cecilia, J.M.; García, J.M. High-throughput parallel blind virtual screening using BINDSURF. BMC Bioinform. 2012, 13 (Suppl. 14), S13. [Google Scholar]
- Rego, N.; Koes, D. 3Dmol.js: Molecular visualization with WebGL. Bioinformatics 2015, 31, 1322–1324. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds reported herein are available from the authors. |
| Compounds | Nitric Oxide (μM) |
|---|---|
| Control | 1.17 ± 0.30 |
| LPS | 15.43 ± 0.58 |
| 17c | 12.00 ± 2.67 |
| 17b | 11.55 ± 0.30 |
| 19c | 9.70 ± 2.46 |
| 19b | 11.34 ± 1.99 |
| 18c | 10.40 ± 1.29 |
| 16a | 10.90 ± 0.56 |
| 18a | 6.70 ± 2.62 |
| 16b | 10.64 ± 1.01 |
| 18b | 7.41 ± 0.34 |
| 19a | 10.16 ± 1.67 |
| 17a | 11.5 ± 0.43 |
| 16c | 12.47 ± 0.97 |
| 23 | 12.59 ± 0.35 |
| 22 | 8.52 ± 1.57 |
| 20 | 8.83 ± 3.20 |
| 21 | 10.31 ± 0.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-Y.; Hung, Y.-L.; Tang, K.-W.; Wang, S.-C.; Tseng, C.-H.; Tzeng, C.-C.; Liu, P.-L.; Li, C.-Y.; Chen, Y.-L. Discovery of 2-Substituted 3-Arylquinoline Derivatives as Potential Anti-Inflammatory Agents Through Inhibition of LPS-Induced Inflammatory Responses in Macrophages. Molecules 2019, 24, 1162. https://doi.org/10.3390/molecules24061162
Yang C-Y, Hung Y-L, Tang K-W, Wang S-C, Tseng C-H, Tzeng C-C, Liu P-L, Li C-Y, Chen Y-L. Discovery of 2-Substituted 3-Arylquinoline Derivatives as Potential Anti-Inflammatory Agents Through Inhibition of LPS-Induced Inflammatory Responses in Macrophages. Molecules. 2019; 24(6):1162. https://doi.org/10.3390/molecules24061162
Chicago/Turabian StyleYang, Cheng-Yao, Yung-Li Hung, Kai-Wei Tang, Shu-Chi Wang, Chih-Hua Tseng, Cherng-Chyi Tzeng, Po-Len Liu, Chia-Yang Li, and Yeh-Long Chen. 2019. "Discovery of 2-Substituted 3-Arylquinoline Derivatives as Potential Anti-Inflammatory Agents Through Inhibition of LPS-Induced Inflammatory Responses in Macrophages" Molecules 24, no. 6: 1162. https://doi.org/10.3390/molecules24061162
APA StyleYang, C.-Y., Hung, Y.-L., Tang, K.-W., Wang, S.-C., Tseng, C.-H., Tzeng, C.-C., Liu, P.-L., Li, C.-Y., & Chen, Y.-L. (2019). Discovery of 2-Substituted 3-Arylquinoline Derivatives as Potential Anti-Inflammatory Agents Through Inhibition of LPS-Induced Inflammatory Responses in Macrophages. Molecules, 24(6), 1162. https://doi.org/10.3390/molecules24061162





