Evaluation of the Anti-Trypanosomal Activity of Vietnamese Essential Oils, with Emphasis on Curcuma longa L. and Its Components
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. PLANTS Collection and Essential Oils Extraction
4.3. Parasites, Cells, and Media
4.4. Anti-Trypanosomal Assay
4.5. Cytotoxicity Assay
4.6. Essential Oils Analysis
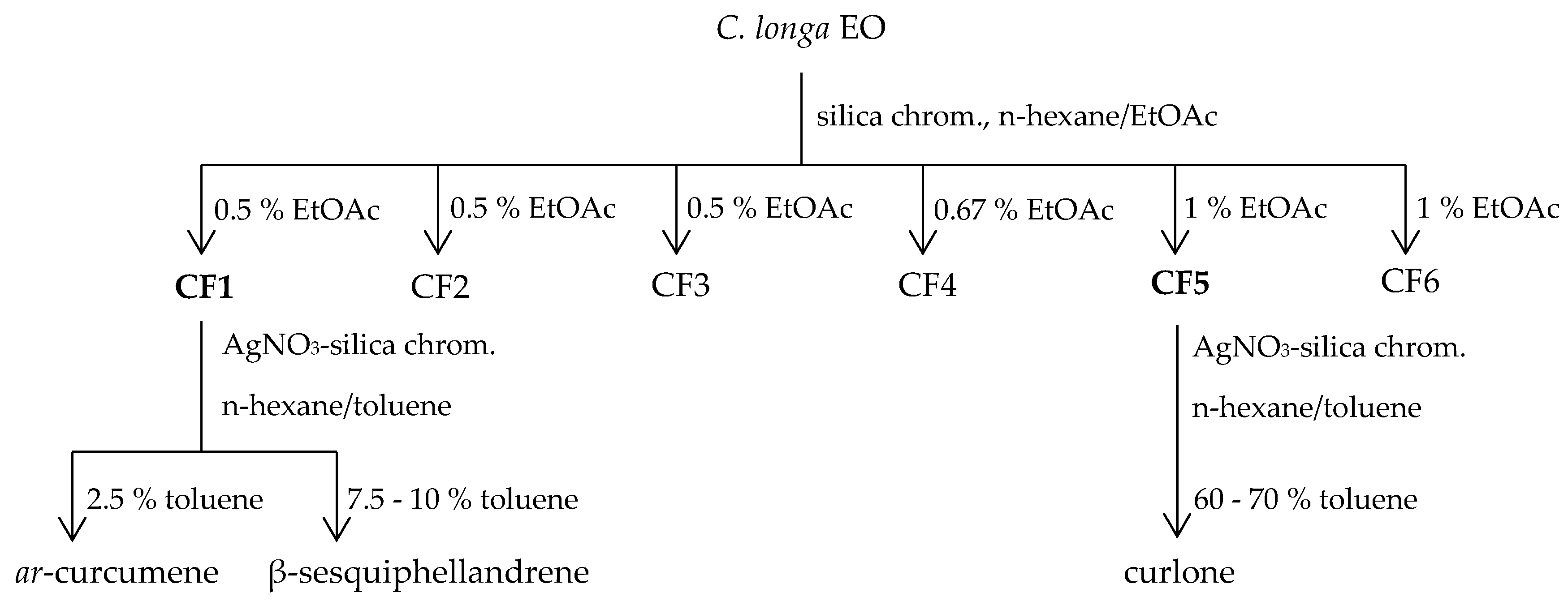
4.7. Components Isolation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Parasites—Sleeping Sickness—Epidemiology & Risk Factors; CDC: Atlanta, GA, USA. Available online: https://www.cdc.gov/parasites/sleepingsickness/epi.html (accessed on 23 January 2019).
- Drugs for Neglected Diseases initiative (DNDi). Diseases & Projects—Sleeping Sickness—Fact Sheet. DNDi: Geneva, Switzerland. Available online: https://www.dndi.org/wp-content/uploads/2018/12/Factsheet2018_HAT.pdf (accessed on 23 January 2019).
- Capewell, P.; Cren-Travaillé, C.; Marchesi, F.; Johnston, P.; Clucas, C.; Benson, R.A.; Gorman, T.A.; Calvo-Alvarez, E.; Crouzols, A.; Jouvion, G.; et al. The skin is a significant but overlooked anatomical reservoir for vector-borne African trypanosomes. Elife 2016, 5, e17716. [Google Scholar] [CrossRef]
- Trindade, S.; Rijo-Ferreira, F.; Carvalho, T.; Pinto-Neves, D.; Guegan, F.; Aresta-Branco, F.; Bento, F.; Young, S.A.; Pinto, A.; Van Den Abbeele, J.; et al. Trypanosoma brucei Parasites Occupy and Functionally Adapt to the Adipose Tissue in Mice. Cell Host Microbe 2016, 19, 837–848. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Human African Trypanosomiasis—The Disease—Symptoms, Diagnosis and Treatment; WHO: Geneva, Switzerland; Available online: http://www.who.int/trypanosomiasis_african/disease/diagnosis/en/ (accessed on 23 January 2019).
- Drugs for Neglected Diseases initiative (DNDi). Diseases & Projects—Portfolio—Fexinidazole (HAT). DNDi: Geneva, Switzerland. Available online: https://www.dndi.org/diseases-projects/portfolio/fexinidazole/ (accessed on 23 January 2019).
- Chiara Cristiano, M.; Cosco, D.; Paolino, D. Technological Aspects of Essential Oils. In Aromatherapy: Basic Mechanisms and Evidence-Based Clinical Use; Bagetta, G., Cosentino, M., Sakurada, T., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 152–153. ISBN 978-1-4822-4663-6. [Google Scholar]
- Heuberger, E. Effects of Essential Oils in the Central Nervous System. In Handbook of Essential Oils: Science, Technology and Applications; Can Baser, K.H., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2010; p. 283. ISBN 978-1-4200-6315-8. [Google Scholar]
- World Health Organization (WHO). Human African Trypanosomiasis—African Trypanosomiasis—Drugs; WHO: Geneva, Switzerland; Available online: http://www.who.int/trypanosomiasis_african/drugs/en/ (accessed on 23 January 2019).
- Le, T.B.; Beaufay, C.; Bonneau, N.; Mingeot-Leclercq, M.-P.; Quetin-Leclercq, J. Anti-protozoal activity of essential oils and their constituents against Leishmania, Plasmodium and Trypanosoma. Phytochimie 2018, 1, 1–33. [Google Scholar]
- Montoro, P.; Masullo, M.; Piacente, S.; Pizza, C. Extraction, Sample Preparation, and Analytical Methods for Quality Issues of Essential Oils. In Aromatherapy: Basic Mechanisms and Evidence-Based Clinical Use; Bagetta, G., Cosentino, M., Sakurada, T., Eds.; CRC Press: Boca Raton, FL, USA, 2016; p. 153. ISBN 978-1-4822-4663-6. [Google Scholar]
- Le, T.B.; Beaufay, C.; Nghiem, D.T.; Mingeot-Leclercq, M.-P.; Quetin-Leclercq, J. In vitro anti-leishmanial activity of essential oils extracted from Vietnamese plants. Molecules 2017, 22, 1071. [Google Scholar] [CrossRef] [PubMed]
- Bero, J.; Kpoviessi, S.; Quetin-Leclercq, J. Anti-Parasitic Activity of Essential Oils and their Constituents against Plasmodium, Trypanosoma and Leishmania. In Novel Plant Bioresource: Applications in Food, Medicine and Cosmetic; Gurib-Fakim, A., Ed.; John Wiley & Sons: Oxford, UK, 2014; pp. 455–469. ISBN 978-1-118-46061-0. [Google Scholar]
- Behar, R.Z.; Davis, B.; Wang, Y.; Bahl, V.; Lin, S.; Talbot, P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. In Vitro 2014, 28, 198–208. [Google Scholar] [CrossRef]
- Hérent, M.F.; De Bie, V.; Tilquin, B. Determination of new retention indices for quick identification of essential oils compounds. J. Pharm. Biomed. Anal. 2007, 43, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Monzote, L.; García, M.; Pastor, J.; Gil, L.; Scull, R.; Maes, L.; Cos, P.; Gille, L. Essential oil from Chenopodium ambrosioides and main components: Activity against Leishmania, their mitochondria and other microorganisms. Exp. Parasitol. 2014, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Cheikh-Ali, Z.; Adiko, M.; Bouttier, S.; Bories, C.; Okpekon, T.; Poupon, E.; Champy, P. Composition, and antimicrobial and remarkable antiprotozoal activities of the essential oil of rhizomes of Aframomum sceptrum K. Schum. (Zingiberaceae). Chem. Biodivers. 2011, 8, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Kpadonou Kpoviessi, B.G.H.; Kpoviessi, S.D.S.; Yayi Ladekan, E.; Gbaguidi, F.; Frédérich, M.; Moudachirou, M.; Quetin-Leclercq, J.; Accrombessi, G.C.; Bero, J. In vitro antitrypanosomal and antiplasmodial activities of crude extracts and essential oils of Ocimum gratissimum Linn from Benin and influence of vegetative stage. J. Ethnopharmacol. 2014, 155, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Si, L.; Chen, Y.; Han, X.; Zhan, Z.; Tian, S.; Cui, Q.; Wang, Y. Chemical composition of essential oils of Litsea cubeba harvested from its distribution areas in China. Molecules 2012, 17, 7057–7066. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y. Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.W.; Feng, Y.C.; Huang, Y.; Li, H.L. Potential cosmetic application of essential oil extracted from Litsea cubeba fruits from China. J. Essent. Oil Res. 2013, 25, 112–119. [Google Scholar] [CrossRef]
- Liu, T.T.; Yang, T.S. Antimicrobial impact of the components of essential oil of Litsea cubeba from Taiwan and antimicrobial activity of the oil in food systems. Int. J. Food Microbiol. 2012, 156, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Chang, W.T.; Hseu, Y.C.; Chen, H.Y.; Chuang, C.H.; Lin, C.C.; Lee, M.S.; Lin, M.K. Immunosuppressive effect of Litsea cubeba L. essential oil on dendritic cell and contact hypersensitivity responses. Int. J. Mol. Sci. 2016, 17, 1319. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.S.; Liou, M.L.; Hu, T.F.; Peng, C.W.; Liu, T.T. Antimicrobial activity of the essential oil of Litsea cubeba on cariogenic bacteria. J. Essent. Oil Res. 2013, 25, 120–128. [Google Scholar] [CrossRef]
- Li, Y.; Kong, W.; Li, M.; Liu, H.; Zhao, X.; Yang, S.; Yang, M. Litsea cubeba essential oil as the potential natural fumigant: Inhibition of Aspergillus flavus and AFB1 production in licorice. Ind. Crops Prod. 2016, 80, 186–193. [Google Scholar] [CrossRef]
- Saikia, A.K.; Chetia, D.; Darrigo, M.; Smeriglio, A.; Strano, T.; Ruberto, G. Screening of fruit and leaf essential oils of Litsea cubeba Pers. from north-east India—Chemical composition and antimicrobial activity. J. Essent. Oil Res. 2013, 25, 330–338. [Google Scholar] [CrossRef]
- Kpoviessi, S.; Bero, J.; Agbani, P.; Gbaguidi, F.; Kpadonou-Kpoviessi, B.; Sinsin, B.; Accrombessi, G.; Frédérich, M.; Moudachirou, M.; Quetin-Leclercq, J. Chemical composition, cytotoxicity and in vitro antitrypanosomal and antiplasmodial activity of the essential oils of four Cymbopogon species from Benin. J. Ethnopharmacol. 2014, 151, 652–659. [Google Scholar] [CrossRef]
- Petrelli, R.; Orsomando, G.; Sorci, L.; Maggi, F.; Ranjbarian, F.; Biapa Nya, P.C.; Petrelli, D.; Vitali, L.A.; Lupidi, G.; Quassinti, L.; et al. Biological activities of the essential oil from Erigeron floribundus. Molecules 2016, 21, 1065. [Google Scholar] [CrossRef] [PubMed]
- Hoet, S.; Stévigny, C.; Hérent, M.-F.; Quetin-Leclercq, J. Antitrypanosomal Compounds from the Leaf Essential Oil of Strychnos spinosa. Planta Med. 2006, 72, 480–482. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of Essential Oils of Curcuma Species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, R.; Ranjbarian, F.; Dall’Acqua, S.; Papa, F.; Iannarelli, R.; Ngahang Kamte, S.L.; Vittori, S.; Benelli, G.; Maggi, F.; Hofer, A.; et al. An overlooked horticultural crop, Smyrnium olusatrum, as a potential source of compounds effective against African trypanosomiasis. Parasitol. Int. 2017, 66, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto-Ribeiro, M.M.G.; Grespan, R.; Kohiyama, C.Y.; Ferreira, F.D.; Mossini, S.A.G.; Silva, E.L.; De Abreu Filho, B.A.; Mikcha, J.M.G.; Machinski Junior, M. Effect of Zingiber officinale essential oil on Fusarium verticillioides and fumonisin production. Food Chem. 2013, 141, 3147–3152. [Google Scholar] [CrossRef] [PubMed]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.B.; Lobaccaro, J.M.A.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- El-Ghorab, A.H.; Nauman, M.; Anjum, F.M.; Hussain, S.; Nadeem, M. A comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J. Agric. Food Chem. 2010, 58, 8231–8237. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.P.; Salgueiro, L.; Gonçalves, M.J.; da Cunha, A.P.; Vila, R.; Cañigueral, S.; Mazzoni, V.; Tomi, F.; Casanova, J. Essential oil composition and antimicrobial activity of three Zingiberaceae from S Tomé e Príncipe. Planta Med. 2001, 67, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, H.; Smith, M.K.; Brooks, L.O.; Myers, S.P.; Leach, D.N. Essential oil composition of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe) grown in Australia. J. Agric. Food Chem. 2006, 54, 1414–1419. [Google Scholar] [CrossRef]
- Singh, G.; Kapoor, I.P.S.; Singh, P.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem. Toxicol. 2008, 46, 3295–3302. [Google Scholar] [CrossRef] [PubMed]
- Buddhakala, N.; Talubmook, C.; Sriyotha, P.; Wray, S.; Kupittayanant, S. Inhibitory effects of ginger oil on spontaneous and PGF2α- induced contraction of rat myometrium. Planta Med. 2008, 74, 385–391. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Sauvain, M.; Deharo, E. Curcuma as a parasiticidal agent: A review. Planta Med. 2011, 77, 672–678. [Google Scholar] [CrossRef]
- Sun, Y.N.; No, J.H.; Lee, G.Y.; Li, W.; Yang, S.Y.; Yang, G.; Schmidt, T.J.; Kang, J.S.; Kim, Y.H. Phenolic constituents of medicinal plants with activity against Trypanosoma brucei. Molecules 2016, 21, 480. [Google Scholar] [CrossRef]
- Lee, Y. Cytotoxicity Evaluation of Essential Oil and its Component from Zingiber officinale Roscoe. Toxicol. Res. 2016, 32, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Activation of apoptotic protein in U937 cells by a component of turmeric oil. BMB Rep. 2009, 42, 96–100. [Google Scholar] [CrossRef]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Essential Oils Exploited in Cytotoxicity Studies for Translation into Safer and More Effective Cancer Therapeutics. In Aromatherapy: Basic Mechanisms and Evidence-Based Clinical Use; Bagetta, G., Cosentino, M., Sakurada, T., Eds.; CRC Press: Boca Raton, FL, USA, 2016; p. 170. ISBN 978-1-4822-4663-6. [Google Scholar]
- Andrade-Ochoa, S.; Sánchez-Aldana, D.; Chacón-Vargas, K.F.; Rivera-Chavira, B.E.; Sánchez-Torres, L.E.; Camacho, A.D.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Oviposition Deterrent and Larvicidal and Pupaecidal Activity of Seven Essential Oils and their Major Components against Culex quinquefasciatus Say (Diptera: Culicidae): Synergism-antagonism Effects. Insects 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Ngahang Kamte, S.L.; Ranjbarian, F.; Cianfaglione, K.; Sut, S.; Dall’Acqua, S.; Bruno, M.; Afshar, F.H.; Iannarelli, R.; Benelli, G.; Cappellacci, L.; et al. Identification of highly effective antitrypanosomal compounds in essential oils from the Apiaceae family. Ecotoxicol. Environ. Saf. 2018, 156, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Sliwowski, J.K.; Caspi, E. An improved method of preparation of plates and sheets for thin-layer argentation chromatography. J. Steroid Biochem. 1977, 8, 42–49. [Google Scholar] [CrossRef]
- Sykes, M.L.; Avery, V.M. Development of an Alamar Blue™ Viability Assay in 384-Well Format for High Throughput Whole Cell Screening of Trypanosoma brucei brucei Bloodstream Form Strain 427. Am. J. Trop. Med. Hyg. 2009, 81, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Ioset, L.-R.; Brun, R.; Wenzler, T.; Kaiser, M.; Yardley, V. Drug Screening for Kinetoplastids Diseases, a Training Manual for Screening in Neglected Diseases; The Pan-Asian Screening Network; DNDi: Geneva, Switzerland, 2009; pp. 20–21. [Google Scholar]
- Hirumi, H.; Himuri, K. Axenic culture of African trypanosome bloodstream forms. Parasitol. Today 1994, 10, 80–84. [Google Scholar] [CrossRef]
- Koch, A.; Basar, S.; Richter, R. TLC of Mono- and Sesquiterpenes. In Thin Layer Chromatography in Phytochemistry; Waksmundzka-Hajnos, M., Sherma, J., Kowalska, T., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 459–461. ISBN 978-1-4200-4677-9. [Google Scholar]
- Williams, C.M.; Mander, L.N. Chromatography with silver nitrate. Tetrahedron 2001, 57, 425–447. [Google Scholar] [CrossRef]
- Morris, L.J. Separations of lipids by silver ion chromatography. J. Lipid Res. 1966, 7, 717–732. [Google Scholar] [PubMed]
- Denyer, C.V.; Jackson, P.; Loakes, D.M.; Ellis, M.R.; Young, D.A.B. Isolation of antirhinoviral sesquiterpenes from ginger (Zingiber officinale). J. Nat. Prod. 1994, 57, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Harrison, L.J.; Tan, B.C. Terpenoids from the liverwort Chandonanthus hirtellus. Tetrahedron 2009, 65, 4035–4043. [Google Scholar] [CrossRef]
- Fujiwaraj, M.; Yagi, N.; Miyazawa, M. Acetylcholinesterase inhibitory activity of volatile oil from Peltophorum dasyrachis Kurz ex Bakar (Yellow Batai) and bisabolane-type sesquiterpenoids. J. Agric. Food Chem. 2010, 58, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Laguardia, M.A.; Rideout, J.A. Antimicrobial sesquiterpenoids and diarylheptanoid from Curcuma domestica. ACGC Chem. Res. Commun. 2005, 18, 21–24. [Google Scholar]
Sample Availability: Samples of essential oils are available from the authors. |

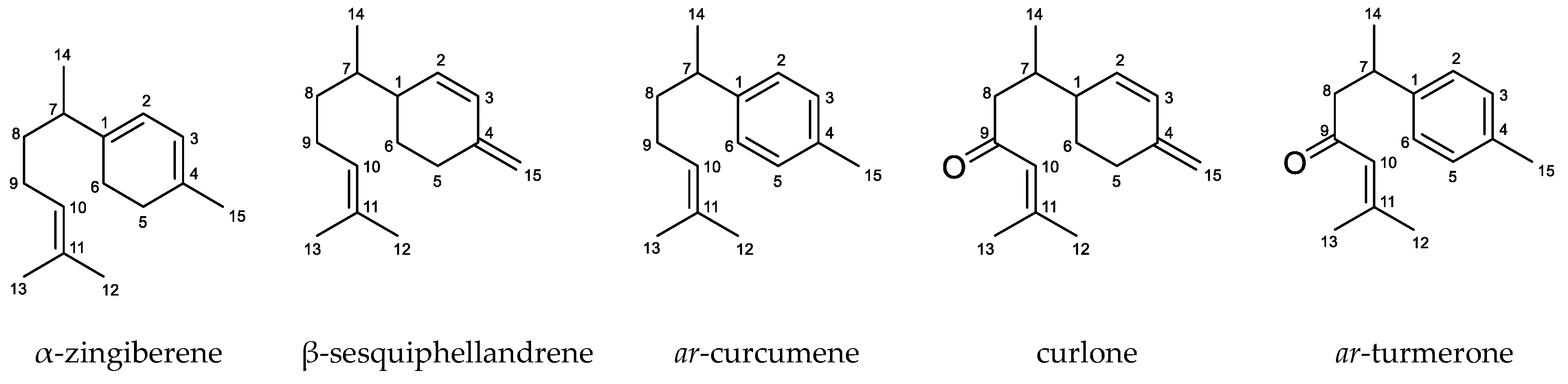
| Plant Species (Studied Parts) | Anti-Trypanosomal Activity (IC50 nL/mL) | Cytotoxicity (IC50 nL/mL) | |||
|---|---|---|---|---|---|
| WI38 | SI | J774 | SI | ||
| Amomum aromaticum (fruits) | 8.75 ± 1.25 | 47.31 ± 0.30 | 5.4 | 22.68 ± 3.22 | 2.6 |
| Artemisia annua (leaves) | 8.99 ± 1.18 | 45.64 ± 1.02 | 5.1 | 38.16 ± 0.21 | 4.2 |
| Cinnamomum cassia (stem barks) | 1.77 ± 0.15 | 11.97 ± 0.93 | 6.8 | 8.97 ± 0.66 | 5.1 |
| Clausena indica (leaves) | 13.22 ± 4.54 | >50.00 | >3.8 | >50.00 | >3.8 |
| Curcuma longa (rhizomes) | 3.17 ± 0.72 | 46.00 ± 0.33 | 14.5 | 44.11 ± 3.13 | 13.9 |
| Curcuma zedoaria (rhizomes) | 2.51 ± 1.08 | 46.64 ± 0.95 | 18.6 | 26.81 ± 1.59 | 10.7 |
| Dysphania ambrosioides (aerial parts) | 2.86 ± 0.32 | >50.00 | >17.5 | 12.29 ± 2.92 | 4.3 |
| Elsholtzia blanda (leaves) | 8.23 ± 1.03 | >50.00 | >6.1 | >50.00 | >6.1 |
| Elsholtzia ciliata (leaves) | 4.26 ± 0.86 | 48.46 ± 0.12 | 11.4 | 13.21 ± 1.48 | 3.1 |
| Elsholtzia communis (leaves) | 18.39 ± 3.32 | >50.00 | >2.7 | 40.68 ± 3.44 | 2.2 |
| Hedychium coronarium (rhizomes) | 9.73 ± 1.43 | >50.00 | >5.1 | 30.00 ± 4.06 | 3.1 |
| Kaempferia galangal (rhizomes) | 15.78 ± 3.29 | >50.00 | >3.2 | >50.00 | >3.2 |
| Litsea cubeba (fruits) | 2.67 ± 1.12 | >50.00 | >18.7 | >50.00 | >18.7 |
| Litsea cubeba (leaves) | 16.47 ± 1.24 | >50.00 | >3.0 | >50.00 | >3.0 |
| Pluchea indica (leaves) | 21.29 ± 1.38 | 27.47 ± 1.49 | 1.3 | 25.05 ± 5.56 | 1.2 |
| Pogostemon cablin (leaves) | 4.07 ± 0.98 | 27.17 ± 3.62 | 6.7 | 28.40 ± 1.81 | 7.0 |
| Vitex trifolia (leaves) | 3.24 ± 0.79 | 31.12 ± 2.83 | 9.6 | 26.64 ± 0.76 | 8.2 |
| Zingiber officinale (rhizomes) | 3.10 ± 0.08 | >50.00 | >16.1 | 37.52 ± 0.05 | 12.1 |
| Zingiber zerumbet (rhizomes) | 6.23 ± 0.73 | 3.65 ± 0.34 | 0.6 | 2.78 ± 0.57 | 0.5 |
| Suramin | 21.53 ± 2.62a | ||||
| Camptothecin | 34.99 ± 9.63 a | 7.32 ± 1.29 a | |||
| No. | Compounds | RI | Relative Percentage (%) | Identification | |||
|---|---|---|---|---|---|---|---|
| L. cubeba | C. zedoaria | Z. officinale | C. longa | ||||
| 1 | α-Pinene m | 536 | 0.74 | 0.11 | 2.29 | - | MS, Co-GC, Ref. |
| 2 | α-Thujene m | 540 | 0.18 | - | - | - | MS, Ref. |
| 3 | Camphene m | 577 | - | 0.26 | 6.94 | - | MS, Ref. |
| 4 | β-Pinene m | 621 | 0.86 | 0.77 | 0.16 | 0.09 | MS, Co-GC, Ref. |
| 5 | Sabinene m | 635 | 0.83 | - | 0.19 | - | MS, Co-GC, Ref. |
| 6 | 3-Carene m | 665 | 0.36 | - | - | - | MS |
| 7 | α-Phellandrene m | 679 | - | - | 0.70 | 0.08 | MS, Co- |
| 8 | Myrcene m | 681 | 1.25 | - | 1.10 | t | MS, Co-GC, Ref. |
| 9 | α-Terpinene m | 697 | 0.51 | - | - | t | MS, Co-GC, Ref. |
| 10 | Limonene m | 714 | 8.72 | 0.18 | 2.06 | 0.19 | MS, Co-GC, Ref. |
| 11 | β-Phellandrene m | 727 | 0.16 | - | 14.78 | - | MS |
| 12 | Eucalyptol m | 727 | 1.37 | 1.61 | 1.79 | 3.15 | MS, Co-GC, Ref. |
| 13 | γ-Terpinene m | 761 | 0.52 | - | t | t | MS, Co-GC, Ref. |
| 14 | p-Cymeme m | 783 | 0.10 | t | t | t | MS, Co-GC, Ref. |
| 15 | Terpinolene m | 798 | 0.31 | - | 0.26 | 1.70 | MS, Co-GC, Ref. |
| 16 | 2-Heptanol | 844 | - | 0.14 | 0.15 | t | MS |
| 17 | 5-Hepten-2-one, 6-methyl- | 854 | 0.35 | - | t | - | MS, Co-GC |
| 18 | 5-Heptenal, 2,6-dimethyl | 867 | 0.68 | - | - | - | MS |
| 19 | 2-Nonanone | 903 | - | 0.43 | 0.15 | t | MS |
| 20 | (E)-2-Octenal | 940 | - | - | t | - | MS |
| 21 | 2-Octanol | 941 | - | t | - | - | MS |
| 22 | p-Cymenene m | 946 | - | - | - | t | MS |
| 23 | 1-Octen-3-ol | 969 | - | t | - | - | MS, Ref. |
| 24 | δ-Elemene s | 980 | - | 0.30 | t | t | MS |
| 25 | Cyclosativene s | 986 | - | - | t | - | MS |
| 26 | Citronellal m | 993 | 43.10 | - | 0.30 | 0.14 | MS, Co-GC, Ref. |
| 27 | α-Copaene s | 999 | - | - | 0.31 | - | MS |
| 28 | Decanone | 1006 | - | t | - | - | MS |
| 29 | Camphor m | 1020 | - | 4.18 | t | - | MS, Co-GC, Ref. |
| 30 | 2-Nonanol | 1038 | - | 2.16 | 0.19 | 0.21 | MS |
| 31 | Linalool m | 1063 | 5.60 | 0.22 | 0.48 | t | MS, Co-GC, Ref. |
| 32 | cis-α-Bergamotene s | 1065 | - | - | t | 0.27 | MS |
| 33 | Pulegol m | 1072 | 6.52 | - | - | - | MS |
| 34 | Isopulegol m | 1082 | 11.10 | - | - | - | MS, Ref. |
| 35 | trans-α-Bergamotene s | 1091 | - | - | - | 0.12 | MS, Ref. |
| 36 | β-Elemene s | 1096 | - | 4.85 | 0.34 | 0.22 | MS |
| 37 | β-Caryophyllene s | 1100 | - | 3.79 | 0.43 | t | MS, Co-GC, Ref. |
| 38 | 2-Undecanone | 1106 | - | - | 0.39 | t | MS |
| 39 | Terpinene-4-ol m | 1109 | 2.58 | 0.31 | 0.22 | 0.13 | MS, Co-GC, Ref. |
| 40 | γ-Elemene s | 1142 | - | 0.32 | - | 0.09 | MS |
| 41 | α-Himachalene s | 1153 | - | - | - | t | MS |
| 42 | γ-Gurjunene s | 1160 | - | - | - | t | MS |
| 43 | α-Humulene s | 1168 | - | 1.28 | - | t | MS, Co-GC, Ref. |
| 44 | (E)-β-Farnesene s | 1174 | - | - | 0.26 | 0.61 | MS |
| 45 | Neral m | 1186 | - | - | 3.16 | - | MS, Co-GC |
| 46 | α-Terpineol m | 1203 | 0.62 | 0.23 | 1.78 | 0.48 | MS, Co-GC, Ref. |
| 47 | Borneol m | 1208 | - | - | 1.35 | - | MS, Co-GC, Ref. |
| 48 | Germacrene D s | 1206 | - | 1.99 | - | t | MS, Ref. |
| 49 | α-Muurolene s | 1217 | - | - | 1.44 | - | MS |
| 50 | β-Selinene s | 1218 | - | 1.76 | - | - | MS |
| 51 | β-Chamigrene s | 1223 | - | 1.47 | - | - | MS |
| 52 | α-Zingiberene s | 1236 | - | - | 27.71 | 25.38 | MS, Co-GC |
| 53 | β-Bisabolene s | 1238 | - | - | 7.27 | 3.38 | MS, Ref. |
| 54 | α-Cubebene s | 1248 | - | - | 0.23 | - | MS |
| 55 | (E,E)-α-Farnesene s | 1259 | - | - | 3.71 | 0.36 | MS |
| 56 | Citronellol m | 1274 | 5.17 | - | - | - | MS, Co-GC, Ref. |
| 57 | β-Sesquiphellandrene s | 1279 | - | - | 8.08 | 18.27 | MS |
| 58 | ar-Curcumene s | 1280 | - | - | 2.71 | 5.22 | MS |
| 59 | ζ-Elemene s | 1323 | - | 2.47 | 0.55 | - | MS |
| 60 | Geraniol m | 1351 | - | - | 0.45 | 0.25 | MS, Co-GC, Ref. |
| 61 | Curzerene s | 1366 | - | 4.87 | - | - | MS |
| 62 | Epiglobulol s | 1468 | - | 0.57 | - | - | MS |
| 63 | (E)-Nerolidol s | 1536 | - | - | 0.32 | - | MS, Co-GC, Ref. |
| 64 | Elemol s | 1569 | - | - | 0.18 | - | MS |
| 65 | Ledene oxide s | 1583 | - | 0.25 | - | - | MS |
| 66 | Bisabolone s | 1595 | - | - | - | 1.08 | MS |
| 67 | Spathulenol s | 1607 | 0.21 | - | - | - | MS |
| 68 | ar-Turmerol s | 1658 | - | - | - | 1.60 | MS |
| 69 | α-Turmerone s | 1667 | - | - | - | 10.28 | MS |
| 70 | Bisabolol s | 1670 | - | - | - | 0.87 | MS |
| 71 | 8,9-Dehydro-9-formyl cycloisolongifolene s | 1703 | - | 29.31 | - | - | MS |
| 72 | Germacrone s | 1710 | - | 8.95 | - | 3.34 | MS |
| 73 | Curlone s | 1723 | - | - | - | 5.15 | MS |
| 74 | ar-Turmerone s | 1739 | - | - | - | 9.93 | MS, Co-GC |
| 75 | Curdione s | 1792 | - | 13.52 | - | - | MS |
| 76 | Farnesol s | 1838 | - | - | 0.20 | - | MS, Ref. |
| Total identified | 91.84 | 86.30 | 92.63 | 92.59 | |||
| Compounds | Anti-Trypanosomal Activity (IC50 µg/mL) | Cytotoxicity (IC50 µg/mL) | |||
|---|---|---|---|---|---|
| WI38 | SI | J774 | SI | ||
| α-Zingiberene | 6.91 ± 2.60 | 28.50 ± 1.43 | 4.1 | 29.64 ± 2.54 | 4.3 |
| β-Sesquiphellandrene | 9.89 ± 1.18 | 19.11 ± 1.58 | 1.9 | 21.02 ± 2.72 | 2.1 |
| ar-Curcumene | 13.38 ± 2.46 | 23.15 ± 1.36 | 1.7 | 24.03 ± 2.64 | 1.8 |
| Curlone | 1.38 ± 0.52 | 43.64 ± 2.45 | 31.7 | 25.06 ± 3.47 | 18.2 |
| ar-Turmerone | 28.83 ± 3.93 | 43.39 ± 3.89 | 1.5 | 44.62 ± 1.41 | 1.6 |
| Suramin | 21.53 ± 2.62 a | ||||
| Camptothecin | 34.99 ± 9.63 a | 7.32 ± 1.29 a | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.B.; Beaufay, C.; Nghiem, D.T.; Pham, T.A.; Mingeot-Leclercq, M.-P.; Quetin-Leclercq, J. Evaluation of the Anti-Trypanosomal Activity of Vietnamese Essential Oils, with Emphasis on Curcuma longa L. and Its Components. Molecules 2019, 24, 1158. https://doi.org/10.3390/molecules24061158
Le TB, Beaufay C, Nghiem DT, Pham TA, Mingeot-Leclercq M-P, Quetin-Leclercq J. Evaluation of the Anti-Trypanosomal Activity of Vietnamese Essential Oils, with Emphasis on Curcuma longa L. and Its Components. Molecules. 2019; 24(6):1158. https://doi.org/10.3390/molecules24061158
Chicago/Turabian StyleLe, Thanh Binh, Claire Beaufay, Duc Trong Nghiem, Tuan Anh Pham, Marie-Paule Mingeot-Leclercq, and Joëlle Quetin-Leclercq. 2019. "Evaluation of the Anti-Trypanosomal Activity of Vietnamese Essential Oils, with Emphasis on Curcuma longa L. and Its Components" Molecules 24, no. 6: 1158. https://doi.org/10.3390/molecules24061158
APA StyleLe, T. B., Beaufay, C., Nghiem, D. T., Pham, T. A., Mingeot-Leclercq, M.-P., & Quetin-Leclercq, J. (2019). Evaluation of the Anti-Trypanosomal Activity of Vietnamese Essential Oils, with Emphasis on Curcuma longa L. and Its Components. Molecules, 24(6), 1158. https://doi.org/10.3390/molecules24061158






