GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Maceration
4.3. Ultrasound Assisted Extraction
4.4. Hydrodistillation
4.5. Gas Chromatography-Mass Spectrometry Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tajuddin, S.; Ahmad, A.; Latif, I.; Qasmi, A.; Amin, K.M.Y. An experimental study of sexual function improving effect of Myristica fragrans Houtt. (nutmeg). BMC Complement. Altern. Med. 2005, 5, 16–23. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Cong, P.N.; Budnikov, H. Ultrasound-assisted micellar extraction of phenolic antioxidants from spices and antioxidant properties of the extracts based on coulometric titration data. Anal. Methods 2016, 8, 7150–7157. [Google Scholar] [CrossRef]
- Adiani, V.; Gupta, S.; Chatterjee, S.; Variyar, P.S.; Sharma, A. Activity guided characterization of antioxidant components from essential oil of Nutmeg (Myristica fragrans). J. Food Sci. Technol. 2015, 52, 221–230. [Google Scholar] [CrossRef]
- D’Souza, S.P.; Chavannavar, S.V.; Kanchanashri, B.; Niveditha, S.B. Pharmaceutical Perspectives of Spices and Condiments as Alternative Antimicrobial Remedy. J. Evid.-Based Complement. Altern. Med. 2017, 22, 1002–1010. [Google Scholar] [CrossRef]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Nagja, T.; Vimal, K.; Sanjeev, A. Myristica Fragrans: A Comprehensive Review. Int. J. Pharm. Pharm. Sci. 2016, 8, 9–12. [Google Scholar]
- Dupuy, N.; Molinet, J.; Mehl, F.; Nanlohy, F.; Le Dréau, Y.; Kister, J. Chemometric analysis of mid infrared and gas chromatography data of Indonesian nutmeg essential oils. Ind. Crop. Prod. 2013, 43, 596–601. [Google Scholar] [CrossRef]
- Morsy, N.F.S. A comparative study of nutmeg (Myristica fragrans Houtt.) oleoresins obtained by conventional and green extraction techniques. J. Food Sci. Technol. 2016, 53, 3770–3777. [Google Scholar] [CrossRef]
- Piaru, S.P.; Mahmud, R.; Majid, A.M.S.; Nassar, Z.D. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac. J. Trop. Med. 2012, 5, 294–298. [Google Scholar]
- Djilani, A.; Dicko, A. The Therapeutic Benefits of Essential Oils. In Nutrition, Well-Being and Health; Bouayed, J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 155–178. [Google Scholar]
- Barceloux, D.G. Nutmeg (Myristica fragrans Houtt.). Dis. Mon. 2009, 55, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Lanari, D.; Marcotullio, M.; Neri, A. A Design of Experiment Approach for Ionic Liquid-Based Extraction of Toxic Components-Minimized Essential Oil from Myristica fragrans Houtt. Fruits. Molecules 2018, 23, 2817. [Google Scholar] [CrossRef] [PubMed]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar]
- Filly, A.; Fabiano-Tixier, A.S.; Louis, C.; Fernandez, X.; Chemat, F. Water as a green solvent combined with different techniques for extraction of essential oil from lavender flowers. C. R. Chim. 2016, 19, 707–717. [Google Scholar] [CrossRef]
- Choi, M.P.K.; Chan, K.K.C.; Leung, H.W.; Huie, C.W. Pressurized liquid extraction of active ingredients (ginsenosides) from medicinal plants using non-ionic surfactant solutions. J. Chromatogr. A 2003, 983, 153–162. [Google Scholar] [CrossRef]
- Feng, L.; Aun, R.; Yan-Wei, W.; Xiu-Quan, X.; Guan-Hua, C. Optimization of Surfactant-Mediated, Ultrasonic-assisted Extraction of Antioxidant Polyphenols from Rattan Tea (Ampelopsis grossedentata) Using Response Surface Methodology. Pharmacogn. Mag. 2017, 13, 446–453. [Google Scholar]
- Zhang, X.; Ban, Q.; Wang, X.; Wang, Z. Green and Efficient PEG-Based Ultrasonic-Assisted Extraction of Polysaccharides from Tree Peony Pods and the Evaluation of Their Antioxidant Activity In Vitro. Biomed. Res. Int. 2018, 2018, 2121385. [Google Scholar] [CrossRef] [PubMed]
- Rezaeepour, R.; Heydari, R.; Ismaili, A. Ultrasound and salt-assisted liquid-liquid extraction as an efficient method for natural product extraction. Anal. Methods 2015, 7, 3253–3259. [Google Scholar] [CrossRef]
- Minjares-Fuentes, R.; Femenia, A.; Garau, M.C.; Meza-Velázquez, J.A.; Simal, S.; Rossello, C. Ultrasound-assisted extraction of pectins from grape pomace using citric acid: A response surface methodology approach. Carbohydr. Polym. 2014, 106, 179–189. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, 1231–1249. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Kara, N.; Erbas, D.; Baydar, H. The Effect of Seawater Used for Hydrodistillation on Essential Oil Yield and Composition of Oil-Bearing Rose ( Rosa damascena Mill.). Int. J. Sec. Metab. 2017, 4, 423–428. [Google Scholar]
- Charchari, S.; Abdelli, M. Enhanced Extraction by Hydrodistillation of Sage (Salvia officinalis L.) Essential Oil Using Water Solutions of Non-ionic Surfactants. J. Essent. Oil Bear. Plants 2015, 5026, 1094–1099. [Google Scholar]
- Hosseinzadeh, R.; Khorsandi, K.; Hemmaty, S. Study of the Effect of Surfactants on Extraction and Determination of Polyphenolic Compounds and Antioxidant Capacity of Fruits Extracts. PLoS ONE 2013, 8, e57353. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kori, S.; Parmar, A. Surfactant mediated extraction of total phenolic contents (TPC) and antioxidants from fruits juices. Food Chem. 2015, 185, 284–288. [Google Scholar] [CrossRef]
- Krupa, A.; Majda, D.; Jachowicz, R.; Mozgawa, W. Solid-state interaction of ibuprofen and Neusilin US2. Thermochim. Acta 2010, 509, 12–17. [Google Scholar] [CrossRef]
- Sander, C.; Holm, P. Porous Magnesium Aluminometasilicate Tablets as Carrier of a Cyclosporine Self-Emulsifying Formulation. AAPS PharmSciTech 2009, 10, 1388. [Google Scholar] [CrossRef]
- Naiserova, M.; Kubova, K.; Vyslouzil, J.; Pavlokova, S.; Vetchy, D.; Urbanova, M.; Brus, J.; Vyslouzil, J.; Kulich, P. Investigation of Dissolution Behavior HPMC/Eudragit®/Magnesium Aluminometasilicate Oral Matrices Based on NMR Solid-State Spectroscopy and Dynamic Characteristics of Gel Layer. AAPS PharmSciTech 2017, 19, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.G.; Bao, L.; Luciani, A.; Panighi, J.; Desjobert, J.M.; Costa, J.; Casanova, J.; Bolla, J.M.; Berti, L. (E)-Methylisoeugenol and Elemicin: Antibacterial Components of Daucus carota L. Essential Oil against Campylobacter jejuni. J. Agric. Food Chem. 2007, 55, 7332–7336. [Google Scholar] [CrossRef]
- Siqueira, H.D.S.; Neto, B.S.; Sousa, D.P.; Gomes, B.S.; da Silva, F.V.; Cunha, F.V.M.; Wanderley, C.W.S.; Pinheiro, G.; Candido, A.G.F.; Wong, D.V.H. α-Phellandrene, a cyclic monoterpene, attenuates inflammatory response through neutrophil migration inhibition and mast cell degranulation. Life Sci. 2016, 160, 27–33. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, Z.G.; Yu, P.J.; Wang, G.F.; Zhang, J.Y.; Li, J.R.; Ai, R.T.; Li, Z.H.; Tian, Y.X.; Zhang, W.X.; et al. Myrislignan attenuates lipopolysaccharide-induced inflammation reaction in murine macrophage cells through inhibition of NF-κB signalling pathway activation. Phyther. Res. 2012, 26, 1320–1326. [Google Scholar] [CrossRef]
- Lu, X.; Yang, L.; Chen, J.; Zhou, J.; Tang, X.; Zhu, Y.; Qiu, H.; Shen, J. The action and mechanism of myrislignan on A549 cells in vitro and in vivo. J. Nat. Med. 2017, 71, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Vanwert, A.; Bogner, R.H. Formation of Physically Stable Amorphous Drugs by Milling with Neusilin. J. Pharm. Sci. 2003, 92, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Khade, S.; Pore, Y. Formulation and Evaluation Of Neusilin® Us2 Adsorbed Amorphous Solid Self-Microemulsifying Delivery System of Atorvastatin Calcium. Asian J. Pharm. Clin. Res. 2016, 9, 93–100. [Google Scholar]
- Mihajilov-Krstev, T.; Jovanovic, B.; Jovic, J.; Ilic, B.; Miladinovic, D.; Matejic, J.; Rajkovic, J.; Dordevic, L.; Cvetkovic, V.; Zlatkovic, B. Antimicrobial, Antioxidative, and Insect Repellent Effects of Artemisia absinthium Essential Oil. Planta Med. 2014, 80, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.; Zuzarte, M.; Goncalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Salgueiro, L.; Cruz, M.T. Antifungal, antioxidant and anti-inflammatory activities of Oenanthe crocata L. essential oil. Food Chem. Toxicol. 2013, 62, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Sun, S. D-limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar] [PubMed]
- Giweli, A.; Dzamic, A.M.; Sokovic, M.; Ristic, M.S.; Marin, P.D. Antimicrobial and antioxidant activities of essential oils of satureja thymbra growing wild in libya. Molecules 2012, 17, 4836–4850. [Google Scholar] [CrossRef]
- Mallavarapu, G.R.; Ramesh, S. Composition of essential oils of nutmeg and mace. J. Med. Arom. Plant Sci 1998, 20, 746–748. [Google Scholar]
- Gupta, M. Pharmacological properties and traditional therapeutic uses of important indian spices: A review. Int. J. Food Prop. 2010, 13, 1092–1116. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical Composition of the Essential Oils of Aerial Parts and Flowers of Chromolaena odorata (L.) R. M. King & H. Rob. from Western Ghats Region of North West Karnataka, India. J. Essent. Oil-Bear. Plants 2013, 16, 71–75. [Google Scholar]
- Chu, S.; Liu, O.; Zhou, L.; Du, S.; Liu, Z. Chemical composition and toxic activity of essential oil of Caryopteris incana against Sitophilus zeamais. Afr. J. Biotechnol. 2013, 10, 8476–8480. [Google Scholar]
- Ekundayo, O.; Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A. Chemical composition of essential oil of myristica fragrans houtt (nutmeg) from nigeria. J. Essent. Oil-Bear. Plants 2003, 6, 21–26. [Google Scholar]
- Du, S.S.; Yang, K.; Wang, C.F.; You, C.X.; Geng, Z.F.; Guo, S.S.; Deng, Z.W.; Liu, Z.L. Chemical constituents and activities of the essential oil from myristica fragrans against cigarette beetle lasioderma serricorne. Chem. Biodivers. 2014, 11, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 1995. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Method | Sample Code | Temperature | Time (h) | Solvent | Solvent: Nutmeg Ratio | Magnesium Aluminometasilicate: Nutmeg Ratio |
|---|---|---|---|---|---|---|
| Maceration | M1 | ambient | 72 | purified water | 20:1 | - |
| Ultrasound-Assisted Extraction | UAE1 | 25 °C | 0.5 | ethanol 50% | 20:1 | - |
| UAE2 | ethanol 70% | 20:1 | - | |||
| UAE3 | ethanol 96% | 20:1 | - | |||
| UAE4 | ethanol 70% + 0.5% magnesium aluminometasilicate | 20:1 | 10:1 | |||
| UAE5 | ethanol 70% + 1% magnesium aluminometasilicate | 20:1 | 5:1 | |||
| UAE6 | ethanol 70% + 2% magnesium aluminometasilicate | 20:1 | 2.5:1 | |||
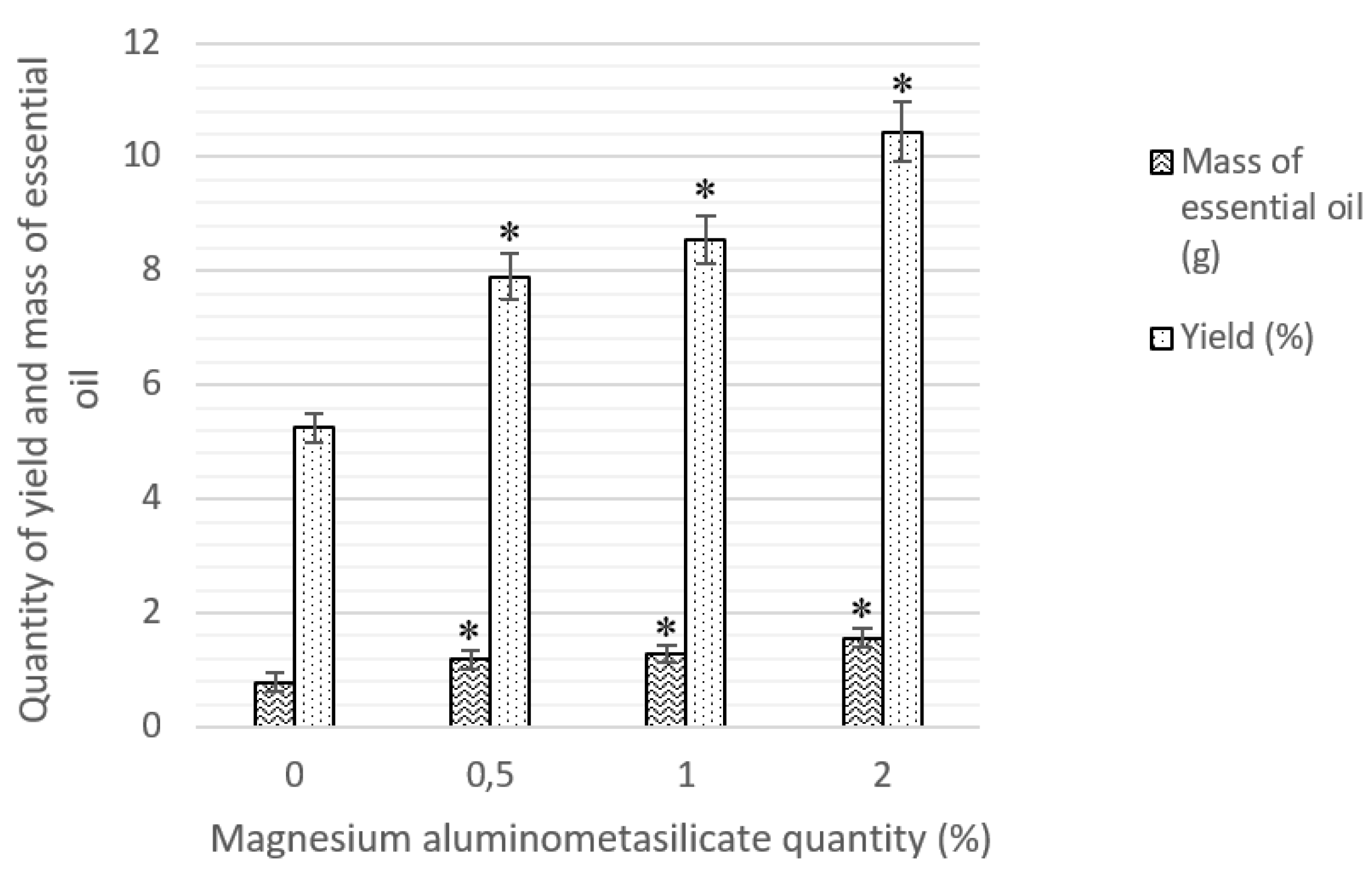
| Hydrodistillation | HD1 | 100 °C | 4 | purified water | 20:1 | - |
| HD2 | purified water + 0.5% magnesium aluminometasilicate | 20:1 | 10:1 | |||
| HD3 | purified water + 1% magnesium aluminometasilicate | 20:1 | 5:1 | |||
| HD4 | purified water + 2% magnesium aluminometasilicate | 20:1 | 2.5:1 |
| Compound | Sample Code a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Essential Oil Compounds | Extracts Compounds | |||||||||||
| RI d | HD e 1 (%) | HD2 (%) | HD3 (%) | HD4 (%) | M f 1 (%) | UAE g 1 (%) | UAE2 (%) | UAE3 (%) | UAE4 (%) | UAE5 (%) | UAE6 (%) | |
| 4-Carene, trans -(+)- | 911 | 7.77 | - | - | - | 3.37 | 1.19 | 6.56 | 13.22 | - | - | - |
| α-Thujene | 928 | 0.99 | 1.04 | 0.93 | 0.99 | - | 2.63 | 0.72 | 1.42 | 0.84 | 0.92 | 0.81 |
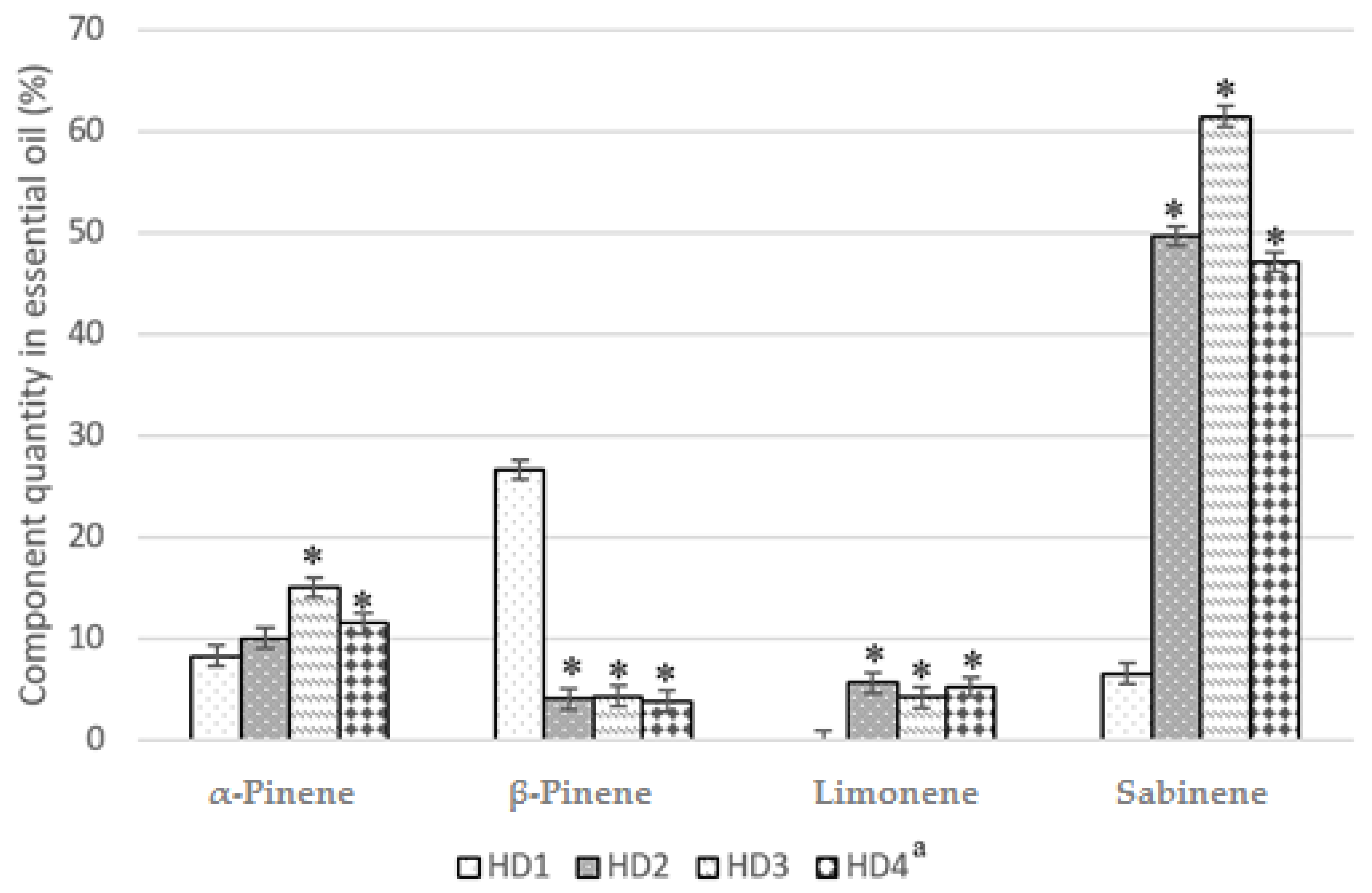
| α-Pinene | 934 | 8.27 | 10.04 | 15.05 | 11.5 | - | 2.4 | 8.3 | 14.67 | 9.65 | 8.13 | 9.94 |
| Camphene | 944 | - | 2.8 | 0.11 | 0.11 | - | - | - | - | - | - | 0.15 |
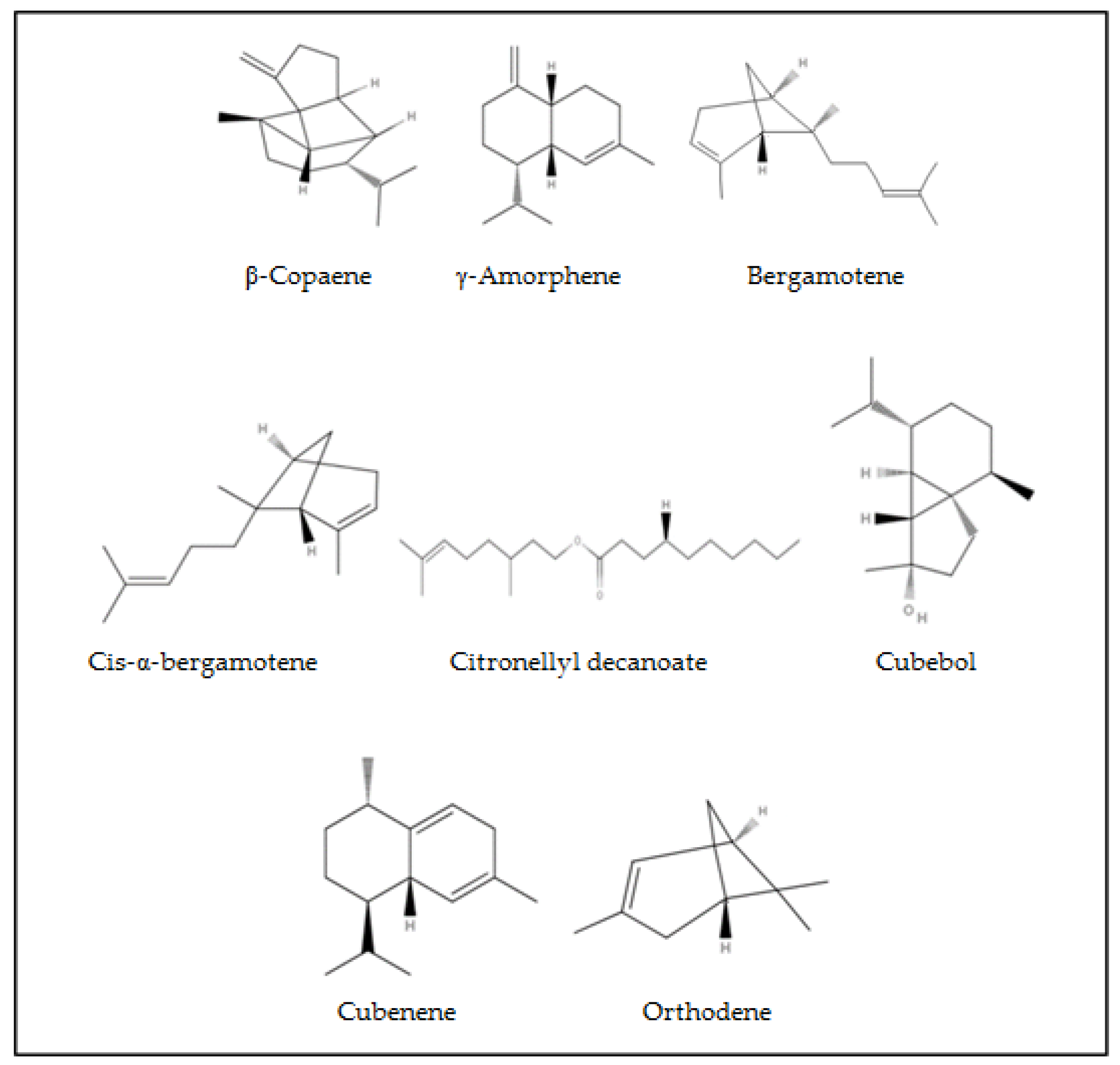
| Orthodene b | 949 | 0.51 | - | - | - | - | - | - | 0.59 | - | - | - |
| 3-Carene | 956 | 0.53 | - | - | - | - | - | 0.42 | 0.78 | - | - | - |
| α-Phellandrene | 957 | - | - | - | - | 13.04 | - | 1.41 | 2.38 | - | - | - |
| β-Myrcene | 958 | - | - | - | - | 4.6 | - | - | - | 9.47 | 7.26 | 9.19 |
| 2-Carene | 960 | 1.99 | - | - | - | - | - | 0.75 | 1.15 | - | - | - |
| Sabinene | 969 | 6.53 | 49.64 | 61.42 | 47.1 | - | - | - | - | 29.9 | 31.74 | 32.69 |
| β-Pinene | 970 | 26.61 | 4.03 | 4.32 | 3.84 | - | - | - | - | - | - | - |
| Myristicin | 981 | 3.26 | 2.34 | 2.15 | 2.16 | - | - | - | - | 1.79 | 1.65 | 1.96 |
| γ-Terpinene | 993 | - | 0.78 | 0.68 | 0.73 | - | - | - | - | 0.68 | 1.65 | 0.71 |
| α-Terpinene | 999 | 1.23 | 1.23 | 0.94 | 1.23 | - | - | 0.6 | 1.07 | 0.67 | 1.18 | 0.69 |
| 3,7,7-trimethylcyclohepta-1,3,5-triene | 1005 | 0.18 | 0.47 | 0.33 | 0.44 | - | - | - | - | 0.37 | - | - |
| Limonene | 1009 | - | 5.62 | 4.2 | 5.23 | - | - | - | - | 4.2 | 4.46 | 4.63 |
| Isoterpinolene | 1030 | - | 2.11 | 1.18 | 2.07 | - | - | - | - | 1.19 | 2.02 | 1.22 |
| Cis-sabinen hydrate | 1037 | 7.76 | 0.58 | 0.3 | 0.83 | - | - | - | - | 1.3 | 2.32 | 1.46 |
| γ-Terpineol | 1043 | - | 0.04 | 0.04 | 0.04 | - | - | - | 0.14 | - | - | 0.09 |
| α-Terpinolene | 1051 | - | 0.7 | 0.38 | 0.7 | - | - | - | - | - | - | 0.54 |
| Sylvestrene | 1059 | - | - | - | - | 1.57 | - | - | - | - | - | - |
| Isomethyleugenol | 1062 | 0.2 | - | - | - | 6.38 | - | - | - | - | - | - |
| Cis-p-menth-2-en-1-ol | 1076 | 2.02 | 0.38 | 0.37 | 0.38 | 0.43 | 3.27 | 5.88 | 3.25 | 1.32 | 2.42 | 1.62 |
| 4-Propenyl syringol | 1083 | - | - | - | - | - | 2.16 | 1.55 | - | - | - | - |
| Citronellyl Decanoate b | 1110 | 0.3 | - | - | - | - | - | 0.43 | 0.16 | - | - | - |
| 1,1-dimethyl-2-[(1E)-3-methylbuta-1,3-dienyl cyclopropane | 1116 | - | - | - | - | - | 8.4 | 29.99 | 47.32 | - | - | - |
| Bicyclogermacrene | 1125 | 0.29 | - | - | - | - | - | - | - | - | - | - |
| 4-Terpineol | 1152 | 0.74 | 0.34 | 0.03 | 0.35 | - | - | 0.77 | 0.3 | 0.47 | 0.47 | 0.47 |
| Cubebol b | 1174 | 0.05 | - | - | - | - | - | - | - | - | - | - |
| Cubenene b | 1177 | 0.07 | - | - | - | - | - | - | - | - | - | - |
| Piperitol | 1189 | 0.09 | - | - | - | - | - | - | - | - | - | - |
| Isoelemicin | 1217 | 5.98 | - | - | - | - | 62.24 | 23.-63 | - | 21.5 | 21.5 | 18.85 |
| Copaene | 1228 | - | 0.25 | 0.01 | 0.25 | - | - | - | - | - | - | - |
| β-Copaene b | 1233 | 0.25 | - | - | - | - | - | - | - | - | - | - |
| γ-Amorphene b | 1245 | - | 0.75 | 0.03 | 0.78 | - | - | - | - | 0.43 | 0.92 | 0.08 |
| Cis-α-bergamotene b | 1283 | - | 0.09 | 0.08 | 0.1 | - | - | - | - | - | - | - |
| Isogermacrene | 1311 | - | 0.99 | 0.01 | 1.15 | 1.61 | - | - | - | 0.86 | 2.44 | 0.82 |
| Licarin B | 1328 | - | - | - | - | - | - | 0.72 | - | 0.71 | - | - |
| Bergamotene b | 1339 | 0.07 | - | - | - | - | - | - | - | - | - | - |
| γ-Asarone | 1361 | 3.69 | 1.21 | 1.22 | 3.51 | 0.79 | 0.84 | 2.07 | - | 0.82 | 1.84 | 1.25 |
| Elemicin | 1542 | - | - | - | - | 13.99 | - | - | - | - | - | - |
| Myrislignan | 2946 | - | - | - | - | 22.59 | - | - | - | - | - | - |
| Total Identified Compounds % c | 79.38 | 85.43 | 93.78 | 83.6 | 68.37 | 83.13 | 83.8 | 86.45 | 86.1 | 90.92 | 87.17 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulyte, I.; Marksa, M.; Ivanauskas, L.; Kalvėnienė, Z.; Lazauskas, R.; Bernatoniene, J. GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules 2019, 24, 1062. https://doi.org/10.3390/molecules24061062
Matulyte I, Marksa M, Ivanauskas L, Kalvėnienė Z, Lazauskas R, Bernatoniene J. GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules. 2019; 24(6):1062. https://doi.org/10.3390/molecules24061062
Chicago/Turabian StyleMatulyte, Inga, Mindaugas Marksa, Liudas Ivanauskas, Zenona Kalvėnienė, Robertas Lazauskas, and Jurga Bernatoniene. 2019. "GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient" Molecules 24, no. 6: 1062. https://doi.org/10.3390/molecules24061062
APA StyleMatulyte, I., Marksa, M., Ivanauskas, L., Kalvėnienė, Z., Lazauskas, R., & Bernatoniene, J. (2019). GC-MS Analysis of the Composition of the Extracts and Essential Oil from Myristica fragrans Seeds Using Magnesium Aluminometasilicate as Excipient. Molecules, 24(6), 1062. https://doi.org/10.3390/molecules24061062