1. Introduction
Plants synthesize numerous natural organic compounds having complex chemical structures. These plant-derived compounds play a crucial role in their ecological functions [
1]. Extensive studies have indicated that terpenoids, phenolics, and nitrogen-containing substances are important phytoalexins which provide a defense system and protect plants against attack by harmful microbes and herbivores [
1,
2]. The plants that release these active compounds are capable to compete and invade other plant species in their vicinity by suppressing their growth (a natural phenomenon known as allelopathy) [
3,
4]. In addition to their physiological functions in plants, numerous phytoalexins have also been reported to possess strong antioxidant, antibacterial, and herbicidal properties. A number of bioactive compounds have been isolated, purified and employed in a wide range of applications including food, pharmaceutical, cosmetic, and agricultural industries [
1,
5,
6,
7]. Therefore, the exploration of active medicinal plants and their natural bioactive molecules has become essential to exploit the possible additional values of natural sources.
Jatropha podagrica is a succulent shrub belonging to the family Euphorbiaceae. It is widely distributed in tropical and subtropical areas worldwide [
8].
J. podagrica is an ornamental plant and one of the most important materials of traditional folklore medicines in Asia, Latin America, and Africa [
9,
10,
11]. In traditional therapies, this plant has been extensively used as an effective treatment for skin infections [
8], jaundice and fever [
12], sexually transmitted diseases like gonorrhea [
8,
13] pain relief [
14], gout [
15], and paralysis [
15,
16]. In addition, its seed oil is applied in African ethnomedical practice as a natural remedy for rheumatic conditions, pruritus, and to alleviate constipation, while its leaves have been used as a hemostatic agent (IPCS-INCHEM). In Nigeria, indigenous people utilize this shrub to cure hepatitis [
17]. The investigations of the biologically active components of
J. podagrica resulted in the isolations of japodic acid, erytrinasinate, fraxidin [
13], steroids and flavonoids [
18], podacycline A and B [
19], diterpenoids, japodagrone, japodagrin [
8], 3-acetylaleuritolic acid, japodagrol [
20], n-heptyl ferulate, and γ-sitosterol [
21]. Although extracts of different parts of
J. podagrica have been reported to possess various biological properties including antiproliferative, antioxidant, antitumor, antibacterial, and antimicrobial [
8,
12,
13], the search for phytochemicals responsible for the observed activities has been conducted sporadically, except for antibacterial capacity. Additionally, little information has been found concerning phytochemicals and biological activity from
J. podagrica stem bark so far [
22]. Several second metabolites including fraxidin, fraxetin, scoparone, 3-acetylaleuritolic acid, β-sitosterol, and sitosterone from the stem bark of this plant were isolated, but their biological activity was not examined [
21]. In another trial, although the antimicrobial activity of
J. podagrica stem bark extracts was evaluated, isolation of the compounds responsible for the studied activity was not achieved. Therefore, the objectives of this research were to establish a simple and effective protocol to isolate the bioactive components present in
J. podagrica stem bark. The bioactive properties including antioxidant, antibacterial, and plant growth inhibitory activities were also evaluated.
3. Discussion
The radical scavenging activity of the isolated fractions was examined using DPPH, which is a frequently used method in natural product antioxidant evaluation [
24]. Previous reports showed that methanol and water extracts of
J. podagrica leaves and seeds exhibited antioxidant activities. By using this method, their antioxidant capacities were evaluated at IC
50 values of 78.19 and 71.34 µg/mL, respectively [
12]. In the genus
Jatropha, the antioxidant capacity of bark extracts has been investigated for
J. curcas [
25]. At a concentration of 1000 µg/mL, the percentages of DPPH and ABTS radical scavenging activity of different extracts were shown as follows: the methanolic extract (91.5% and 89%, respectively), the aqueous extract (80.5% and 86.8%, respectively), and the ethanolic extract (78.2% and 87.78%, respectively). However, the IC
50 values of the antioxidant activity of these extracts were not mentioned. In this study, for the first time, we found that the
J. podagrica stem bark extracts possessed remarkable antioxidant capacity. At a concentration of 500 µg/mL, the inhibition percentage of hexane, EtOAc, and aqueous was >95% in both the DPPH and ABTS methods. The oxidation process is involved with multiple reaction characteristics and different mechanisms; hence, no single method can accurately evaluate the antioxidants in complex botanical extracts. The result indicated that this compound could easily donate an electron to Fe (III) most effectively, thus reducing it to Fe (II) [
24,
26]. Therefore, three different assays including DPPH, ABTS radical scavenging activities, and reducing power assay were employed to measure the antioxidant activity of various extracts of
J. podagrica. The result obtained from these methods showed consistently that the EtOAc extract had the strongest antioxidant ability (
Table 1), indicating that the antioxidants of this sub-woody shrub have been effectively enriched in this extraction. Compared with
J. podagrica leaves and seeds, the extract of stem bark demonstrated a stronger DPPH radical scavenging activity (IC
50 = 46.7 µg/mL,
Table 1). Moreover, the antioxidant capacity of
J. podagrica seed extract has been reported to be greater than that of ascorbic acid [
12]. Our results suggest that the EtOAc extract of stem bark might contain the major antioxidants of
J. podagrica.
Due to the considerable therapeutic values, intensive investigations of
J. podagrica have documented the presence of many classes of plant secondary metabolites such as diterpenoids, flavonoids, steroids, cyclic peptides [
8,
13,
18,
19,
20] (
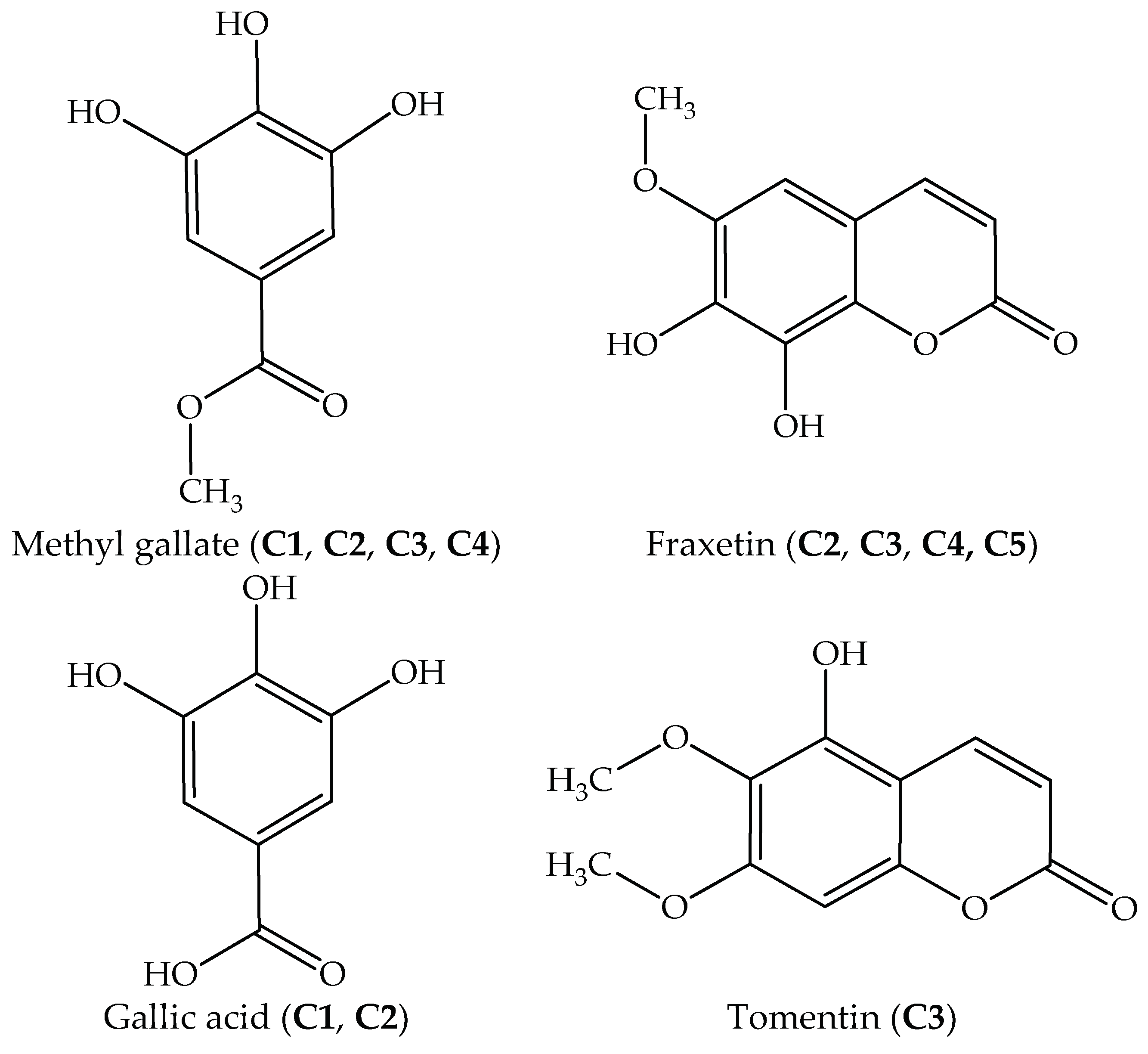
Table 7). However, they have primarily focused thus far on the root, leaf, and seed of this plant, while studies on stem bark have been desultory. In this study, four compounds were isolated and identified from the most effective EtOAc extract of stem bark, namely fraxetin, gallic acid, methyl gallate, and tomentin. They are biologically active constituents belonging to coumarin and phenolic acids. The antioxidant capacities of a purified compound (
C5) and the mixtures
C1,
C2,
C3, and
C4 were significantly stronger than the EtOAc extract and the standard (BHT), except for the DPPH radical scavenging activity of
C1 (methyl gallate, gallic acid) which was lower than that of BHT. Specifically, the antioxidant abilities of a mixture
C2 (fraxetin, gallic acid, and methyl gallate) and a pure compound
C5 (fraxetin) measured by DPPH and ABTS assays, were significantly higher than BHT, approximately 3 to 18 folds, respectively [
27,
28,
29,
30,
31]. Fraxetin was successfully isolated from
Fraxinus rhinchophylla and reported to have potential anti-oxidative effects [
32], while gallic acid and methyl gallate have been separated from
Givotia rottleriformis and reported to have anti-proliferative effects [
33].
In addition to antioxidant potential, isolated constituents of
J. podagrica showed considerable herbicide activity and antibacterial property. Although the antibacterial capacity of extracts and isolated compounds of
J. podagrica has been investigated so far [
8], the growth inhibitory activity of these plants was acknowledged for the first time in this study. According to the allelopathic assay, all fractions inhibited the growth of the studied plants at various levels. Generally, the level of inhibition on root lengths was higher than on shoot elongations in all plants (
Table 6). The reduction in root growth might be due to the sensitivity of roots to allelochemicals. The strongest inhibitory effect was observed in the growth reduction of
L. sativa. The results suggest that these fractions isolated from
J. podagrica might be potent candidates for the development of novel herbicides. Allelochemicals other than the identified components of
J. podagrica in this study and their interference mechanisms need further elaboration.
Dilution is one of the most appropriate techniques for determining the MIC value [
34]. By using this method, we can estimate the lowest concentration of antimicrobial agents that will inhibit the visible growth of a microorganism. Antibacterial agents of
J. podagrica have been reported thus far including fraxidin, fraxetin, erythrinasinate, japodgrin, japodagrone, 4z-jatrogrosidentadion, 15-epi-4z–jatrogrossidentadion, 2-hydroxyisojatrogrossidion, and 2-epihydroxyisojatrogrossidion. Those compounds exerted antibacterial activity toward
S. aureus and
B. subtilis with inhibition zones ranging from 12 to 35 mm at a concentration of 20 µg/disk (
Table 7). However, other pathogenic bacteria such as
E. coli and
Pseudomonas aeruginosa were not sensitive to these compounds at the same concentration. In this study, the antibacterial effects of five isolated fractions were evaluated against the growth of six different pathogens including
S. aureus, E. coli, K. pneumoniae, L. monocytogenes, B. subtilis, and
P. mirabilis. The studied microorganisms are ubiquitous bacteria causing an array of serious nosocomial infections worldwide. The result revealed that most of the bacteria strains were susceptible to the fractions at the tested concentrations. Among the isolated fractions, a combination of fraxetin and methyl gallate in the mixture
C4 caused the strongest antibacterial effect on
S. aureus, E. coli, L. monocytogenes, B. subtilis, and
P. mirabilis (
Table 5). Therefore, the reaction mechanism of this synergic effect should be further investigated. It was observed that gram-negative bacteria
K. pneumoniae and
P. mirabilis exhibited high resistance to treatment by
C1 and
C2. A possible explanation for this might be that the outer membrane of gram-negative bacteria acts as a barrier which is able to protect them against the penetration of compounds, and the periplasmic space carries enzymes which can break down foreign molecules introduced from outside [
35].
In this study, phytochemical investigation of stem bark of
J. podagrica led to identification of four constituents. Among the isolated compounds, fraxetin (
C5) was previously identified from
J. podagrica by Rumzhum et al. [
21] and has been known as an antibacterial and antidiabetic agent [
36,
37]. Other constituents including gallic acid, methyl gallate, and tomentin were isolated for the first time in the stem bark of
J. podagrica herein. The content of pure fraxetin (
C5) was quantified as 13.04 µg/g DW (
Table 3). Gallic acid and methyl gallate have been documented as medicinally important components found in most plants and possess a wide range of pharmacological activities such as antioxidant, anticancer, anti-HIV, antiulcerogenic, anti-inflammatory, and antifungal [
38,
39]. Tomentin was firstly identified in the root of
J. curcas which is another member of the Euphorbiaceae family [
40]. The biological activities of this substance have been described as a potent anti-inflammatory capacity [
41]. The result in
Figure 1 suggests that the combination of hexane and ethyl acetate at 8:2, 7:3, and 6:4 was the most efficient elution to yield bioactive components from
J. podagrica stem bark. This is the first report of methyl gallate, gallic acid, and tomentin from
J. podagrica. This research provided practical information about purification of fraxetin by effective and simple methods. However, further work is required to establish more efficacious solvent systems to purify the methyl gallate, tomentin, and gallic acid in the
J. podagrica stem bark.