Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Solidago Canadensis Essential Oil
2.2. In Vitro Antifungal Activity
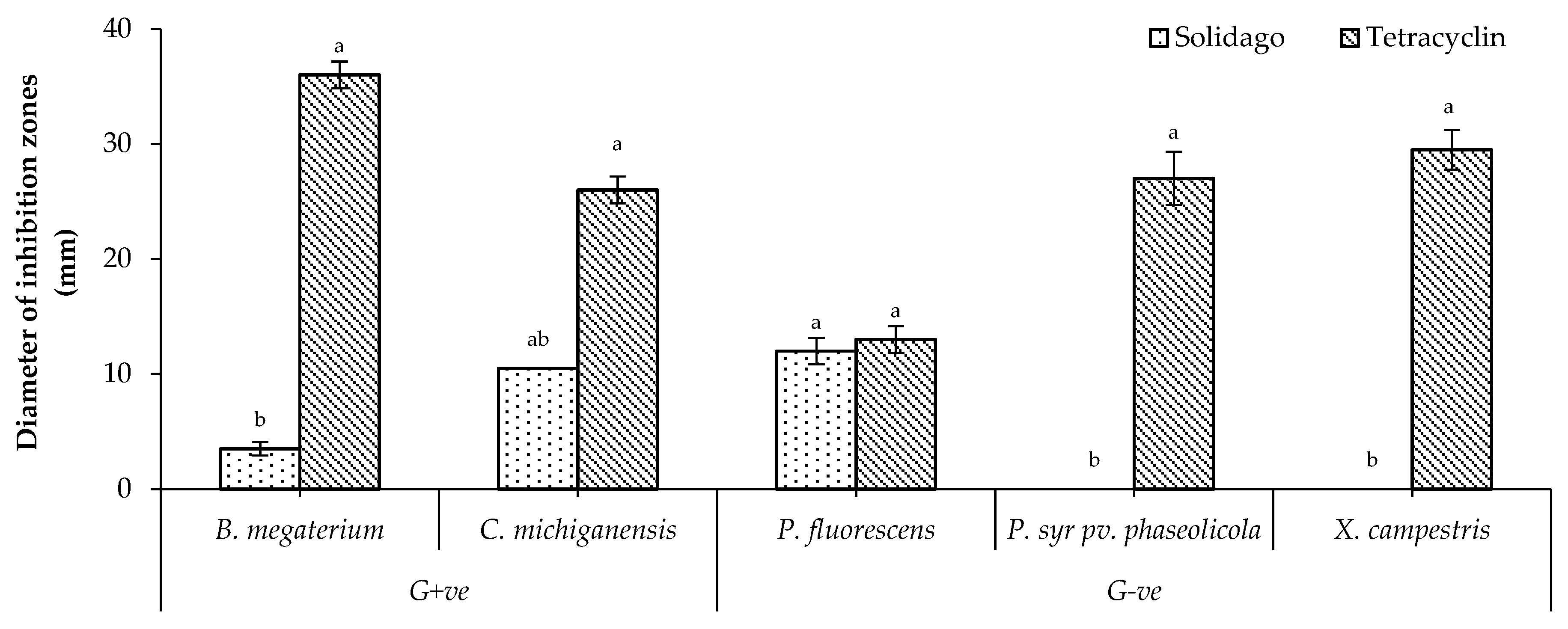
2.3. In Vitro Antibacterial Activity
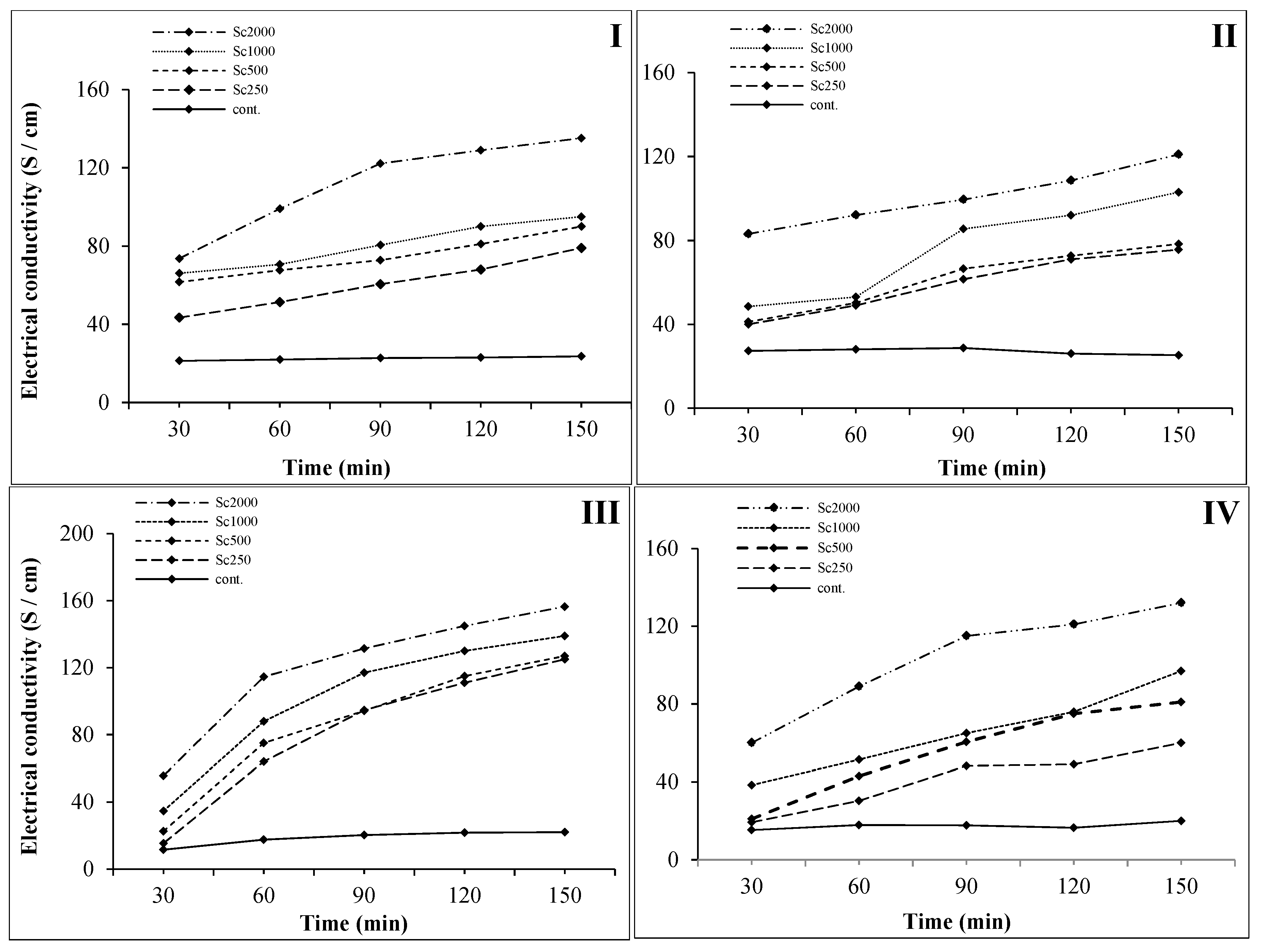
2.4. Cell Membrane Permeability Assay
2.5. Determination of Minimum Inhibitory Concentration (96-Well Microplate Method)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material
5.2. Isolation of Essential Oil
5.3. Gas-Chromatography-Mass Spectrometry Analysis of Essential Oil
5.4. Antifungal Activity
5.4.1. Tested fungal isolates
5.4.2. Fungicidal assay
5.5. Antibacterial Activity
5.5.1. Tested Bacterial Strains
5.5.2. Bactericidal Assay
5.6. Cell Membrane Permeability
5.7. Determination of Minimum Inhibitory Concentration (96-Well Microplate Method)
5.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Mancini, E.; Camele, I.; Elshafie, H.S.; De Martino, L.; Pellegrino, C.; Grulova, D.; De Feo, V. Chemical Composition and Biological Activity of the Essential Oil of Origanum vulgare ssp. hirtum from Different Areas in the Southern Apennines (Italy). Chem. Biodivers. 2014, 11, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. Investigating the effects of plant essential oils on post-harvest fruit decay. In Fungal Pathogenicity; INTECH: Rijeka, Croatia, 2016; ISBN 978-953-51-4624-7. [Google Scholar]
- Lopez-Reyes, J.G.; Spadaro, D.; Prelle, A.; Garibaldi, A.; Gullino, M.L. Efficacy of plant essential oils on postharvest control of rots caused by fungi on different stone fruits in vivo. J. Food Protect. 2013, 76, 631–639. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An overview of The Biological Effects of Some Mediterranean Essential Oils on Human Health (Review article). BioMed Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gruľová, D.; Mudrončeková, S.; Zheljazkov, V.D.; Šalamon, I.; Rondon, S.I. Effect of Plant Essential Oils against Rophalosiphum padi on Wheat and Barley. Nat. Prod. Commun. 2017, 12, 1517–1520. [Google Scholar]
- Matoušková, M.; Jurová, J.; Grul’ová, D.; Wajs-Bonikowska, A.; Renčo, M.; Sedlák, V.; Poráčová, J.; Gogaľová, Z.; Kalemba, D. Phytotoxic Effect of Invasive Heracleum Mantegazzianum Essential Oil on Dicot and Monocot Species. Molecules 2019, 24, 425. [Google Scholar] [CrossRef]
- Walck, J.L.; Baskin, J.M.; Baskin, C.C. Relative competitive abilities and growth characteristics of a narrowly endemic and a geographically widespread Solidago species (Asteraceae). Am. J. Bot. 1999, 86, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Šutovská, M.; Capek, P.; Kocmálová, M.; Fraňová, S.; Pawlaczyk, L.; Gancarz, R. Characterization and biological activity of Solidago canadensis complex. Int. J. Biol. Macromol. 2013, 52, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Gruľová, D.; Pľuchtová, M.; Fejér, J.; De Martino, L.; Caputo, L.; Sedlák, V.; De Feo, V. Influence of the six essential oils on invasive Solidago canadensis L. seed germination. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall, H.; Christiansen, C.; Kalemba, D.; Góra, J. Constituents of the Essential Oil of Solidago canadensis (“Goldenrod”) from Poland—A Correction. Planta Med. 1993, 59, 281–282. [Google Scholar] [CrossRef]
- Kalemba, D.; Thiem, B. Constituents of the essential oils of four micropropagated Solidago species. Flavour Fragr. J. 2004, 19, 40–43. [Google Scholar] [CrossRef]
- Mishra, D.; Joshi, S.; Bisht, G.; Pilkhwal, S. Chemical composition and antimicrobial activity of Solidago canadensis Linn. root essential oil. J. Basic Clin. Pharm. 2010, 1, 187–190. [Google Scholar] [PubMed]
- Mishra, D.; Joshi, S.; Sah, S.P.; Bisht, G. Chemical composition, analgesic and antimicrobial activity of Solidago canadensis essential oil from India. J. Pharm. Res. 2011, 4, 63–66. [Google Scholar]
- Huang, B.; Lei, Y.; Qin, L.; Liu, J. Chemical Composition and Cytotoxic Activities of the Essential Oil from the Inflorescences of Solidago canadensis L., an Invasive Weed in Southeastern China. J. Essent. Oil Bear. Plants 2012, 15, 667–671. [Google Scholar] [CrossRef]
- El-Sherei, M.; Khaleel, A.; Motaal, A.A.; Abd-Elbaki, P. Effect of seasonal variation on the composition of the essential oil of Solidago canadensis cultivated in Egypt. J. Essent. Oil Bear. Plants 2014, 17, 891–898. [Google Scholar] [CrossRef]
- Gruľová, D.; Baranová, B.; Ivanova, V.; De Martino, L.; Mancini, E.; De Feo, V. Composition and biological activity of essential oils of Solidago species and the possible impact on their invasions. Allelopath. J. 2016, 39, 129–142. [Google Scholar]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops. J. Pest Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Shelepova, O.; Vinogradova, Y.; Zaitchik, B.; Ruzhitsky, A.; Grygorieva, O.; Brindza, J. Constituents of the essential oil in Solidago canadensis L. from Eurasia. Potravin. Slovak J. Food Sci. 2018, 12, 20–25. [Google Scholar] [CrossRef]
- Apati, P.; Szentmihalyi, K.; Kristo, S.T.; Papp, I.; Vinkeber, P.; Szoke, E.; Kery, A. Herbal remedies of Solidago—Correlation of phytochemical characteristics and antioxidative properties. J. Pharm. Biomed. Anal. 2003, 32, 1045–1053. [Google Scholar] [CrossRef]
- Kołodziej, B.; Kowalski, R.; Kędzia, B. Antibacterial and antimutagenic activity of extracts aboveground parts of three Solidago species: Solidago virgaurea L., Solidago canadensis L. and Solidago gigantea Ait. J. Med. Plant. Res. 2011, 5, 6770–6779. [Google Scholar] [CrossRef]
- Huang, Y.; Bai, Y.; Wang, Y.; Kong, H. Allelopathic effects of the extracts from an invasive species Solidago canadensis L. on Microcystis aeruginosa. Lett. Appl. Microbiol. 2013, 57, 451–458. [Google Scholar] [CrossRef]
- Anzlovar, S.; Koce, J.D. Antibacterial and Antifungal Activity of Aqueous and Organic Extracts from Indigenous and Invasive Species of Goldenrod (Solidago spp.) Grown in Slovenia. Phyton-Ann. Rei Bot. A 2014, 54, 135–147. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, Y.; Padilla-Zakour, O.; Yang, G. Polyphenols, antioxidant and antimicrobial activities of leaf and bark extracts of Solidago canadensis L. Ind. Crop. Prod. 2015, 74, 803–809. [Google Scholar] [CrossRef]
- Liu, S.; Shao, X.; Wei, Y.; Li, Y.; Xu, F.; Wang, H. Solidago canadensis L. essential oil vapor effectively inhibits Botrytis cinerea Growth and preserves postharvest quality of strawberry as a food model system. Fornt. Microbiol. 2016, 7, 1179. [Google Scholar] [CrossRef] [PubMed]
- Paré, M.C.; Legault, J.; Pichette, A.; Tremblay, C.; Aubut, M.-F. Canadian goldenrod residues and extracts inhibit the growth of Streptomyces scabies, the causal agent of potato common scab. Can. J. Plant Pathol. 2017, 40, 70–75. [Google Scholar] [CrossRef]
- Sakr, S.H.; Elshafie, H.S.; Camele, I.; Sadeek, S.A. Synthesis, Spectroscopic, and Biological Studies of Mixed Ligand Complexes of Gemifloxacin and Glycine with Zn(II), Sn(II), and Ce(III). Molecules 2018, 23, 1182. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured: Carol Stream, IL, USA, 2007. [Google Scholar]
- Kalemba, D.; Góra, J.; Kurowska, A. Analysis of the essential oil of Solidago canadensis. Planta Med. 1990, 56, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Gruľová, D.; De Martino, L.; Mancini, E.; Salamon, I.; De Feo, V. Seasonal variability of the main components in essential oil of Mentha piperita L. J. Sci. Food Agric. 2015, 95, 621–627. [Google Scholar] [CrossRef]
- Kasalia, A.A.; Ekundayo, O.; Paul, C.; König, W.A. epi-Cubebanes from Solidago canadensis. Phytochemistry 2002, 59, 805–810. [Google Scholar] [CrossRef]
- Thiem, B.; Wesolowska, M. Phenolic compounds in two Solidago, L. species from in vitro culture. Acta Pol. Pharm. 2001, 58, 277–281. [Google Scholar]
- Deepa, N.; Velayutham, R. Antimicrobial activity of extractives of Solidago canadensis L. Int. J. Res. Pharm. Sci. 2010, 1, 411–413. [Google Scholar]
- Yue, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef]
- Cvachová, A.; Gojdičová, E. Regulation for Invasive Plant Species Removal; SOPSR, COPK: Banská Bystrica, Slovakia, 2003; p. 37. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Soylu, E.M.; Kurt, S.; Soylu, S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Mancini, E.; Camele, I.; De Martino, L.; De Feo, V. In vivo antifungal activity of two essential oils from Mediterranean plants against postharvest brown rot disease of peach fruit. Ind. Crop. Prod. 2015, 66, 11–15. [Google Scholar] [CrossRef]
- Zygadlo, J.A.; Guzman, C.A.; Grosso, N.R. Antifungal properties of the leaf oils of Tagetes minuta L. and Tagetes filifolia Lag. J. Essent. Oil Res. 1994, 6, 617–621. [Google Scholar] [CrossRef]
- Bhunia, M.C.; Johnson, B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidolactici. J. Appl. Bacteriol. 1988, 8, 261–268. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Ghanney, N.; Mang, S.M.; Ferchichi, A.; Camele, I. An in vitro attempt for controlling severe phyto and human pathogens using essential oils from Mediterranean plants of genus Schinus. J. Med. Food. 2016, 19, 166–173. [Google Scholar] [CrossRef]
- King, E.O.; Ward, M.K.; Raney, D.E. Two simple media for demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 1954, 44, 301–307. [Google Scholar] [PubMed]
- MacFaddin, J.F. Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria; Williams and Wilkins: London, UK, 1985; Volume 1, pp. 634–636. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Mancini, E.; De Martino, L.; Pellegrino, C.; Camele, I.; De Feo, V. Antifungal activity of some constituents of Origanum vulgare L. essential oil against postharvest disease of peach fruit. J. Med. Food 2015, 18, 929–934. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Sakr, S.H.; Sadeek, S.A.; Camele, I. Biological investigations and spectroscopic studies of new Moxifloxacin/Glycine-Metal complexes. Chem. Biodivers. 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]

| No. | Compound Name | [%] | Ki | Ki lit. | Identif. |
|---|---|---|---|---|---|
| 1. | α-Pinene | 11.6 | 922 | 936 | Ki; S; MS |
| 2. | Camphene | 1.0 | 943 | 950 | Ki; MS |
| 3. | Sabinene | 3.9 | 964 | 973 | Ki; MS |
| 4. | β-Pinene | 3.1 | 972 | 978 | Ki; S; MS |
| 5. | α -Phellandrene | 0.3 | 997 | 1002 | Ki; MS |
| 6. | m-Cymene | 0.1 | 1013 | 1013 | Ki; MS |
| 7. | Limonene | 12.5 | 1014 | 1025 | Ki; S; MS |
| 8. | β-trans-Ocimene | 0.4 | 1034 | 1029 | Ki; MS |
| 9. | γ-Terpinen | 0.1 | 1047 | 1051 | Ki; S; MS |
| 10. | Cyclohexane, 2-ethenyl-1,1-dimethyl-3-methylene- | 0.2 | 1071 | Ki; MS | |
| 11. | α-Campholenal | 0.2 | 1106 | 1105 | Ki; MS |
| 12. | trans-Pinocarveol | 0.2 | 1131 | 1126 | Ki; MS |
| 13. | trans-Verbenol | 0.1 | 1136 | 1136 | Ki; MS |
| 14. | 1-Terpinen-4-ol | 0.1 | 1137 | 1137 | Ki; MS |
| 15. | 3-Thujene-10-al | 0.2 | 1158 | 1158 | Ki; MS |
| 16. | Myrtenal | 0.5 | 1175 | 1172 | Ki; MS |
| 17. | Carveol | 0.1 | 1188 | 1200 | Ki; MS |
| 18. | Verbenone | 0.1 | 1204 | 1183 | Ki; MS |
| 19. | trans-Carveol | 0.3 | 1207 | 1210 | Ki; MS |
| 20. | Carvone | 0.2 | 1210 | 1214 | Ki; S; MS |
| 21. | Bornyl acetate | 6.3 | 1270 | 1270 | Ki; S; MS |
| 22. | a-Terpinyl acetate | 0.8 | 1334 | 1335 | Ki; MS |
| 23. | β-Cubebene | 0.2 | 1355 | 1355 | Ki; MS |
| 24. | α-Copaene | 0.4 | 1376 | 1379 | Ki; MS |
| 25. | β-Elemen | 7.1 | 1387 | 1389 | Ki; MS |
| 26. | β-Caryophyllene | 3.0 | 1421 | 1421 | Ki; S; MS |
| 27. | Sesquisabinene A | 1.5 | 1435 | 1435 | Ki; MS |
| 28. | α-Caryophyllene | 1.1 | 1456 | 1454 | Ki; MS |
| 29. | Epi-bicyclosesquiphellandrene | 2.5 | 1470 | 1487 | Ki; MS |
| 30. | Germacrene D | 34.9 | 1480 | 1480 | Ki; S; MS |
| 31. | γ-Cadinene | 2.1 | 1507 | 1507 | Ki; MS |
| 32. | β-Cadinene | 2.8 | 1526 | 1526 | Ki; MS |
| Total | 97.7 | ||||
| Hydrocarbon monoterpenes | 32.9 | ||||
| Oxygenated monoterpenes | 9.1 | ||||
| Sesquiterpene hydrocarbons | 55.5 | ||||
| Oxygenated sesquiterpenes | 0.0 | ||||
| Others | 0.2 |
| Absorbance of Fungal Mycelium Growth at 450 nm | |||||
|---|---|---|---|---|---|
| 3 days b | 4 days | 5 days | 6 days | 7 days | |
| PDB + F | 0.03 ± 0.02c | 1.31 ± 0.25b | 1.40 ± 0.25b | 1.58 ± 0.32a | 1.63 ± 0.32a |
| 800 µg/mL a | 0.03 ± 0.00b | 0.71 ± 0.05a | 0.75 ± 0.05a | 0.74 ± 0.15a | 0.76 ± 0.17a |
| 1000 µg/mL | 0.02 ± 0.00c | 0.46 ± 0.10b | 0.51 ± 0.05b | 0.48 ± 0.03b | * 0.22 ± 0.03a |
| 1200 µg/mL | 0.02 ± 0.00c | 0.35 ± 0.05b | 0.38 ± 0.04b | 0.44 ± 0.02b | * 0.21 ± 0.03a |
| 1400 µg/mL | 0.02 ± 0.00c | 0.36 ± 0.04b | 0.35 ± 0.07b | * 0.25 ± 0.05a | 0.22 ± 0.03a |
| 1600 µg/mL | 0.01 ± 0.00b | * 0.21 ± 0.01a | 0.18 ± 0.03a | 0.14 ± 0.05ab | 0.15 ± 0.05ab |
| PDB | 0.00 ± 0.00 | 0.15 ± 0.0 | 0.15 ± 0.0 | 0.15 ± 0.0 | 0.16 ± 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshafie, H.S.; Gruľová, D.; Baranová, B.; Caputo, L.; De Martino, L.; Sedlák, V.; Camele, I.; De Feo, V. Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules 2019, 24, 1206. https://doi.org/10.3390/molecules24071206
Elshafie HS, Gruľová D, Baranová B, Caputo L, De Martino L, Sedlák V, Camele I, De Feo V. Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules. 2019; 24(7):1206. https://doi.org/10.3390/molecules24071206
Chicago/Turabian StyleElshafie, Hazem S., Daniela Gruľová, Beáta Baranová, Lucia Caputo, Laura De Martino, Vincent Sedlák, Ippolito Camele, and Vincenzo De Feo. 2019. "Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia" Molecules 24, no. 7: 1206. https://doi.org/10.3390/molecules24071206
APA StyleElshafie, H. S., Gruľová, D., Baranová, B., Caputo, L., De Martino, L., Sedlák, V., Camele, I., & De Feo, V. (2019). Antimicrobial Activity and Chemical Composition of Essential Oil Extracted from Solidago canadensis L. Growing Wild in Slovakia. Molecules, 24(7), 1206. https://doi.org/10.3390/molecules24071206










